Responses of N-Cycling Enzyme Activities and Functional Diversity of Soil Microorganisms to Soil Depth, Pedogenic Processes and Cultivated Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Soil Sampling
2.2. Physicochemical Properties
2.3. Potential Enzyme Activities
2.4. Microbial Biomass C and N Content
2.5. Nitrogen Substrate Use Capacity Expressed as Microbial Metabolic Potential Diversity
2.6. Evaluation of Root Biomass and Morphology
2.7. Data Analyses
3. Results
3.1. Physical and Chemical Properties of the Soil Profiles
3.2. Soil Microbial Biomass C and N
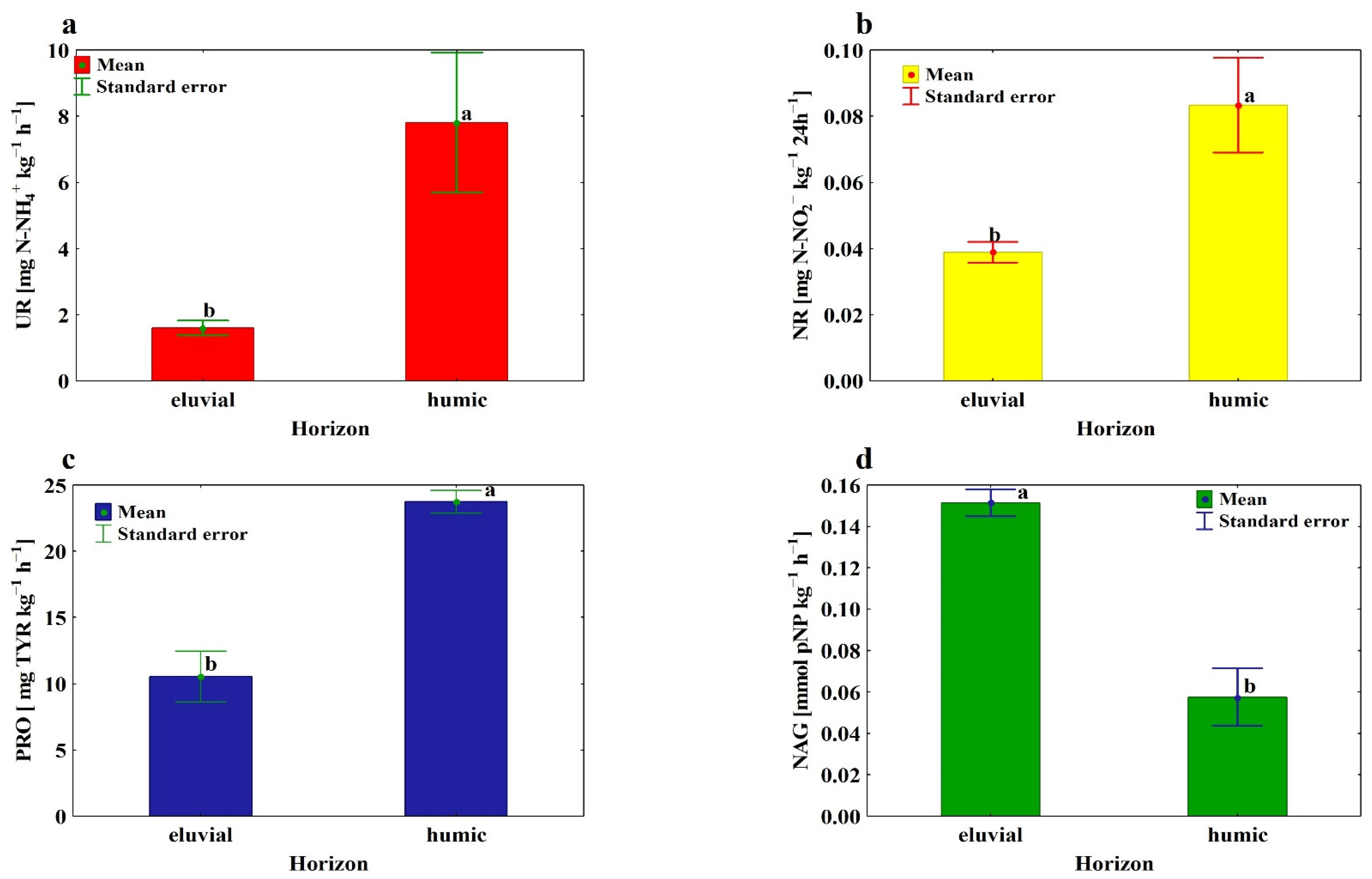
3.3. Absolute and Specific Enzymatic Activity
3.4. Potential Microbial Metabolic Diversity Expressed in Terms of N-Substrate Utilization
3.5. Root Biomass and Morphology
3.6. Relationship between the Studied Properties—Analysis of Correlation and PCA
4. Discussion
4.1. Changes in the Soil Properties along the Soil Profiles
4.2. Changes Associated with Soil Type, Genetic Horizons and Soil-Forming Processes
4.3. Changes of Microbial Functional Diversity under Different Plants Cultivation and Various Soil Depth
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Darby, B.A.; Goodale, C.L.; Chin, G.A.; Fuss, C.B.; Lang, A.K.; Ollinger, S.V.; Lovett, G.M. Depth patterns and connections between gross nitrogen cycling and soil exoenzyme activities in three northern hardwood forests. Soil Biol. Biochem. 2020, 147, 107836. [Google Scholar] [CrossRef]
- James, J.; Knight, E.; Gamba, V.; Harrison, R. Deep soil: Quantification, modeling, and significance of subsurface nitrogen. For. Ecol. Manag. 2015, 336, 194–202. [Google Scholar] [CrossRef]
- Matejek, B.; Huber, C.; Dannenmann, M.; Kohlpaintner, M.; Gasche, R.; Göttlein, A.; Papen, H. Microbial nitrogen turnover processes within the soil profile of a nitrogen saturated spruce forest and their relation to the small-scale pattern of seepage-water nitrate. J. Plant Nutr. Soil Sci. 2010, 173, 224–236. [Google Scholar] [CrossRef]
- Iversen, C.M.; Hooker, T.D.; Classen, A.T.; Norby, R.J. Net mineralization of N at deeper soil depths as a potential mechanism for sustained forest production under elevated [CO2]. Glob. Chang. Biol. 2011, 17, 1130–1139. [Google Scholar] [CrossRef]
- Jobbagy, E.G.; Jackson, R.B. The distribution of soils nutrients with depth: Global patterns and the imprint of plants. Biogeochemistry 2001, 53, 51–77. [Google Scholar] [CrossRef]
- Mooshammer, M.; Wanek, W.; Zechmeisgter-Boltenstern, S.; Richter, A. Stoichiometric imbalances between terrestrial decomposer communities and their resources: Mechanisms, and implications of microbial adaptations to their resources. Front. Microbiol. 2014, 5, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dessureault-Rompre, J.; Zebarth, B.J.; Butron, D.L.; Grant, C.A. Depth distribution of mineralizable nitrogen pools in contrasting soils in a semi-arid climate. Can. J. Soil Sci. 2016, 96, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Pajewski, T. Water pollution as a negative effect of agricultural activity. Sci. Ann. Assoc. Agric. Agribus. Econ. 2016, 18, 191–195. (In Polish) [Google Scholar]
- Zhang, Y.; Dong, X.; Yang, X.; Munyampirwa, T.; Shen, Y. The lagging movement of soil nitrate in comparison to that of soil water in the 500-cm soil profile. Agric. Ecosyst. Environ. 2022, 326, 107811. [Google Scholar] [CrossRef]
- Podlasek, A.; Koda, E.; Vaverkova, M.D. The variability of nitrogen forms in soils due to traditional and precisian agriculture: Case studies in Poland. Int. J. Environ. Res. Public Health 2021, 18, 465. [Google Scholar] [CrossRef]
- Zhu, X.; Fu, W.; Kong, X.; Cgen, C.; Liu, Z.; Chen, Z.; Zhou, J. Nitrate accumulation in the soil profile is the main fate of surplus nitrogen after land-use from cereal cultivation to apple orchards on the Loess Plateau. Agric. Ecosyst. Environ. 2021, 319, 107574. [Google Scholar] [CrossRef]
