Multipurpose Agricultural Reuse of Microalgae Biomasses Employed for the Treatment of Urban Wastewater
Abstract
:1. Introduction
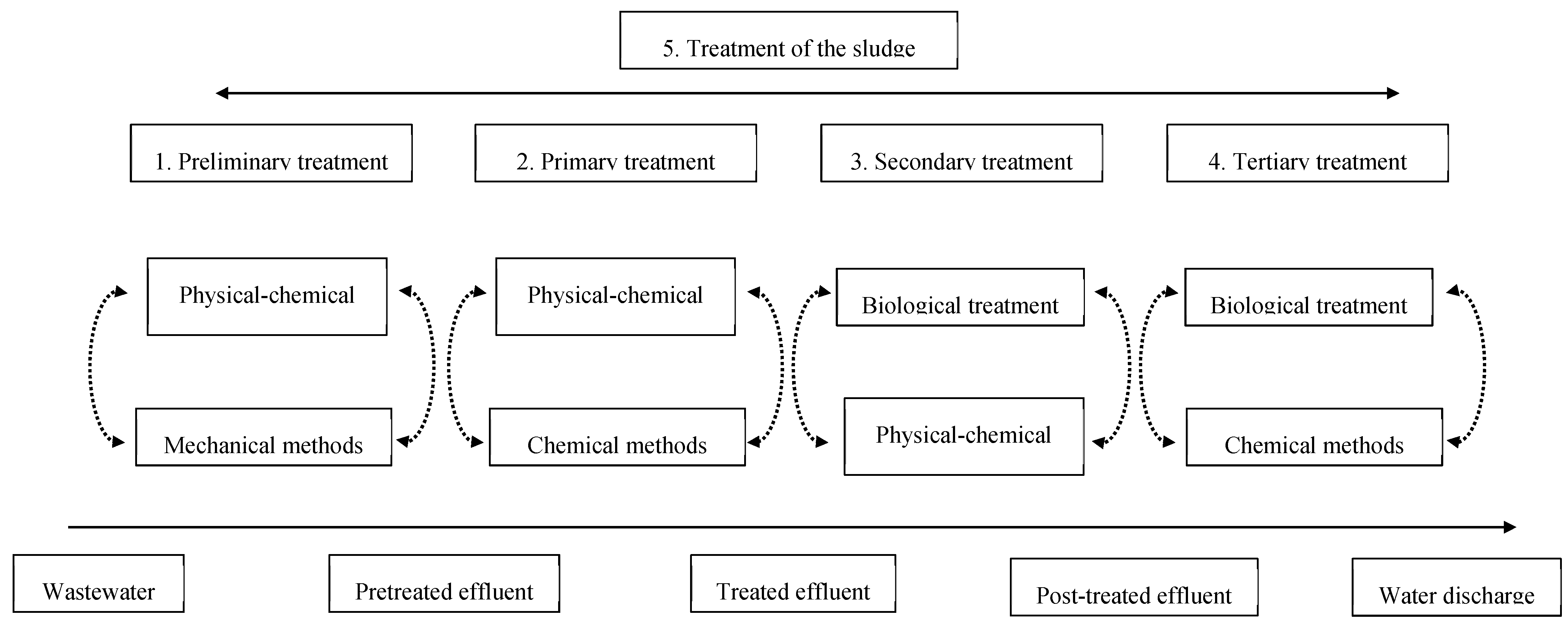
2. Conventional Purification Methods of Wastewaters
- Preliminary treatment (physical and mechanical) includes screening, grinding, grit removal, flotation, equalization, and flocculation. The primary objective of this treatment consists of the removal of solids and other large substances often present in raw wastewater [8]. This step aims to remove or reduce, in size, the solids.
- Primary treatment (physiochemical and chemical) involves the physical processes of screening, comminution, and sedimentation. This stage is aimed to remove solid substances, both organic and inorganic, from wastewater [9]. Some forms of organic nitrogen, organic phosphorous, and heavy metals associated with solids are also removed during this process [8].
- Secondary treatment or purification (chemical and biological) is based on the use of microorganisms to remove the contaminants. Several aerobic biological processes are used in the way in which the oxygen is supplied to the microorganisms, and in the rate at which organisms metabolize the organic matter [8]. The main purpose of these treatments is the removal of fine suspended and dispersed solids, and dissolved organics.
- Tertiary or final treatment (physical and chemical) is the final process that enhances the quality of wastewater before it is reused or discharged to the environment, and treatment of the sludge formed.
- Treatment of the sludge (supervised tipping, recycling, incineration) consists of the sustainable management of the sludge in order to reduce the impact on the environment.
3. Phytoremediation
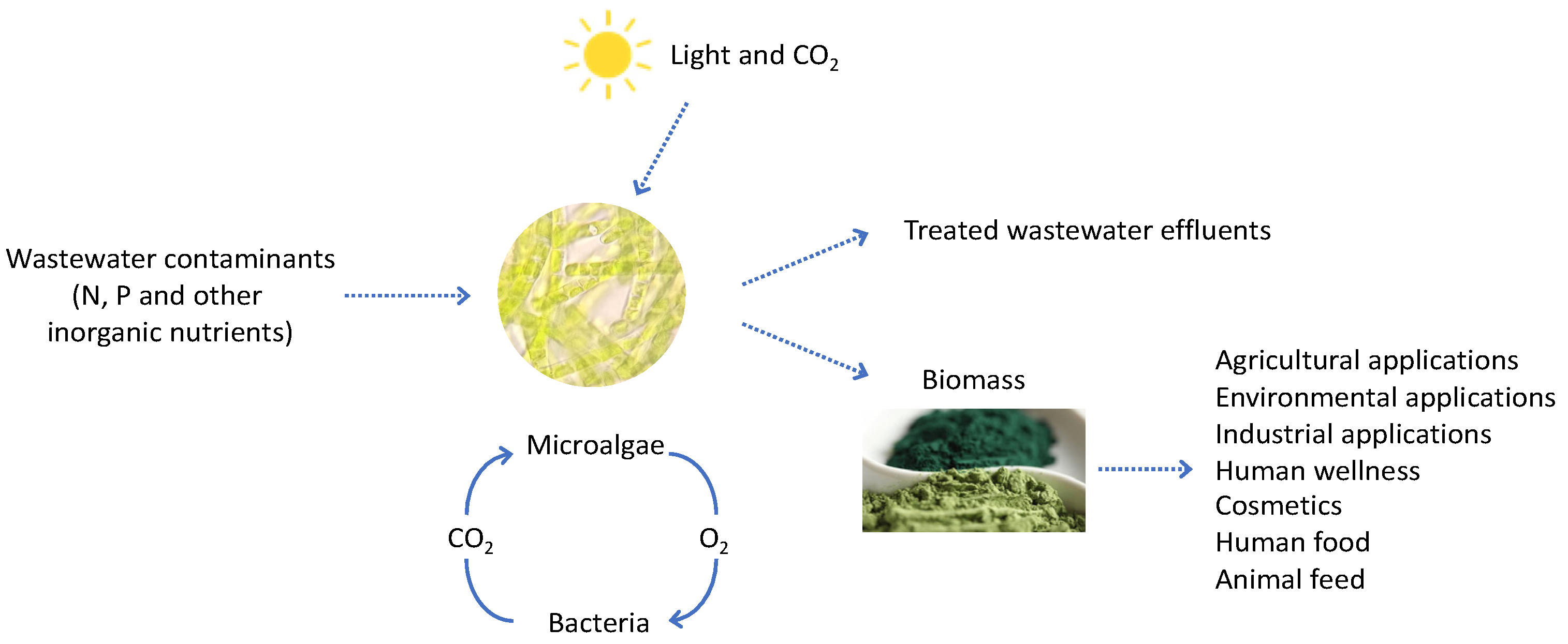
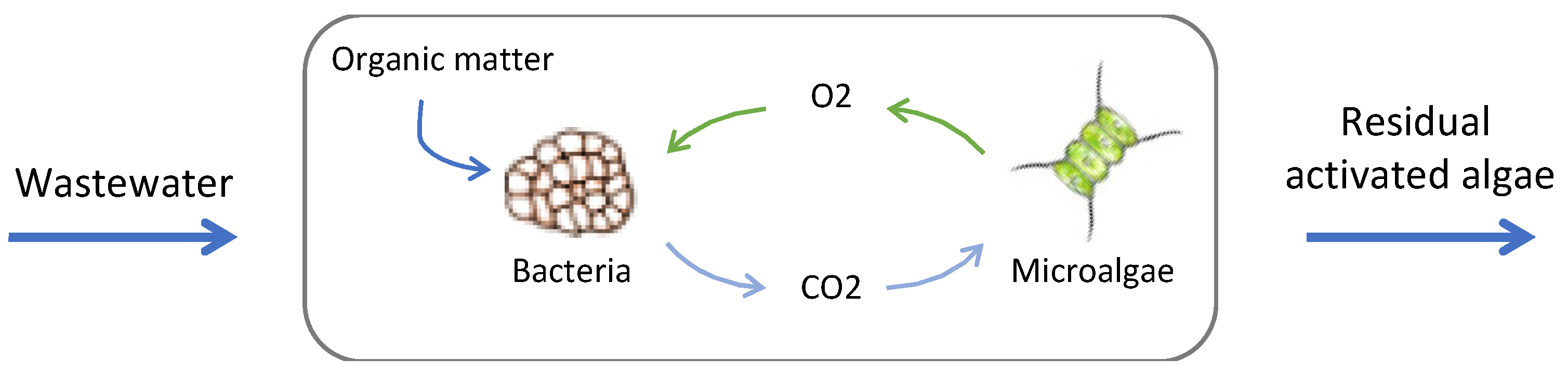
4. Phycoremediation
- -
- Autotrophic: microalgae grow by obtaining energy through the absorption of light energy for the reduction of CO2 by oxidation of the substrates with the release of O2.
- -
- Heterotrophic: microalgae grow using organic carbon in the dark, solving problems related to the presence and distribution of light and CO2.
- -
- Mixotrophic: microalgae grow depending on the environmental conditions in their regime, during which CO2 and organic carbon may be assimilated, depending on their availability, under either autotrophic or heterotrophic conditions.
4.1. Chlorella sp.
4.2. Ankistrodesmus sp.
4.3. Scenedesmus sp.
4.4. Other Species
5. Employ of Microalgae in Agriculture
5.1. Biostimulants
5.2. Biofertilizers
5.3. Biopesticides
6. Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Chai, W.S.; Tan, W.G.; Halimatul Munawaroh, H.S.; Gupta, V.K.; Ho, S.H.; Show, P.L. Multifaceted Roles of Microalgae in the Application of Wastewater Biotreatment: A Review. Environ. Pollut. 2021, 269, 116236. [Google Scholar] [CrossRef]
- Wollmann, F.; Dietze, S.; Ackermann, J.U.; Bley, T.; Walther, T.; Steingroewer, J.; Krujatz, F. Microalgae Wastewater Treatment: Biological and Technological Approaches. Eng. Life Sci. 2019, 19, 860–871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolognesi, S.; Bernardi, G.; Callegari, A.; Dondi, D.; Capodaglio, A.G. Biochar Production from Sewage Sludge and Microalgae Mixtures: Properties, Sustainability and Possible Role in Circular Economy. Biomass Convers. Biorefinery 2021, 11, 289–299. [Google Scholar] [CrossRef]
- Rizzo, L.; Malato, S.; Antakyali, D.; Beretsou, V.G.; Đolić, M.B.; Gernjak, W.; Heath, E.; Ivancev-Tumbas, I.; Karaolia, P.; Lado Ribeiro, A.R.; et al. Consolidated vs New Advanced Treatment Methods for the Removal of Contaminants of Emerging Concern from Urban Wastewater. Sci. Total Environ. 2019, 655, 986–1008. [Google Scholar] [CrossRef] [PubMed]
- Puzowski, P.; Skoczko, I. Investigation on Magnetic Field Usage for Urban Water Treatment. Proceedings 2020, 51, 1031. [Google Scholar] [CrossRef]
- Skoczko, I.; Puzowski, P.; Szatyłowicz, E. Experience from the Implementation and Operation of the Biological Membrane Reactor (Mbr) at the Modernized Wastewater Treatment Plant in Wydminy†. Water 2020, 12, 3410. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and Disadvantages of Techniques Used for Wastewater Treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Sonune, A.; Ghate, R. Developments in Wastewater Treatment Methods. Desalination 2004, 167, 55–63. [Google Scholar] [CrossRef]
- Zinicovscaia, I. Conventional Methods of Wastewater Treatment. In Cyanobacteria for Bioremediation of Wastewaters; Springer: Berlin/Heidelberg, Germany, 2016; pp. 17–25. ISBN 9783319267517. [Google Scholar]
- Sharma, S.; Singh, B.; Manchanda, V.K. Phytoremediation: Role of Terrestrial Plants and Aquatic Macrophytes in the Remediation of Radionuclides and Heavy Metal Contaminated Soil and Water. Environ. Sci. Pollut. Res. 2015, 22, 946–962. [Google Scholar] [CrossRef]