- Matczak, D.; Siczek, A. Effectiveness of the use of urease inhibitors in agriculture: A review. Int. Agrophys. 2021, 35, 197–208. [Google Scholar] [CrossRef]
- Barford, C.; Lajtha, K. Nitrification and nitrate reductase activity along a secondary successional gradient. Plant Soil 1992, 145, 1–10. [Google Scholar] [CrossRef]
- Geisseler, D.; Horwath, W.R. Regulation of extracellular protease activity in soil in response to different sources and concentrations of nitrogen and carbon. Soil Biol. Biochem. 2008, 40, 3040–3048. [Google Scholar] [CrossRef]
- Vranova, V.; Rejsek, K.; Formanek, P. Proteolytic activity in soil: A review. Appl. Soil Ecol. 2013, 70, 23–32. [Google Scholar] [CrossRef]
- Adamczyk, B. Root-derived proteases as a plant tool to access soil organic nitrogen; Current stage of knowledge and controversies. Plants 2021, 10, 731. [Google Scholar] [CrossRef]
- Dove, N.C.; Arogyaswamy, K.; Billings, S.A.; Botthoff, J.K.; Carey, C.J.; Cisco, C.; De Forest, J.L.; Fairbanks, D.; Fierer, N.; Gallery, R.E.; et al. Continental-scale patterns of extracellular enzyme activity in the subsoil: An overlooked reservoir of microbial activity. Environ. Res. Lett. 2020, 15, 1040a1. [Google Scholar] [CrossRef]
- Loeppmann, S.; Forbush, K.; Chend, W.; Pasuch, J. Subsoil biogeochemical properties induce shift in carbon allocation pattern and soil C dynamics in wheat. Plant Soil 2019, 442, 369–383. [Google Scholar] [CrossRef]
- Herold, N.; Schöning, I.; Berner, D.; Haslwimmer, H.; Kandeler, E.; Michalyik, B.; Schrumpf, M. Vertical gradient of potential enzymes activities in soil profiles of European beech, Norwaz spruce and Scots pine dominated forest sites. Pedobiol. J. Soil Ecol. 2014, 57, 181–189. [Google Scholar] [CrossRef]
- Stone, M.M.; De Forest, J.L.; Plante, A.F. Changes in extracellular enzyme activity and microbial community structure with soil depth at the Luquillo Critical Zone Observatory. Soil Biol. Biochem. 2014, 75, 237–247. [Google Scholar] [CrossRef]
- Zhou, Z.; Yu, M.; Ding, G.; Gao, G.; He, Y. Diversity and structural differences of bacterial microbial communities in rhizocompartments of desert leguminous plants. PLoS ONE 2020, 15, e0241057. [Google Scholar]
- Xiong, Q.; Hu, J.; Wei, H.; Zhang, H.; Zhu, J. Relationship between plant roots, rhizosphere microorganisms, and nitrogen and its special focus on rice. Agriculture 2021, 11, 234. [Google Scholar] [CrossRef]
- Kompała-Bąba, A.; Bierza, W.; Sierka, E.; Błońska, A.; Besenyei, L.; Woźniak, G. The role of plants and soil properties in the enzyme activities of substrates on hard coal mine spoil heaps. Sci. Rep. 2021, 11, 5155. [Google Scholar] [CrossRef]
- Ko, D.; Yoo, G.; Yun, S.T.; Jun, S.C.; Chung, H. Bacterial and fungal community composition across the soil depth profiles in a fallow field. J. Ecol. Environ. 2017, 41, 34. [Google Scholar] [CrossRef] [Green Version]
- Yao, Y.; Shao, M.; Fu, X.; Wang, X.; Wei, X. Effects of shrubs on soil nutrients and enzymatic activities over a 0–100 cm soil profile in the desert-loess transition zone. Catena 2019, 174, 362–370. [Google Scholar] [CrossRef]
- Min, K.; Slessarev, E.; Kan, M.; McFarlane, K.; Oerter, E.; Pett-Ridge, J.; Nuccio, E.; Berhe, A.A. Active microbial biomass decreases, but microbial growth potential remains similar across soil depth profiles under deeply-vs. shallow-rooted plants. Soil Biol. Biochem. 2021, 162, 108401. [Google Scholar] [CrossRef]
- Marinari, S.; Antisari, L.V. Effect of lithological substrate on microbial biomass and enzyme activity in brown soil profiles in the northern Apennines (Italy). Pedobiologia 2010, 53, 313–320. [Google Scholar] [CrossRef]
- Antisari, L.V.; Agnelli, A.; Corti, G.; Falsone, G.; Ferronato, C.; Marinari, S.; Vianello, G. Modern and ancient pedogenesis as revealed by Holocene fire—Northern Apennines, Italy. Quat. Int. 2018, 467, 264–276. [Google Scholar] [CrossRef]
- Marinari, S.; Marabottini, R.; Falsone, G.; Vianello, G.; Antisari, L.V.; Agnelli, A.; Massaccesi, L.; Cocco, S.; Cardelli, V.; Serrani, D.; et al. Mineral weathering and lessivage affect microbial community and enzyme activity in mountain soils. Appl. Soil Ecol. 2021, 167, 104024. [Google Scholar] [CrossRef]
- Wilson, S.G.; Lambert, J.J.; Nanzyo, M.; Dahlgren, R.A. Soil genesis and mineralogy across a volcanic lithosequence. Geoderma 2017, 285, 301–312. [Google Scholar] [CrossRef] [Green Version]
- Jackson, C.R.; Thomson, J.A.; Kolka, R.K. Wetland Soils, Hydrology, and Geomorphology. In Ecology of Freshwater and Estuarine Wetlands Soil; Batzer, D., Sharitz, R., Eds.; University of California Press: Berkeley, CA, USA, 2014; pp. 23–60. [Google Scholar]
- Pankin, Z.; Malyk, S.; Yamelynets, T. Diagnostic criteria for lessivage of profile-differentiated soils of the Precarpathian region (Ukraine). J. Land Manag. Food Environ. 2019, 70, 201–207. [Google Scholar]
- Piotrowska-Dlugosz, A.; Kobierski, M.; Długosz, J. Enzymatic activity and physicochemical properties of soil profiles of Luvisols. Materials 2021, 14, 6364. [Google Scholar] [CrossRef]
- International Union of Soil Sciences Working Group World Reference Base. World Reference Base for Soil Resource 2014. International Soil Classification System for Naming Soils and Creating Legend for Soil Maps. Update 2015. Worlds Soil Resources Report, No. 106; Food and Agriculture Organization: Rome, Italy, 2015. [Google Scholar]
- Świtoniak, M.; Mroczek, P.; Bednarek, R. Luvisols or Cambisols? Micromorphological study of soil truncation in Young morainic landscapes—Case study: Brodnica and Chełmno Lake Districts (North Poland). Catena 2016, 137, 583–595. [Google Scholar] [CrossRef]
- Polish Norm PN-ISO 11277; Soil Quality—Determination of Particle Size Distribution in Mineral Soil Material—Method by Sieving and Sedimentation. Polish Committee for Standardization: Warsaw, Poland, 2005.
- Polish Norm PN-ISO 10390; Soil Quality—Determination of Soil pH. Polish Committee for Standardization: Warsaw, Poland, 1997.
- Bashour, I.I.; Sayegh, A.H. Methods of Analysis for Soils of Arid and Semi-Arid Regions; Food and Agriculture Organization of the United States: Rome, Italy, 2007; 128p. [Google Scholar]
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonia. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Kandeler, E. Enzymes Involved in Nitrogen Metabolism. In Methods in Soil Biology; Scinner, F., Öhlinger, R., Kandeler, E., Margesin, R., Eds.; Springer: Berlin/Heidelberg, Germany, 1995; pp. 163–184. [Google Scholar]
- Ladd, J.N.; Butler, J.H.A. Short-term assays of soil proteolytic enzyme activities using proteins and peptide derivates as substrates. Soil Biol. Biochem. 1972, 4, 19–30. [Google Scholar] [CrossRef]
- Deng, S.; Popova, I. Carbohydrate hydrolases. In Methods of Soil Enzymology; Dick, R.P., Ed.; Soil Science Society of America: Madison, WI, USA, 2011; Volume 9, pp. 185–207. [Google Scholar]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method for measuring microbial biomass in soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinsen, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen—Total. In Methods of Soil Analysis Part 2; Page, A.L., Miller, R.H., Keeny, D.R., Eds.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 594–624. [Google Scholar]
- Joergensen, R.G.; Mueller, T. The fumigation-extraction method to estimate soil microbial biomass: Calibration of the kEN value. Soil Biol. Biochem. 1996, 28, 33–37. [Google Scholar] [CrossRef]
- Anderson, T.H.; Domsch, K.H. Ratios of microbial biomass carbon to total organic carbon in arable soils. Soil Biol. Biochem. 1989, 21, 471–479. [Google Scholar] [CrossRef]
- Hallama, M.; Pekrun, C.; Pilz, S.; Jarosch, K.A.; Frąc, M.; Uksa, M.; Marhan, S.; Kandeler, E. Interactions between cover crops and soil microorganisms increase phosphorus availability in conservation agriculture. Plant Soil 2021, 463, 307–328. [Google Scholar] [CrossRef]
- Frąc, M.; Oszust, K.; Lipiec, J. Community level physiological profiles (CLPP), characterization and microbial activity of soil amended with dairy sewage sludge. Sensors 2012, 12, 3253–3268. [Google Scholar] [CrossRef] [Green Version]