- Dar, S.H.; Kumawat, D.M.; Singh, N.; Wani, K.A. Sewage Treatment Potential of Water Hyacinth (Eichhornia Crassipes). Res. J. Environ. Sci. 2011, 5, 377. [Google Scholar] [CrossRef]
- Rahman, M.A.; Hasegawa, H. Aquatic Arsenic: Phytoremediation Using Floating Macrophytes. Chemosphere 2011, 83, 633–646. [Google Scholar] [CrossRef] [Green Version]
- Malaviya, P.; Singh, A. Constructed Wetlands for Management of Urban Stormwater Runoff. Crit. Rev. Environ. Sci. Technol. 2012, 42, 2153–2214. [Google Scholar] [CrossRef]
- Zhang, D.; Gersberg, R.M.; Ng, W.J.; Tan, S.K. Removal of Pharmaceuticals and Personal Care Products in Aquatic Plant-Based Systems: A Review. Environ. Pollut. 2014, 184, 620–639. [Google Scholar] [CrossRef]
- Ahmad, J.; Abdullah, S.R.S.; Hassan, H.A.; Rahman, R.A.A.; Idris, M. Saringan Tumbuhan Akuatik Tropika Tempatan Untuk Rawatan Penyudahan Sisa Pulpa Dan Kertas. Malays. J. Anal. Sci. 2017, 21, 105–112. [Google Scholar] [CrossRef]
- Luo, L.; He, H.; Yang, C.; Wen, S.; Zeng, G.; Wu, M.; Zhou, Z.; Lou, W. Nutrient Removal and Lipid Production by Coelastrella Sp. in Anaerobically and Aerobically Treated Swine Wastewater. Bioresour. Technol. 2016, 216, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Raouf, N.; Al-Homaidan, A.A.; Ibraheem, I.B.M. Microalgae and Wastewater Treatment. Saudi J. Biol. Sci. 2012, 19, 257–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Zhang, C.; Tan, L.; Wang, J. Toxicity of Co Nanoparticles on Three Species of Marine Microalgae. Environ. Pollut. 2018, 236, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Koppel, D.J.; Adams, M.S.; King, C.K.; Jolley, D.F. Chronic Toxicity of an Environmentally Relevant and Equitoxic Ratio of Five Metals to Two Antarctic Marine Microalgae Shows Complex Mixture Interactivity. Environ. Pollut. 2018, 242, 1319–1330. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xiao, X.; Wang, S.; Zhang, X.; Li, J.; Pavlostathis, S.G.; Luo, X.; Luo, S.; Zeng, G. Synergistic Removal of Cadmium and Organic Matter by a Microalgae-Endophyte Symbiotic System (MESS): An Approach to Improve the Application Potential of Plant-Derived Biosorbents. Environ. Pollut. 2020, 261, 114177. [Google Scholar] [CrossRef]
- Suganya, T.; Varman, M.; Masjuki, H.H.; Renganathan, S. Macroalgae and Microalgae as a Potential Source for Commercial Applications along with Biofuels Production: A Biorefinery Approach. Renew. Sustain. Energy Rev. 2016, 55, 909–941. [Google Scholar] [CrossRef]
- Quijano, G.; Arcila, J.S.; Buitrón, G. Microalgal-Bacterial Aggregates: Applications and Perspectives for Wastewater Treatment. Biotechnol. Adv. 2017, 35, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Rawat, I.; Ranjith Kumar, R.; Mutanda, T.; Bux, F. Dual Role of Microalgae: Phycoremediation of Domestic Wastewater and Biomass Production for Sustainable Biofuels Production. Appl. Energy 2011, 88, 3411–3424. [Google Scholar] [CrossRef]
- Zhu, Y.; Tu, X.; Chai, X.S.; Wei, Q.; Guo, L. Biological Activities and Nitrogen and Phosphorus Removal during the Anabaena Flos-Aquae Biofilm Growth Using Different Nutrient Form. Bioresour. Technol. 2018, 251, 7–12. [Google Scholar] [CrossRef]
- Hena, S.; Znad, H.; Heong, K.T.; Judd, S. Dairy Farm Wastewater Treatment and Lipid Accumulation by Arthrospira Platensis. Water Res. 2018, 128, 267–277. [Google Scholar] [CrossRef]
- Renuka, N.; Sood, A.; Ratha, S.K.; Prasanna, R.; Ahluwalia, A.S. nutrient sequestration, biomass production by microalgae and phytoremediation of sewage water. Int. J. Phytoremediation 2013, 15, 789–800. [Google Scholar] [CrossRef]
- Rasoul-Amini, S.; Montazeri-Najafabady, N.; Shaker, S.; Safari, A.; Kazemi, A.; Mousavi, P.; Mobasher, M.A.; Ghasemi, Y. Removal of Nitrogen and Phosphorus from Wastewater Using Microalgae Free Cells in Bath Culture System. Biocatal. Agric. Biotechnol. 2014, 3, 126–131. [Google Scholar] [CrossRef]
- Colak, O.; Kaya, Z. A Study on the Possibilities of Biological Wastewater Treatment Using Algae. Doga. Biyoloji. Serisi. 1988, 12, 18–29. [Google Scholar]
- Wang, L.; Min, M.; Li, Y.; Chen, P.; Chen, Y.; Liu, Y.; Wang, Y.; Ruan, R. Cultivation of Green Algae Chlorella Sp. in Different Wastewaters from Municipal Wastewater Treatment Plant. Appl. Biochem. Biotechnol. 2010, 162, 1174–1186. [Google Scholar] [CrossRef]
- Lau, P.S.; Tam, N.F.Y.; Wong, Y.S. Wastewater Nutrients Removal by Chlorella Vulgaris: Optimization Through Acclimation. Environ. Technol. 1996, 17, 183–189. [Google Scholar] [CrossRef]
- Baglieri, A.; Sidella, S.; Barone, V.; Fragalà, F.; Silkina, A.; Nègre, M.; Gennari, M. Cultivating Chlorella Vulgaris and Scenedesmus Quadricauda Microalgae to Degrade Inorganic Compounds and Pesticides in Water. Environ. Sci. Pollut. Res. 2016, 23, 18165–18174. [Google Scholar] [CrossRef]
- Martinez, M.E.; Sanchez, S.; Jimenez, J.M.; el Yousfi, F.; Munoz, L. Nitrogen and Phosphorus Removal from Urban Wastewater by the Microalga Scenedesmus Obliquus. Bioresour. Technol. 2000, 73, 263–272. [Google Scholar] [CrossRef]
- Xin, L.; Hong-ying, H.; Jia, Y. Lipid Accumulation and Nutrient Removal Properties of a Newly Isolated Freshwater Microalga, Scenedesmus Sp. LX1, Growing in Secondary Effluent. New Biotechnol. 2010, 27, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Baglieri, A.; Reyneri, A.; Gennari, M.; Nègre, M. Organically Modified Clays as Binders of Fumonisins in Feedstocks. J. Environ. Sci. Health Part B 2013, 48, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Lananan, F.; Mohd Yunos, F.H.; Mohd Nasir, N.; Abu Bakar, N.S.; Lam, S.S.; Jusoh, A. Optimization of Biomass Harvesting of Microalgae, Chlorella Sp. Utilizing Auto-Flocculating Microalgae, Ankistrodesmus Sp. as Bio-Flocculant. Int. Biodeterior. Biodegrad. 2016, 113, 391–396. [Google Scholar] [CrossRef]
- Ruiz-Marin, A.; Mendoza-Espinosa, L.G.; Stephenson, T. Growth and Nutrient Removal in Free and Immobilized Green Algae in Batch and Semi-Continuous Cultures Treating Real Wastewater. Bioresour. Technol. 2010, 101, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Wang, B.; Wang, Q.; Zhang, S.; Zhao, B. Ammonia-Nitrogen and Orthophosphate Removal by Immobilized Scenedesmus Sp. Isolated from Municipal Wastewater for Potential Use in Tertiary Treatment. Bioresour. Technol. 2008, 99, 3787–3793. [Google Scholar] [CrossRef]
- Kurade, M.B.; Kim, J.R.; Govindwar, S.P.; Jeon, B.H. Insights into Microalgae Mediated Biodegradation of Diazinon by Chlorella Vulgaris: Microalgal Tolerance to Xenobiotic Pollutants and Metabolism. Algal Res. 2016, 20, 126–134. [Google Scholar] [CrossRef]
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural sustainability and Intensive Production Practices. Nature 2002, 418, 671–677. [Google Scholar] [CrossRef]
- Puglisi, I.; Nicolosi, E.; Vanella, D.; lo Piero, A.R.; Stagno, F.; Saitta, D.; Roccuzzo, G.; Consoli, S.; Baglieri, A. Physiological and Biochemical Responses of Orange Trees to Different Deficit Irrigation Regimes. Plants 2019, 8, 423. [Google Scholar] [CrossRef] [Green Version]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in Plant Science: A Global Perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef] [Green Version]
- Kocira, A.; Świeca, M.; Kocira, S.; Złotek, U.; Jakubczyk, A. Enhancement of Yield, Nutritional and Nutraceutical Properties of Two Common Bean Cultivars Following the Application of Seaweed Extract (Ecklonia Maxima). Saudi J. Biol. Sci. 2018, 25, 563–571. [Google Scholar] [CrossRef] [Green Version]
- du Jardin, P. Plant Biostimulants: Definition, Concept, Main Categories and Regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Hultberg, M.; Carlsson, A.S.; Gustafsson, S. Treatment of Drainage Solution from Hydroponic Greenhouse Production with Microalgae. Bioresour. Technol. 2013, 136, 401–406. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for Biodiesel Production and Other Applications: A Review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef] [Green Version]
- Gong, Y.; Jiang, M. Biodiesel Production with Microalgae as Feedstock: From Strains to Biodiesel. Biotechnol. Lett. 2011, 33, 1269–1284. [Google Scholar] [CrossRef] [PubMed]
- Barone, V.; Puglisi, I.; Fragalà, F.; lo Piero, A.R.; Giuffrida, F.; Baglieri, A. Novel Bioprocess for the Cultivation of Microalgae in Hydroponic Growing System of Tomato Plants. J. Appl. Phycol. 2019, 31, 465–470. [Google Scholar] [CrossRef]
- Puglisi, I.; Barone, V.; Sidella, S.; Coppa, M.; Broccanello, C.; Gennari, M.; Baglieri, A. Biostimulant Activity of Humic-like Substances from Agro-Industrial Waste on Chlorella Vulgaris and Scenedesmus Quadricauda. Eur. J. Phycol. 2018, 53, 433–442. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Zhou, Q. Co-Cultivation of Chlorella Spp and Tomato in a Hydroponic System. Biomass Bioenergy 2017, 97, 132–138. [Google Scholar] [CrossRef]
- Gonçalves, A.L. The Use of Microalgae and Cyanobacteria in the Improvement of Agricultural Practices: A Review on Their Biofertilising, Biostimulating and Biopesticide Roles. Appl. Sci. 2021, 11, 871. [Google Scholar] [CrossRef]
- Chiaiese, P.; Corrado, G.; Colla, G.; Kyriacou, M.C.; Rouphael, Y. Renewable Sources of Plant Biostimulation: Microalgae as a Sustainable Means to Improve Crop Performance. Front. Plant Sci. 2018, 9, 1782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ronga, D.; Biazzi, E.; Parati, K.; Carminati, D.; Carminati, E.; Tava, A. Microalgal Biostimulants and Biofertilisers in Crop Productions. Agronomy 2019, 9, 192. [Google Scholar] [CrossRef] [Green Version]