- Gryta, A.; Frąc, M.; Oszust, K. The application of the biology EcoPlate approach in Ecotoxicological evaluation of dairy sewage sludge. Appl. Biochem. Biotechnol. 2014, 174, 1434–1443. [Google Scholar] [CrossRef] [Green Version]
- Oszust, K.; Frąc, M.; Lipiec, J. Soil microbial functionality in response to dairy sewage sludge and mineral fertilisers application under winter rape. Int. J. Environ. Sci. Technol. 2015, 12, 3675–3684. [Google Scholar] [CrossRef] [Green Version]
- Arsenault, J.L.; Poulcur, S.; Messier, C.; Guay, R. WIN-RHIZO a root-measuring system with a unique overlap correction method. HortScience 1995, 30, 906. [Google Scholar] [CrossRef] [Green Version]
- Hao, J.; Chai, Y.N.; Lopes, L.D.; Ordóñez, R.A.; Wright, E.E.; Archontoulis, S.; Schachtman, D.P. The effects of soil depth on the structure of microbial communities in agricultural soils in Iowa, USA. Appl. Environ. Microbiol. 2020, 87, e02673-20. [Google Scholar]
- Schnecker, J.; Wild, B.; Takriti, M.; Alves, R.J.E.; Gentsch, N.; Gittel, A.; Hofer, A.; Klaus, K.; Knoltsch, A.; Lashchinskiy, N.; et al. Microbial community composition shapes enzyme patterns in topsoil and subsoil horizons along a latitudinal transect in Western Siberia. Soil Biol. Biochem. 2015, 83, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Rumpel, C.; Kőgel-Knabner, I. Deep soil organic matter—A key but poorly understood component of terrestrial C cycle. Plant Soil 2011, 338, 143–158. [Google Scholar] [CrossRef]
- Parvin, S.; Blagodatskaya, E.; Becker, J.N.; Kuzyakov, Y.; Uddin, S.; Dorodnikov, M. Depth rather than microrelief controls microbial biomass and kinetics of C-, N-, P- and S-cycle enzymes in peatland. Geoderma 2018, 324, 67–76. [Google Scholar] [CrossRef]
- Andersson, M.; Kjøller, A.; Struwe, S. Microbial enzyme activities in leaf litter, humus and mineral soil layers of European forests. Soil Biol. Biochem. 2004, 36, 1527–1537. [Google Scholar] [CrossRef]
- Miller, M.; Palojärvi, A.; Rangger, A.; Reeslav, M.; Kjøller, A. The use of fluorogenic substrates to measure fungal presence and activity in soil. Appl. Environ. Microbiol. 1998, 64, 613–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frąc, M.; Lipiec, J.; Usowicz, B.; Oszust, K.; Brzezińska, M. Structural and functional microbial diversity of sandy soil under cropland and grassland. Peer J. 2020, 8, e9501. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.R.; Xue, D.; Yao, H.Y. Microbial biomass, community diversity, and enzyme activities in response to urea application in tea orchard soils. Commun. Soil Sci. Plant Anal. 2010, 41, 797–810. [Google Scholar] [CrossRef]
- Brewer, T.E.; Aronson, E.L.; Arogyaswamy, K.; Billings, S.A.; Botthoff, J.K.; Campbell, A.N.; Dove, N.C.; Fairbanks, D.; Gallery, R.E.; Hart, S.C.; et al. Ecological and genomic attributes of novel bacterial taxa that thrive in subsurface soil horizons. mBio 2019, 10, e1318-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joergensen, R.G.; Emmerling, C. Methods for evaluating human impact on soil microorganisms based on their activity, biomass, and diversity in agricultural soils. J. Plant Nutr. Soil Sci. 2006, 169, 295–309. [Google Scholar] [CrossRef]
- Du, C.; Geng, Z.; Wang, Q.; Zhang, T.; He, W.; Hou, L.; Wang, Y. Variations in bacterial and fungal communities through soil depth profiles in a Betula albosinensis forest. J. Microbiol. 2017, 55, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Kramer, S.; Marhan, S.; Haslwimmer, H.; Ruess, L.; Kandeler, E. Temporal variation in surface and subsoil abundance and function of the soil microbial community in an arable soil. Soil Biol. Biochem. 2013, 61, 76–85. [Google Scholar] [CrossRef]
- Gelsomino, A.; Azzellino, A. Multivariate analysis of soils: Microbial biomass, metabolic activity, and bacterial-community structure and their relationships with soil depth and type. J. Plant Nutr. Soil Sci. 2011, 174, 381–394. [Google Scholar] [CrossRef]