- Win, T.T.; Barone, G.D.; Secundo, F.; Fu, P. Algal Biofertilizers and Plant Growth Stimulants for Sustainable Agriculture. Ind. Biotechnol. 2018, 14, 203–211. [Google Scholar] [CrossRef]
- Kusvuran, A.; Kusvuran, S. Using of Microbial Fertilizer as Biostimulant Alleviates Damage from Drought Stress in Guar (Cyamopsis Tetragonoloba (L.) Taub.) Seedlings. Int. Lett. Nat. Sci. 2019, 76, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Reddy, C.A.; Saravanan, R.S. Polymicrobial Multi-Functional Approach for Enhancement of Crop Productivity. In Advances in Applied Microbiology; Academic Press Inc.: London, UK, 2013; Volume 82, pp. 53–113. [Google Scholar]
- Bulgari, R.; Cocetta, G.; Trivellini, A.; Vernieri, P.; Ferrante, A. Biostimulants and Crop Responses: A Review. Biol. Agric. Hortic. 2015, 31, 1–17. [Google Scholar] [CrossRef]
- Parrado, J.; Bautista, J.; Romero, E.J.; García-Martínez, A.M.; Friaza, V.; Tejada, M. Production of a Carob Enzymatic Extract: Potential Use as a Biofertilizer. Bioresour. Technol. 2008, 99, 2312–2318. [Google Scholar] [CrossRef] [PubMed]
- Ertani, A.; Pizzeghello, D.; Baglieri, A.; Cadili, V.; Tambone, F.; Gennari, M.; Nardi, S. Humic-like Substances from Agro-Industrial Residues Affect Growth and Nitrogen Assimilation in Maize (Zea Mays L.) Plantlets. J. Geochem. Explor. 2013, 129, 103–111. [Google Scholar] [CrossRef]
- Baglieri, A.; Cadili, V.; Mozzetti Monterumici, C.; Gennari, M.; Tabasso, S.; Montoneri, E.; Nardi, S.; Negre, M. Fertilization of Bean Plants with Tomato Plants Hydrolysates. Effect on Biomass Production, Chlorophyll Content and N Assimilation. Sci. Hortic. 2014, 176, 194–199. [Google Scholar] [CrossRef]
- Alam, M.Z.; Braun, G.; Norrie, J.; Hodges, D.M. Ascophyllum Extract Application Can Promote Plant Growth and Root Yield in Carrot Associated with Increased Root-Zone Soil Microbial Activity. Can. J. Plant Sci. 2014, 94, 337–348. [Google Scholar] [CrossRef] [Green Version]
- Rouphael, Y.; Giordano, M.; Cardarelli, M.; Cozzolino, E.; Mori, M.; Kyriacou, M.C.; Bonini, P.; Colla, G. Plant-and Seaweed-Based Extracts Increase Yield but Differentially Modulate Nutritional Quality of Greenhouse Spinach through Biostimulant Action. Agronomy 2018, 8, 126. [Google Scholar] [CrossRef] [Green Version]
- Colla, G.; Nardi, S.; Cardarelli, M.; Ertani, A.; Lucini, L.; Canaguier, R.; Rouphael, Y. Protein Hydrolysates as Biostimulants in Horticulture. Sci. Hortic. 2015, 196, 28–38. [Google Scholar] [CrossRef]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed Extracts as Biostimulants in Horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Stirk, W.A.; Ördög, V.; Novák, O.; Rolčík, J.; Strnad, M.; Bálint, P.; van Staden, J. Auxin and Cytokinin Relationships in 24 Microalgal Strains1. J. Phycol. 2013, 49, 459–467. [Google Scholar] [CrossRef]
- Tate, J.J.; Gutierrez-Wing, M.T.; Rusch, K.A.; Benton, M.G. The Effects of Plant Growth Substances and Mixed Cultures on Growth and Metabolite Production of Green Algae Chlorella Sp.: A Review. J. Plant Growth Regul. 2013, 32, 417–428. [Google Scholar] [CrossRef]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial Applications of Microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [Green Version]
- Barone, V.; Baglieri, A.; Stevanato, P.; Broccanello, C.; Bertoldo, G.; Bertaggia, M.; Cagnin, M.; Pizzeghello, D.; Moliterni, V.M.C.; Mandolino, G.; et al. Root Morphological and Molecular Responses Induced by Microalgae Extracts in Sugar Beet (Beta Vulgaris L.). J. Appl. Phycol. 2018, 30, 1061–1071. [Google Scholar] [CrossRef]
- Puglisi, I.; Barone, V.; Fragalà, F.; Stevanato, P.; Baglieri, A.; Vitale, A. Effect of Microalgal Extracts from Chlorella Vulgaris and Scenedesmus Quadricauda on Germination of Beta Vulgaris Seeds. Plants 2020, 9, 675. [Google Scholar] [CrossRef]
- Barone, V.; Puglisi, I.; Fragalà, F.; Stevanato, P.; Baglieri, A. Effect of Living Cells of Microalgae or Their Extracts on Soil Enzyme Activities. Arch. Agron. Soil Sci. 2019, 65, 712–726. [Google Scholar] [CrossRef]
- Puglisi, I.; La Bella, E.; Rovetto, E.I.; lo Piero, A.R.; Baglieri, A. Biostimulant Effect and Biochemical Response in Lettuce Seedlings Treated with a Scenedesmus Quadricauda Extract. Plants 2020, 9, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La Bella, E.; Baglieri, A.; Rovetto, E.I.; Stevanato, P.; Puglisi, I. Foliar Spray Application of Chlorella Vulgaris Extract: Effect on the Growth of Lettuce Seedlings. Agronomy 2021, 11, 308. [Google Scholar] [CrossRef]