- Allison, S.D.; Weintraub, M.N.; Gartner, T.B.; Waldrop, M.P. Evolutionary economic principles as regulators of soil enzyme production and ecosystem function. In Soil Enzymology; Shukla, G.C., Varma, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 229–243. [Google Scholar]
- Senga, Y.; Hiroki, M.; Nakamura, Y.; Watarasi, Y.; Watanabe, Y.; Nohara, S. Vertical profiles of DIN, DOC, and microbial activities in the wetland soil of Kushiro Mire, northeastern Japan. Limnology 2011, 12, 17–23. [Google Scholar] [CrossRef]
- Gale, P.M.; Dévai, I.; Reddy, K.R.; Graetz, D.A. Denitrification potential of soils from constructed and natural wetlands. Ecol. Eng. 1993, 2, 119–130. [Google Scholar] [CrossRef]
- Wu, H.; Song, X.; Zhao, X.; Peng, X.; Hallett, P.D.; Hodson, M.E.; Zhang, G.L. Accumulation of nitrate and dissolved organic nitrogen at depth in a red soil Critical Zone. Geoderma 2019, 337, 1175–1185. [Google Scholar] [CrossRef]
- Bai, X.; Jiang, Y.; Miao, H.; Xue, H.; Chen, Z.; Zhou, J. Intensive vegetable production results in high nitrate accumulation in deep soil profiles in China. Environ. Pollut. 2021, 287, 117598. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Ju, X.; Yang, H. Nitrate leching in a winter wheat-summer maize rotation on a calcareous soil as affected by nitrogen and straw management. Sci. Rep. 2017, 7, 42247. [Google Scholar] [CrossRef] [Green Version]
- Hamdi, W.; Gamaoun, F.; Pelster, D.E.; Seffen, M. Nitrate sorption in an agricultural soil profile. Appl. Environ. Soil Sci. 2013, 2013, 597824. [Google Scholar] [CrossRef] [Green Version]
- Rice, C.W.; Tiedje, J.M. Regulation of nitrate assimilation by ammonium in soils and in isolated soil microorganisms. Soil Biol. Biochem. 1989, 21, 597–602. [Google Scholar] [CrossRef]
- Frąc, M.; Hannula, S.E.; Bełka, M.; Jędryczka, M. Fungal Biodiversity and Their Role in Soil Health. Front. Microbiol. 2018, 9, 707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egamberdieva, D.; Renella, G.; Wirth, S.; Islam, R. Enzyme activities in the Rhizosphere of Plants. In Soil Enzymology; Shukla, G., Varma, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 149–166. [Google Scholar]
- Greenfield, L.M.; Hill, P.W.; Paterson, E.; Baggs, E.M.; Jones, D.L. Do plants use root derived proteases to promote the uptake of soil organic nitrogen? Plant Soil 2020, 456, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Meier, I.C.; Finzi, A.C.; Phillips, R.P. Root exudates increase N availability by stimulating microbial turnover of fast-cycling N pools. Soil Biol. Biochem. 2017, 106, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Fierer, N. Embracing the unknown: Disentangling the complexities of the soil microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Fenner, N.; Freeman, C. Drought-induced carbon loss in peatlands. Nat. Geosci. 2011, 4, 895–900. [Google Scholar] [CrossRef]
- Hall, S.J.; Treffkorn, J.; Silver, W.L. Breaking the enzymatic latch: Impacts of reducing conditions on hydrolytic enzyme activity in tropical forest soils. Ecology 2014, 95, 2964–2973. [Google Scholar] [CrossRef]
- Myers, M.; McGarity, J.W. The urease activity in profiles of five great soil groups from Northern New South Wales. Plant Soil 1968, 28, 25–37. [Google Scholar] [CrossRef]
- Błońska, E.; Lasota, J. Enzymatic activity of forest Luvisols. Electron. J. Pol. Agric. Univ. 2013, 16, 1. [Google Scholar]
- An, N.; Zhou, C.H.; Zhuang, X.Y.; Tong, D.S.; Yu, W.H. Immobilization of enzymes on clay minerals for biocatalysts and biosensors. Appl. Clay Sci. 2015, 114, 283–296. [Google Scholar] [CrossRef]
- Tzanakakis, V.A.; Monokrousos, N.; Chatzistathis, T. Effects of clinoptilolite zeolite and vermiculite on nitrification and nitrogen and phosphorus acquiring enzymes in a nitrogen applied agricultural soil. J. Soil Sci. Plant Nutr. 2021, 21, 2791–2802. [Google Scholar] [CrossRef]