- Puglisi, I.; La Bella, E.; Rovetto, E.I.; Stevanato, P.; Fascella, G.; Baglieri, A. Morpho-biometric and Biochemical Responses in Lettuce Seedlings Treated by Different Application Methods of Chlorella Vulgaris Extract: Foliar Spray or Root Drench? J. Appl. Phycol. 2021. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, J.; Sommerfeld, M. Biofertilizer and Biostimulant Properties of the Microalga Acutodesmus Dimorphus. J. Appl. Phycol. 2016, 28, 1051–1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plaza, B.M.; Gómez-Serrano, C.; Acién-Fernández, F.G.; Jimenez-Becker, S. Effect of Microalgae Hydrolysate Foliar Application (Arthrospira Platensis and Scenedesmus Sp.) on Petunia x Hybrida Growth. J. Appl. Phycol. 2018, 30, 2359–2365. [Google Scholar] [CrossRef]
- Mutale-joan, C.; Redouane, B.; Najib, E.; Yassine, K.; Lyamlouli, K.; Laila, S.; Zeroual, Y.; Hicham, E.A. Screening of Microalgae Liquid Extracts for Their Bio Stimulant Properties on Plant Growth, Nutrient Uptake and Metabolite Profile of Solanum Lycopersicum L. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chien, S.H.; Prochnow, L.I.; Cantarella, H. Chapter 8 Recent Developments of Fertilizer Production and Use to Improve Nutrient Efficiency and Minimize Environmental Impacts. Adv. Agron. 2009, 102, 267–322. [Google Scholar]
- Dineshkumar, R.; Kumaravel, R.; Gopalsamy, J.; Sikder, M.N.A.; Sampathkumar, P. Microalgae as Bio-Fertilizers for Rice Growth and Seed Yield Productivity. Waste Biomass Valorization 2018, 9, 793–800. [Google Scholar] [CrossRef]
- Coppens, J.; Grunert, O.; van den Hende, S.; Vanhoutte, I.; Boon, N.; Haesaert, G.; de Gelder, L. The Use of Microalgae as a High-Value Organic Slow-Release Fertilizer Results in Tomatoes with Increased Carotenoid and Sugar Levels. J. Appl. Phycol. 2016, 28, 2367–2377. [Google Scholar] [CrossRef]
- Schreiber, C.; Schiedung, H.; Harrison, L.; Briese, C.; Ackermann, B.; Kant, J.; Schrey, S.D.; Hofmann, D.; Singh, D.; Ebenhöh, O.; et al. Evaluating Potential of Green Alga Chlorella Vulgaris to Accumulate Phosphorus and to Fertilize Nutrient-Poor Soil Substrates for Crop Plants. J. Appl. Phycol. 2018, 30, 2827–2836. [Google Scholar] [CrossRef]
- Shaaban, M.M. Nutritional Status and Growth of Maize Plants as Affected by Green Microalgae as Soil Additives. J. Biol. Sci. 2001, 1, 475–479. [Google Scholar] [CrossRef] [Green Version]
- Faheed, F.A.; Abdel Fattah, Z. Effect of Chlorella Vulgaris as Bio-Fertilizer on Growth Parameters and Metabolic Aspects of Lettuce Plant. J. Agric. Soc. Sci. 2008, 4, 165–169. [Google Scholar]
- Wuang, S.C.; Khin, M.C.; Chua, P.Q.D.; Luo, Y.D. Use of Spirulina Biomass Produced from Treatment of Aquaculture Wastewater as Agricultural Fertilizers. Algal Res. 2016, 15, 59–64. [Google Scholar] [CrossRef]
- Elhafiz, A.A.; Gaur, A.E.S.S.; Osman, N.H.M.; Lakshmi, T.R. Chlorella Vulgaris and Chlorella Pyrenoidosa Cells Appear to Be Promising Sustainable to Grow Rice, Lettuce, Cucumber and eggplant in the UAE Soils. Recent Res. Sci. Technol. 2015, 7, 14–21. [Google Scholar] [CrossRef]
- Costa, J.A.V.; Freitas, B.C.B.; Cruz, C.G.; Silveira, J.; Morais, M.G. Potential of Microalgae as Biopesticides to Contribute to Sustainable Agriculture and Environmental Development. J. Environ. Sci. Health—Part B Pestic. Food Contam. Agric. Wastes 2019, 54, 366–375. [Google Scholar] [CrossRef]
- Mnif, I.; Ghribi, D. Potential of Bacterial Derived Biopesticides in Pest Management. Crop Prot. 2015, 77, 52–64. [Google Scholar] [CrossRef]
- Gupta, V.; Ratha, S.K.; Sood, A.; Chaudhary, V.; Prasanna, R. New Insights into the Biodiversity and Applications of Cyanobacteria (Blue-Green Algae)-Prospects and Challenges. Algal Res. 2013, 2, 79–97. [Google Scholar] [CrossRef]
- Prasanna, R.; Nain, L.; Tripathi, R.; Gupta, V.; Chaudhary, V.; Middha, S.; Joshi, M.; Ancha, R.; Kaushik, B.D. Evaluation of Fungicidal Activity of Extracellular Filtrates of Cyanobacteria—Possible Role of Hydrolytic Enzymes. J. Basic Microbiol. 2008, 48, 186–194. [Google Scholar] [CrossRef]
- Chaudhary, V.; Prasanna, R.; Nain, L.; Dubey, S.C.; Gupta, V.; Singh, R.; Jaggi, S.; Bhatnagar, A.K. Bioefficacy of Novel Cyanobacteria-Amended Formulations in Suppressing Damping off Disease in Tomato Seedlings. World J. Microbiol. Biotechnol. 2012, 28, 3301–3310. [Google Scholar] [CrossRef]
- Chandel, S.T. Nematicidal Activity of the Cyanobacterium, Aulosira Fertilissima on the Hatch of Meloidogyne Triticoryzae and Meloidogyne Incognita. Arch. Phytopathol. Plant Prot. 2009, 42, 32–38. [Google Scholar] [CrossRef]
- Renuka, N.; Guldhe, A.; Prasanna, R.; Singh, P.; Bux, F. Microalgae as Multi-Functional Options in Modern Agriculture: Current Trends, Prospects and Challenges. Biotechnol. Adv. 2018, 36, 1255–1273. [Google Scholar] [CrossRef]
- Babu, S.; Bidyarani, N.; Chopra, P.; Monga, D.; Kumar, R.; Prasanna, R.; Kranthi, S.; Saxena, A.K. Evaluating Microbe-Plant Interactions and Varietal Differences for Enhancing Biocontrol Efficacy in Root Rot Disease Challenged Cotton Crop. Eur. J. Plant Pathol. 2015, 142, 345–362. [Google Scholar] [CrossRef]