- Mahmud, K.; Makaju, S.; Ibrahim, R.; Missaoui, A. Current progress in nitrogen fixing plants and microbiome research. Plants 2020, 9, 97. [Google Scholar] [CrossRef] [Green Version]
- Tian, J.; Bu, L.; Zhang, M.; Yuan, J.; Zhang, Y.; Wei, G.; Wang, H. Soil bacteria with distinct diversity and functions mediates the soil nutrients after introducing leguminous shrub in desert ecosystems. Glob. Ecol. Conserv. 2021, 31, e01841. [Google Scholar] [CrossRef]
- Song, X.; Fang, C.; Yuan, Z.Q.; Li, F.M. Long-term growth of alfalfa increased soil organic matter accumulation and nutrient mineralization in a semi-arid environment. Front. Environ. Sci. 2021, 9, 649346. [Google Scholar] [CrossRef]
- Fahey, C.; Koyama, A.; Antunes, P.M.; Dunfield, K.; Flory, S.L. Plant communities mediate the interactive effects of invasion and drought on soil microbial communities. ISME J. 2020, 14, 1396–1409. [Google Scholar] [CrossRef]
- Zuo, Y.L.; He, C.; He, X.L.; Li, X.; Xue, Z.K.; Li, X.M.; Wang, S.J. Plant cover of Ammopiptanthus mongolicus and soil factors shape soil microbial community and catabolic functional diversity in the arid desert in Northwest China. Appl. Soil Ecol. 2020, 147, 103389. [Google Scholar] [CrossRef]
- Kang, E.Z.; Li, Y.; Zhang, X.D.; Yan, Z.Q.; Wu, H.D.; Li, M.; Yan, L.; Zhang, K.R.; Wang, J.Z.; Kang, X.M. Soil pH and nutrients shape the vertical distribution of microbial communities in an alpine wetland. Sci. Total Environ. 2021, 774, 145780. [Google Scholar] [CrossRef]
- Giner-Chavez, B.I.; Van Soest, P.J.; Robertson, J.B.; Lascano, C.; Pell, A.N. Comparison of the Precipitation of Alfalfa Leaf Protein and Bovine Serum Albumin by Tannins in the Radial Diffusion Method. J. Sci. Food Agric. 1997, 74, 513–523. [Google Scholar] [CrossRef]
- Siddiqi, R.A.; Singh, T.P.; Rani, M.; Sogi, D.S.; Bhat, M.A. Diversity in Grain, Flour, Amino Acid Composition, Protein Profiling, and Proportion of Total Flour Proteins of Different Wheat Cultivars of North India. Front. Nutr. 2020, 7, 141. [Google Scholar] [CrossRef] [PubMed]






| Genetic Horizon | Depth | Munsell Color | Texture * | Structure # | Bulk Density | |
|---|---|---|---|---|---|---|
| Dry | Moist | [g cm3] | ||||
| Profile 1. Haplic Luvisol (Cutanic) | ||||||
| Ap | 0–30 | 5/3 2.5Y | 3/3 2.5Y | L | AB, ME, 2 | 1.64 |
| E1 | 30–55 | 6/3 10YR | 4/4 10YR | SL | AB, FI, 1 | 1.62 |
| E2 | 55–82 | 6/3 10YR | 4/4 10YR | SL | AB, FI, 1 | 1.72 |
| Bt | 82–144 | 6/4 10YR | 4/6 10YR | SiL | SB, CO, 3 | 1.77 |
| BC | 144–150 | 5/6 10YR | 4/6 10YR | L | SB, CO, 3 | 1.83 |
| Profile 2. Mollic Stagnic Gleyosol | ||||||
| Ap | 0–30 | 4/1 10YR | 2/1 10YR | L | AB, ME, 2 | 1.67 |
| A2 | 30–53 | 6/2 5Y | 3/2 5Y | L | AB, VC, 3 | 1.69 |
| 2ACgg | 53–70 | 5/2 5Y | 4/2 5Y | L | AB, CO, 3 | 1.77 |
| 3G1 | 70–110 | 5/2 5Y | 4/3 5Y | L | AB, CO, 3 | 1.78 |
| 3G2 | 110–150 | 4/1 10YR | 2/1 10YR | L | AB, ME, 2 | 1.79 |
| Profile 3. Haplic Luvisol (Cutanic) | ||||||
| Ap | 0–32 | 5/3 10YR | 3/3 10YR | SL | AB CO 2 | 1.55 |
| E | 32–46 | 6/4 10YR | 4/4 10YR | L | AB ME 1 | 1.63 |
| EB | 46–58 | 6/4 10YR | 4/6 10YR | L | SB ME 2 | 1.71 |
| Bt | 58–135 | 5/4 10YR | 3/6 10YR | L | SB CO 3 | 1.73 |
| Ck | 135–150 | 6/4 10YR | 4/6 10YR | L | AB CO 2 | 1.73 |
| Profile 4. Cambic Stagnic Phaeozem | ||||||
| Ap | 0–35 | 3/2 10YR | 2/1 10YR | L | AB ME 2 | 1.46 |
| BCkg | 35–67 | 7/2 2.5Y | 6/3 2.5Y | L | SB VC 3 | 1.61 |
| Ck | 67–90 | 7/2 2.5Y | 6/3 2.5Y | L | AB CO2 | 1.71 |
| 2Ck1 | 90–123 | 7/1 2.5Y | 6/2 2.5Y | FSL | AB ME 2 | 1.72 |
| 2Ck2 | 123–150 | 7/2 2.5Y | 5/3 2.5Y | FS | AB FI 1 | 1.75 |
| Profile | Genetic Horizon | pH KCl | Clay | TOC | TN | TOC/TN | N-NO3− | N-NH4+ | CEC |
|---|---|---|---|---|---|---|---|---|---|
| % | g kg−1 | mg kg−1 | mmol kg−1 | ||||||
| Haplic Luvisol (Cutanic) | Ap | 5.71 ± 0.18 A | 8 ± 0.3 C | 10.8 ± 1.23 A | 1.32 ± 0.32 A | 10.8 ± 0.30 A | 1.31 ± 0.01 A | 2.46 ± 0.04 A | 46 ± 1.7 B |
| E1 | 5.94 ± 0.14 A | 7 ± 0.2 C | 2.70 ± 0.56 B | 0.31 ± 0.09 B | 8.7 ± 0.20 B | 0.40 ± 0.01 D | 0.62 ± 0.01 B | 36 ± 1.0 B | |