- Swain, S.S.; Paidesetty, S.K.; Padhy, R.N. Antibacterial, Antifungal and Antimycobacterial Compounds from Cyanobacteria. Biomed. Pharmacother. 2017, 90, 760–776. [Google Scholar] [CrossRef]
- El-Mougy, N.S.; Abdel-Kader, M.M. Effect of Commercial Cyanobacteria Products on the Growth and Antagonistic Ability of Some Bioagents under Laboratory Conditions. J. Pathog. 2013, 2013, 1–11. [Google Scholar] [CrossRef]
- Scaglioni, P.T.; de Oliveira Garcia, S.; Badiale-Furlong, E. Inhibition of in Vitro Trichothecenes Production by Microalgae Phenolic Extracts. Food Res. Int. 2019, 124, 175–180. [Google Scholar] [CrossRef]
- Ranglová, K.; Lakatos, G.E.; Câmara Manoel, J.A.; Grivalský, T.; Suárez Estrella, F.; Acién Fernández, F.G.; Molnár, Z.; Ördög, V.; Masojídek, J. Growth, Biostimulant and Biopesticide Activity of the MACC-1 Chlorella Strain Cultivated Outdoors in Inorganic Medium and Wastewater. Algal Res. 2021, 53, 102136. [Google Scholar] [CrossRef]

| Advantages | Disadvantages |
|---|---|
| Cheaper than conventional methods | Limited to shallow contaminants |
| Low energy requirement, solar driven | Phytotoxicity of contaminants |
| High public acceptance | Slower than conventional methods |
| Use of natural and renewable source | Unknown effects of biodegradation products |
| Less secondary waste generation | |
| Less carbon footprint | |
| Reclamation of wastewater and nutrient recovery | |
| Generation of feedstock for different applications | |
| Possibility of harvesting the plants for the extraction of absorbed and accumulated contaminants such as toxic heavy metals for recycling |
| Microalgae Species | Wastewater Type | Treatment Efficiency (%) | Reference |
|---|---|---|---|
| Anabaena flos-aquae | Ammonium form nitrogen group | N: 94.9 | [24] |
| P: 96.8 | |||
| Anabaena flos-aquae | Orthophosphate form phosphorous group | P: 97.7 | [24] |
| Ankistrodesmus falcatus | Aquaculture wastewater | NO3−: 80.85 | [15] |
| NO2−: 99.73 | |||
| NH4+: 86.45 | |||
| P: 98.52 | |||
| COD: 61 | |||
| Arthrospira platensis | Dairy farm wastewater | NO3–N: 99.6 | [25] |
| NH4–N: ~100 | |||
| PO4–P: 98.8 | |||
| COD: 98.4 | |||
| Calothrix sp. | Sewage water | N: 57 | [26] |
| P: 74 | |||
| Chlamydomonas sp. (YG04) | Municipal wastewater | N: 77.57 | [27] |
| P: 100 | |||
| Chlamydomonas sp. (YG05) | Municipal wastewater | N: 74.49 | [27] |
| P: 100 | |||
| Chlorella sp. | Domestic wastewater | N: 50.2 | [28] |
| P: 85.7 | |||
| BOD5: 68.4 | |||
| COD: 67.2 | |||
| Chlorella sp. | Municipal wastewater before primary settling | NH4–N: 82.4 | [29] |
| P: 83.2 | |||
| COD: 50.9 | |||
| Chlorella sp. | Municipal wastewater after primary settling | NH4–N: 74.7 | [29] |
| P: 90.6 | |||
| COD: 56.5 | |||
| Chlorella sp. | Municipal wastewater after activated sludge tank | NH4–N: 62.5 | [29] |
| P: 4.7 | |||
| Chlorella sp. | Municipal wastewater generated in sludge centrifuge | NH4–N: 78.3 | [29] |
| P: 85.6 | |||
| COD: 83 | |||
| Chlorella sp. | Sewage water | N: 78 | [26] |
| P: 45 | |||
| Chlorella sp. (YG01) | Municipal wastewater | N: 84.11 | [27] |
| P: 82.36 | |||
| Chlorella sp. (YG02) | Municipal wastewater | N: 68.23 | [27] |
| P: 99 | |||
| Chlorella vulgaris | Wastewater from the Shatin sewage treat. | N: 86 | [30] |
| P: 78 | |||
| Chlorella vulgaris | Agricultural wastewater | NH4–N: 99 | [31] |
| NO3–N:83 | |||
| P: 88 | |||
| Lyngbya sp. | Sewage water | N: 59 | [26] |
| P: 92 | |||
| Oocystis sp. (YG03) | Municipal wastewater | N: 83.32 | [27] |
| P: 99.01 | |||
| Scenedesmus obliquus | Secondary effluent—without stirring (20 °C) | N: 94 | [32] |
| P: 97 | |||
| Scenedesmus obliquus | Secondary effluent—without stirring (25 °C) | N: 99 | [32] |
| P: 98 | |||
| Scenedesmus obliquus | Secondary effluent—without stirring (30 °C) | N: 99 | [32] |
| P: 94 | |||
| Scenedesmus obliquus | Secondary effluent—without stirring (35 °C) | N: 79 | [32] |
| P: 54 | |||
| Scenedesmus obliquus | Secondary effluent—with stirring (20 °C) | N: 80 | [32] |
| P: 98 | |||
| Scenedesmus obliquus | Secondary effluent—with stirring (25 °C) | N: 100 | [32] |
| P: 98 | |||
| Scenedesmus obliquus | Secondary effluent—with stirring (30 °C) | N: 99 | [32] |
| P: 97 | |||
| Scenedesmus obliquus | Secondary effluent—with stirring (35 °C) | N: 82 | [32] |
| P: 62 | |||
| Scenedesmus quadricauda | Agricultural wastewater | NH4–N: 99 | [31] |
| NO3–N: 5 | |||
| P: 94 | |||
| Scenedesmus sp. LX1 | Secondary effluent | N: 98 | [33] |
| P: 98 | |||
| Ulothrix sp. | Sewage water | N: 67 | [26] |
| P: 85 |
| Microalgae Species | Extract/Biomass | Application | Effects | Reference |
|---|---|---|---|---|
| A. dimorphus | Cellular extracts, growth medium and culture | Solanum lycopersicum cv Roma | Improving seed germination. Increasing plant growth through foliar application | [73] |