| E2 | 6.03 ± 0.11 A | 6 ± 0.1 C | 1.59 ± 0.24 C | 0.26 ± 0.07 C | 6.1 ± 0.14 C | 0.58 ± 0.01 C | 0.27 ± 0.01 C | 28 ± 0.7 C | |
| Bt | 5.96 ± 0.08 A | 16 ± 0.6 A | 1.34 ± 0.45 C | 0.35 ± 0.10 B | 3.8 ± 0.15 D | 0.91 ± 0.02 B | 0.07 ± 0.01 D | 108 ± 4.0 A | |
| BC | 5.58 ± 0.11 A | 13 ± 0.4 B | 1.22 ± 0.31 C | 0.35 ± 0.13 B | 3.5 ± 0.10 D | 0.34 ± 0.01 E | 0.26 ± 0.01 C | 97 ± 3.1 A | |
| Molic Eutric Stagnosol | Ap | 6.91 ± 0.09 A | 10 ± 0.3 C | 17.7 ± 1.54 A | 1.96 ± 0.34 A | 9.0 ± 0.35 B | 1.40 ± 0.04 A | 2.23 ± 0.05 A | 142 ± 4.9 A |
| A2 | 6.49 ± 0.11 C | 12 ± 0.2 C | 9.66 ± 0.87 B | 0.90 ± 0.11 B | 10.7 ± 0.22 A | 0.36 ± 0.01 B | 0.54 ± 0.01 B | 126 ± 2.7 A | |
| 2ACgg | 6.46 ± 0.05 C | 25 ± 0.7 A | 4.02 ± 0.35 C | 0.42 ± 0.09 C | 9.6 ± 0.25 AB | 0.21 ± 0.01 C | 0.34 ± 0.01 C | 139 ± 4.0 A | |
| 3G1 | 6.67 ± 0.05 B | 14 ± 0.3 B | 2.34 ± 25 D | 0.37 ± 0.09 C | 6.3 ± 0.09 C | 0.13 ± 0.01 C | 0.21 ± 0.01 D | 96 ± 2.3 B | |
| 3G2 | 6.74 ± 0.03 B | 14 ± 0.5 B | 2.13 ± 0.33 D | 0.29 ± 0.05 D | 7.3 ± 0.31 C | 0.07 ± 0.01 D | 0.22 ± 0.01 D | 92 ± 3.5 B | |
| Haplic Luvisol (Cutanic) | Ap | 6.86 ± 0.15 A | 9 ± 0.2 C | 10.1 ± 0.41 A | 1.25 ± 0.02 A | 8.0 ± 0.18 A | 9.80 ± 0.40 B | 2.40 ± 0.04 A | 70 ± 1.0 B |
| E | 5.81 ± 0.19 B | 8 ± 0.2 C | 3.07 ± 0.10 B | 0.41 ± 0.01 B | 7.3 ± 0.19 A | 7.85 ± 0.09 C | 0.49 ± 0.01 B | 57± 1.5 C | |
| EB | 5.72 ± 0.20 B | 14 ± 0.4 B | 2.17 ± 0.02 C | 0.37 ± 0.01 B | 6.2 ± 0.22 B | 23.5 ± 0.69 A | 0.26 ± 0.01 C | 64 ± 2.6 BC | |
| Bt | 5.63 ± 0.21 B | 18 ± 0.4 A | 1.60 ± 0.04 C | 0.36 ± 0.01 B | 4.4 ± 0.17 C | 8.13 ± 0.18 B | 0.25 ± 0.01 C | 109 ± 2.3 B | |
| Ck | 7.46 ± 0.30 A | 15 ± 0.4 B | 1.72 ± 0.03 C | 0.30 ± 0.01 C | 5.9 ± 0.24 B | 2.19 ± 0.05 D | 0.20 ± 0.01 C | 132 ± 4.3 A | |
| Cambic Stagnic Phaeozem | Ap | 7.41 ± 0.36 A | 9 ± 0.2 C | 19.1 ± 0.21 A | 2.43 ± 0.06 A | 7.9 ± 0.38 A | 3.36 ± 0.14 D | 2.78 ± 0.05 A | 287 ± 10.7 C |
| BCkg | 7.82 ± 0.08 A | 21 ± 0.5 B | 2.97 ± 0.03 B | 0.36 ± 0.01 B | 8.3 ± 0.08 A | 34.4 ± 0.56 C | 0.48 ± 0.01 B | 499 ±14.4 B | |
| Gk | 7.71 ± 0.27 A | 24 ± 0.4 A | 2.28 ± 0.05 B | 0.36 ± 0.01 B | 6.3 ± 0.22 B | 77.3 ± 0.77 A | 0.52 ± 0.01 B | 576 ±13.5 A | |
| 2Gk1 | 8.36 ± 0.14 A | 7 ± 0.2 C | 0.68 ± 0.02 C | 0.08 ± 0.00 C | 8.9 ± 0.15 A | 41.8 ± 1.25 B | 0.17 ± 0.01 C | 156 ± 2.9 D | |
| 2Gk2 | 8.19 ± 0.48 A | 2 ± 0.10 D | 0.32 ± 0.01 C | 0.08 ± 0.00 C | 4.2 ± 0.25 C | 26.6 ± 0.92 C | 0.04 ± 0.01 D | 60 ± 1.9 E | |
| Genetic Horizon | Depth | MBC | MBN | MBC/MBN | MBC/TOC | MBN/TN |
|---|---|---|---|---|---|---|
| (mg kg−1) | (%) | |||||
| Profile 1. Haplic Luvisol | ||||||
| Ap | 0–30 | 123.3 ± 6.67 A | 25.6 ± 2.97 A | 4.81 ± 0.38 C | 1.15 ± 0.09 B | 1.94 ± 0.12 C |
| E1 | 30–55 | 47.1 ± 3.98 B | 10.5 ± 1.22 B | 4.47 ± 0.29 CD | 1.74 ± 0.15 A | 3.40 ± 0.29 A |
| E2 | 55–82 | 27.2 ± 1.89 C | 6.56 ± 0.41 C | 4.01 ± 0.34 D | 1.71 ± 0.16 A | 2.52 ± 0.19 B |
| Bt | 82–144 | 18.5 ± 1.75 D | 3.42 ± 0.37 D | 5.41 ± 0.28 B | 1.38 ± 0.16 B | 0.98 ± 0.18 D |
| BC | >144 | 15.3 ± 1.44 D | 2.45 ± 0.32 D | 6.24 ± 0.23 A | 1.25 ± 0.14 B | 0.70 ± 0.13 D |
| Profile 2. Mollic Stagnic Gleyosol | ||||||
| Ap | 0–30 | 145.6 ± 5.9 A | 30.2 ± 1.82 A | 4.83 ± 0.69 C | 0.82 ± 0.09 C | 1.54 ± 0.09 B |
| A2 | 30–53 | 113.6 ± 5.4 B | 25.5 ± 0.93 A | 4.46 ± 0.77 C | 1.18 ± 0.15 A | 2.83 ± 0.12 A |
| 2ACgg | 53–70 | 53.1 ± 4.38 C | 10.5 ± 0.41 B | 5.06 ± 0.82 B | 1.32 ± 0.14 A | 2.50 ± 0.18 A |
| 3G1 | 70–110 | 25.6 ± 3.37 D | 4.12 ± 0.67 C | 6.21 ± 0.89 A | 1.09 ± 0.12 B | 1.11 ± 0.13 C |
| 3G2 | 110–150 | 16.5 ± 1.02 E | 2.56 ±0.35 D | 4.44 ± 0.50 C | 0.78 ± 0.14 C | 0.88 ± 0.11 D |
| Profile 3. Haplic Luvisol | ||||||
| Ap | 0–32 | 113.9 ± 1.12 A | 23.2 ± 0.34 A | 4.93 ± 0.05 A | 1.12 ± 0.02 B | 1.83 ± 0.02 B |
| E | 32–46 | 57.1 ± 0.31 B | 12.1 ± 0.32 B | 4.72 ± 0.17 A | 1.86 ± 0.05 A | 2.84 ± 0.10 A |
| EB | 46–58 | 39.0 ± 1.57 C | 9.1 ± 0.37 C | 4.32 ± 0.23 B | 1.78 ± 0.08 A | 2.54 ± 0.12 A |