| A. maxima | Crude Bio-Extracts (CBEs) | Solanum lycopersicum | Improving chlorophyll contents, nutrient uptake, root and shoot length and dry weight | [75] |
| A. platensis | Hydrolysate extracts | Petunia x hybrida | Increasing root dry weight, flower dry weight and fresh weight. Improving plant nutrition status | [74] |
| A. platensis | Crude Bio-Extracts (CBEs) | Solanum lycopersicum | Improving chlorophyll contents, nutrient uptake, root and shoot length and dry weight | [75] |
| Aphanothese sp. | Crude Bio-Extracts (CBEs) | Solanum lycopersicum | Improving chlorophyll contents, nutrient uptake, root and shoot length and dry weight | [75] |
| C. ellipsoidae | Crude Bio-Extracts (CBEs) | Solanum lycopersicum | Improving chlorophyll contents, nutrient uptake, root and shoot length and dry weight | [75] |
| C. marina | Crude Bio-Extracts (CBEs) | Solanum lycopersicum | Improving chlorophyll contents, nutrient uptake, root and shoot length and dry weight | [75] |
| C. pyrenoidosa | Crude Bio-Extracts (CBEs) | Solanum lycopersicum | Improving chlorophyll contents, nutrient uptake, root and shoot length and dry weight | [75] |
| C. reinhardtii | Crude Bio-Extracts (CBEs) | Solanum lycopersicum | Improving chlorophyll contents, nutrient uptake, root and shoot length and dry weight | [75] |
| C. sorokiniana | Crude Bio-Extracts (CBEs) | Solanum lycopersicum | Improving chlorophyll contents, nutrient uptake, root and shoot length and dry weight | [75] |
| C. vulgaris | Cellular extracts | Beta vulgaris cv Shannon | Improving germination rates and root development | [68] |
| C. vulgaris | Cellular extracts | Lettuce seedlings | Increasing dry matter, chlorophylls, carotenoids, proteins, and influencing the activities of several enzymes | [71] |
| C. vulgaris | Cellular extracts | Lettuce seedlings | Increasing dry matter, chlorophylls, carotenoids, proteins, and influencing the activities of several enzymes | [72] |
| C. vulgaris | Cellular extracts and living cells | Application on soil | Increasing values of the biochemical index of potential soil fertility | [69] |
| C. vulgaris | Crude Bio-Extracts (CBEs) | Solanum lycopersicum | Improving chlorophyll contents, nutrient uptake, root and shoot length and dry weight | [75] |
| D. salina | Crude Bio-Extracts (CBEs) | Solanum lycopersicum | Improving chlorophyll contents, nutrient uptake, root and shoot length and dry weight | [75] |
| I. galbana | Crude Bio-Extracts (CBEs) | Solanum lycopersicum | Improving chlorophyll contents, nutrient uptake, root and shoot length and dry weight | [75] |
| N. gaditana | Crude Bio-Extracts (CBEs) | Solanum lycopersicum | Improving chlorophyll contents, nutrient uptake, root and shoot length and dry weight | [75] |
| Porphyridium sp. | Crude Bio-Extracts (CBEs) | Solanum lycopersicum | Improving chlorophyll contents, nutrient uptake, root and shoot length and dry weight | [75] |
| S. almeriensis | Hydrolysate extracts | Petunia x hybrida | Increasing root dry weight, flower dry weight and fresh weight. Improving plant nutrition status | [74] |
| S. dimorphus | Crude Bio-Extracts (CBEs) | Solanum lycopersicum | Improving chlorophyll contents, nutrient uptake, root and shoot length and dry weight | [75] |
| S. obliquus | Crude Bio-Extracts (CBEs) | Solanum lycopersicum | Improving chlorophyll contents, nutrient uptake, root and shoot length and dry weight | [75] |
| S. quadricauda | Cellular extracts | Beta vulgaris cv Shannon | Improving germination rates and root development | [68] |
| S. quadricauda | Cellular extracts and living cells | Application on soil | Increasing values of the biochemical index of potential soil fertility | [69] |
| S. quadricauda | Cellular extracts | Lettuce seedlings | Increasing dry matter, chlorophylls, carotenoids, proteins, and influencing the activities of several enzymes | [70] |
| T. marina | Crude Bio-Extracts (CBEs) | Solanum lycopersicum | Improving chlorophyll contents, nutrient uptake, root and shoot length and dry weight | [75] |
| T. suecica | Crude Bio-Extracts (CBEs) | Solanum lycopersicum | Improving chlorophyll contents, nutrient uptake, root and shoot length and dry weight | [75] |
| Tetraselmis sp. | Crude Bio-Extracts (CBEs) | Solanum lycopersicum | Improving chlorophyll contents, nutrient uptake, root and shoot length and dry weight | [75] |
| Microalgae Species | Biomass/Solution | Application On | Effects | Reference |
|---|---|---|---|---|
| C. pyrenoidosa | Solution | Lactuca sativa (lettuce) | Improving germination process and salinity tolerance, and enhancing chlorophyll content | [83] |
| C. pyrenoidosa | Solution | Oryza sp. (rice) | Improving germination process and salinity tolerance, and enhancing chlorophyll content | [83] |