| Bt | 58–135 | 26.6 ± 0.77 D | 6.1 ± 0.13 D | 4.42 ± 0.13 B | 1.64 ± 0.12 A | 1.64 ± 0.08 B |
| Ck | 135–150 | 10.0 ± 0.43 E | 2.1 ± 0.11 E | 4.76 ± 0.21 A | 0.58 ± 0.09 C | 0.71 ± 0.06 C |
| Profile 4. Cambic Stagnic Phaeozem | ||||||
| Ap | 0–35 | 302.1 ± 9.57 A | 61.8 ± 1.07 A | 4.63 ± 0.09 B | 1.51 ± 0.03 D | 2.61 ± 0.05 E |
| BCkg | 35–67 | 106.1 ± 5.99 B | 22.9 ± 0.34 B | 4.71 ± 0.12 B | 3.65 ± 0.04 C | 6.36 ± 0.32 C |
| Gk | 67–90 | 88.8 ± 3.40 C | 13.8 ± 0.34 C | 6.04 ± 0.18 A | 3.74 ± 0.13 C | 4.01 ± 0.08 D |
| 2Gk1 | 90–123 | 74 2 ± 2.03 D | 12.0 ± 0.23 CD | 5.96 ± 0.16 A | 10.8 ± 0.25 B | 16.0 ± 0.41 A |
| 2Gk2 | 123–150 | 46.7 ± 0.55 E | 9.8 ± 0.15 D | 4.66 ± 0.12 B | 13.8 ± 0.19 A | 12.8 ± 0.31 B |
| Genetic Horizon | Depth | UR activity | NR Activity | PRO Activity | NAG Activity |
|---|---|---|---|---|---|
| Profile 1. Haplic Luvisol (Cutanic) | |||||
| Ap | 0–30 | 6.32 ± 0.325 A | 0.33 ± 0.002 A | 13.7 ± 0.344 A | 0.26 ± 0.004 A |
| E1 | 30–55 | 2.09 ± 0.072 C | 0.05 ± 0.002 B | 11.2 ± 0.342 B | 0.17 ± 0.001 B |
| E2 | 55–82 | 3.31 ± 0.120 B | 0.03 ± 0.001 C | 11.1 ± 0.338 B | 0.07 ± 0.002 C |
| Bt | 82–144 | 2.61 ± 0.125 BC | 0.02 ± 0.001 C | 6.15 ± 0.339 C | 0.02 ± 0.001 D |
| BC | 144–150 | 1.88 ± 0.064 C | 0.02 ± 0.001 C | 2.54 ± 0.127 D | 0.01 ± 0.000 D |
| Profile 2. Mollic Stagnic Gleyosol | |||||
| Ap | 0–30 | 42.1 ± 0.127 A | 1.38 ± 0.043 A | 29.9 ± 1.487 A | 0.20 ± 0.032 A |
| A2 | 30–53 | 12.4 ± 0.628 B | 0.05 ± 0.005 C | 22.2 ± 1.021 B | 0.08 ± 0.010 B |
| 2ACgg | 53–70 | 3.82 ± 0.315 C | 0.19 ± 0.090 B | 26.0 ± 0.714 AB | 0.03 ± 0.001 C |
| 3G1 | 70–110 | 1.73 ± 0.050 D | 0.02 ± 0.001 C | 3.34 ± 0.084 C | 0.02 ± 0.000 C |
| 3G2 | 110–150 | 0.77 ± 0.063 D | 0.01 ± 0.000 C | 3.22 ± 0.108 C | 0.01 ± 0.000 C |
| Profile 3. Haplic Luvisol (Cutanic) | |||||
| Ap | 0–32 | 4.37 ± 0.176 A | 5.87 ± 0.002 A | 27.8 ± 1.140 A | 0.54 ± 0.010 A |
| E | 32–46 | 1.11 ± 0.058 C | 0.03 ± 0.001 B | 9.85 ± 1.207 C | 0.14 ± 0.001 B |
| EB | 46–58 | 3.04 ± 0.060 B | 0.01 ± 0.001 B | 18.3 ± 0.003 B | 0.10 ± 0.001 C |
| Bt | 58–135 | 0.42 ± 0.008 D | 0.01 ± 0.001 B | 11.1 ± 0.378 C | 0.10 ± 0.001 C |
| Ck | 135–150 | 0.33 ± 0.063 D | 0.01 ± 0.001 B | 4.10 ± 0.005 D | 0.04 ± 0.001 D |
| Profile 4. Cambic Stagnic Phaeozem | |||||
| Ap | 0–35 | 65.6 ± 15.80 A | 3.83 ± 0.030 A | 61.2 ± 0.388 A | 0.47 ± 0.006 A |
| BCkg | 35–67 | 3.20 ± 0.900 B | 0.11 ± 0.003 B | 25.3 ± 0.395 B | 0.04 ± 0.022 B |
| Ck | 67–90 | 3.19 ± 0.262 B | 0.08 ± 0.003 B | 7.01 ± 0.810 D | 0.03 ± 0.001 B |
| 2Ck1 | 90–123 | 1.25 ± 0.069 C | 0.04 ± 0.002 C | 7.18 ± 0.003 D | 0.01 ± 0.000 B |
| 2Ck2 | 123–150 | 0.91 ± 0.101 C | 0.01 ± 0.001 C | 10.4 ± 0.861 C | 0.02 ± 0.001 B |
| Variable | PCA1 | PCA2 | Variable | PCA1 | PCA2 |
|---|---|---|---|---|---|
| UR | 0.870 | 0.006 | clay | −0.242 | 0.535 |
| NR | 0.782 | −0.100 | TOC | 0.936 | −0.097 |
| PRO | 0.905 | 0.091 | TN | 0.951 | −0.108 |
| NAG | 0.875 | −0.247 | CEC | 0.166 | 0.939 |
| MBC | 0.947 | 0.238 | BD | −0859 | −0.072 |
| MBN | 0.955 | 0.179 | N-NO3− | −0.130 | 0.852 |
| pH in KCl | 0.140 | 0.711 | N-NH4+ | 0.920 | −0.134 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piotrowska-Długosz, A.; Długosz, J.; Gryta, A.; Frąc, M. Responses of N-Cycling Enzyme Activities and Functional Diversity of Soil Microorganisms to Soil Depth, Pedogenic Processes and Cultivated Plants. Agronomy 2022, 12, 264. https://doi.org/10.3390/agronomy12020264
Piotrowska-Długosz A, Długosz J, Gryta A, Frąc M. Responses of N-Cycling Enzyme Activities and Functional Diversity of Soil Microorganisms to Soil Depth, Pedogenic Processes and Cultivated Plants. Agronomy. 2022; 12(2):264. https://doi.org/10.3390/agronomy12020264
Chicago/Turabian StylePiotrowska-Długosz, Anna, Jacek Długosz, Agata Gryta, and Magdalena Frąc. 2022. "Responses of N-Cycling Enzyme Activities and Functional Diversity of Soil Microorganisms to Soil Depth, Pedogenic Processes and Cultivated Plants" Agronomy 12, no. 2: 264. https://doi.org/10.3390/agronomy12020264