| C. pyrenoidosa | Solution | Solanum melongena (eggplant) | Improving germination process and salinity tolerance, and enhancing chlorophyll content | [83] |
| C. pyrenoidosa | Solution | Cucumis sativus (cucumber) | Improving germination process and salinity tolerance, and enhancing chlorophyll content | [83] |
| C. vulgaris | Biomass | Oryza sp. | Improving biological activity and chemical properties of the soil and increasing the availability of macronutrients | [77] |
| Microalgal bacterial flocs | Biomass | Solanum lycopersicum | Improving fruit quality through an increase in sugar and carotenoid content | [78] |
| Nannochloropsis sp. | Biomass | Solanum lycopersicum | Improving fruit quality through an increase in sugar and carotenoid content | [78] |
| S. platensis | Biomass | Eruca sativa | Enhancing plant growth and improving germination process | [82] |
| S. platensis | Biomass | Amaranthus gangeticus | Enhancing plant growth and improving germination process | [82] |
| S. platensis | Biomass | Brassica rapa spp. chinensis | Enhancing plant growth and improving germination process | [82] |
| S. platensis | Biomass | Brassica oleracea alboglabra | Enhancing plant growth and improving germination process | [82] |
| S. platensis | Biomass | Oryza sp. | Improving biological activity and chemical properties of the soil and increasing the availability of macronutrients | [77] |
| Microalgae Species | Application | Microorganism Target | Effects | Reference |
|---|---|---|---|---|
| Anabaena laxa RPAN8 | In vivo on Gossypium hirsutum F1861 and Gossypium arboretum CISA 310 | Rhizoctonia spp. | Enhancing the levels of defense enzyme activities, reducing mortality, and improving growth and yield | [91] |
| C. vulgaris MACC-1 (cultivated in BG-11) | In vitro | Fusarium oxysporum f.sp. melonis | Inhibiting microorganism development | [95] |
| C. vulgaris MACC-1 (cultivated in BG-11) | In vitro | Rhizoctonia solani | Inhibiting microorganism development | [95] |
| C. vulgaris MACC-1 (cultivated in BG-11) | In vitro | Phytophthora capsici | Inhibiting microorganism development | [95] |
| C. vulgaris MACC-1 (cultivated in BG-11) | In vitro | Clavibacter michiganensis subsp. michiganensis | Inhibiting microorganism development | [95] |
| C. vulgaris MACC-1 (cultivated in urban wastewater) | In vitro | Fusarium oxysporum f.sp. melonis | Inhibiting microorganism development | [95] |
| C. vulgaris MACC-1 (cultivated in urban wastewater) | In vitro | Rhizoctonia solani | Inhibiting microorganism development | [95] |
| C. vulgaris MACC-1 (cultivated in urban wastewater) | In vitro | Phytophthora capsici | Inhibiting microorganism development | [95] |
| C. vulgaris MACC-1 (cultivated in urban wastewater) | In vitro | Phytium ultimum | Inhibiting microorganism development | [95] |
| C. vulgaris MACC-1 (cultivated in urban wastewater) | In vitro | Clavibacter michiganensis subsp. michiganensis | Inhibiting microorganism development | [95] |
| C. vulgaris MACC-1 (cultivated in urban wastewater) | In vitro | Xanthomonas campestris pv. vesicatoria | Inhibiting microorganism development | [95] |
| C. vulgaris MACC-1 (cultivated in urban wastewater) | In vitro | Pseudomonas syringae pv. tomato | Inhibiting microorganism development | [95] |
| C. vulgaris MACC-1 (cultivated in urban wastewater) | In vitro | Pectobacterium carotovorum | Inhibiting microorganism development | [95] |
| Calothrix sp. | In vivo on Gossypium hirsutum F1861 and Gossypium arboretum CISA 310 | Rhizoctonia spp. | Enhancing the levels of defense enzyme activities, reducing mortality, and improving growth and yield | [91] |
| Nannochloropsis sp. | In vitro | Fusarium graminearum species complex | Reducing mycelial halo formation and ergosterol production, inhibiting the production of the acetylates and the production of trichothecenes | [94] |
| Spirulina sp. | In vitro | Fusarium graminearum species complex | Reducing mycelial halo formation and ergosterol production, inhibiting the production of the acetylates and the production of trichothecenes | [94] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
La Bella, E.; Baglieri, A.; Fragalà, F.; Puglisi, I. Multipurpose Agricultural Reuse of Microalgae Biomasses Employed for the Treatment of Urban Wastewater. Agronomy 2022, 12, 234. https://doi.org/10.3390/agronomy12020234
La Bella E, Baglieri A, Fragalà F, Puglisi I. Multipurpose Agricultural Reuse of Microalgae Biomasses Employed for the Treatment of Urban Wastewater. Agronomy. 2022; 12(2):234. https://doi.org/10.3390/agronomy12020234
Chicago/Turabian StyleLa Bella, Emanuele, Andrea Baglieri, Ferdinando Fragalà, and Ivana Puglisi. 2022. "Multipurpose Agricultural Reuse of Microalgae Biomasses Employed for the Treatment of Urban Wastewater" Agronomy 12, no. 2: 234. https://doi.org/10.3390/agronomy12020234








