Growth of Tea Nursery Plants as Influenced by Different Rates of Protein Hydrolysate Derived from Chicken Feathers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Chicken Feather
2.2. Production of Chicken Feather Protein Hydrolysate
2.3. Analysis of Chicken Feather and CFPH
2.4. Amino Acid Profile
2.5. Plant Materials and Treatment Application
2.6. Plant Growth Measurements
2.7. Root Growth Measurements
2.8. Leaf Gas Exchange
2.9. Chlorophyll Content
2.10. Leaf Nutrient Analysis
2.11. Statistical Analysis
3. Results
3.1. Production and Characterization of CFPH
3.2. Shoot Growth
3.3. Root Growth
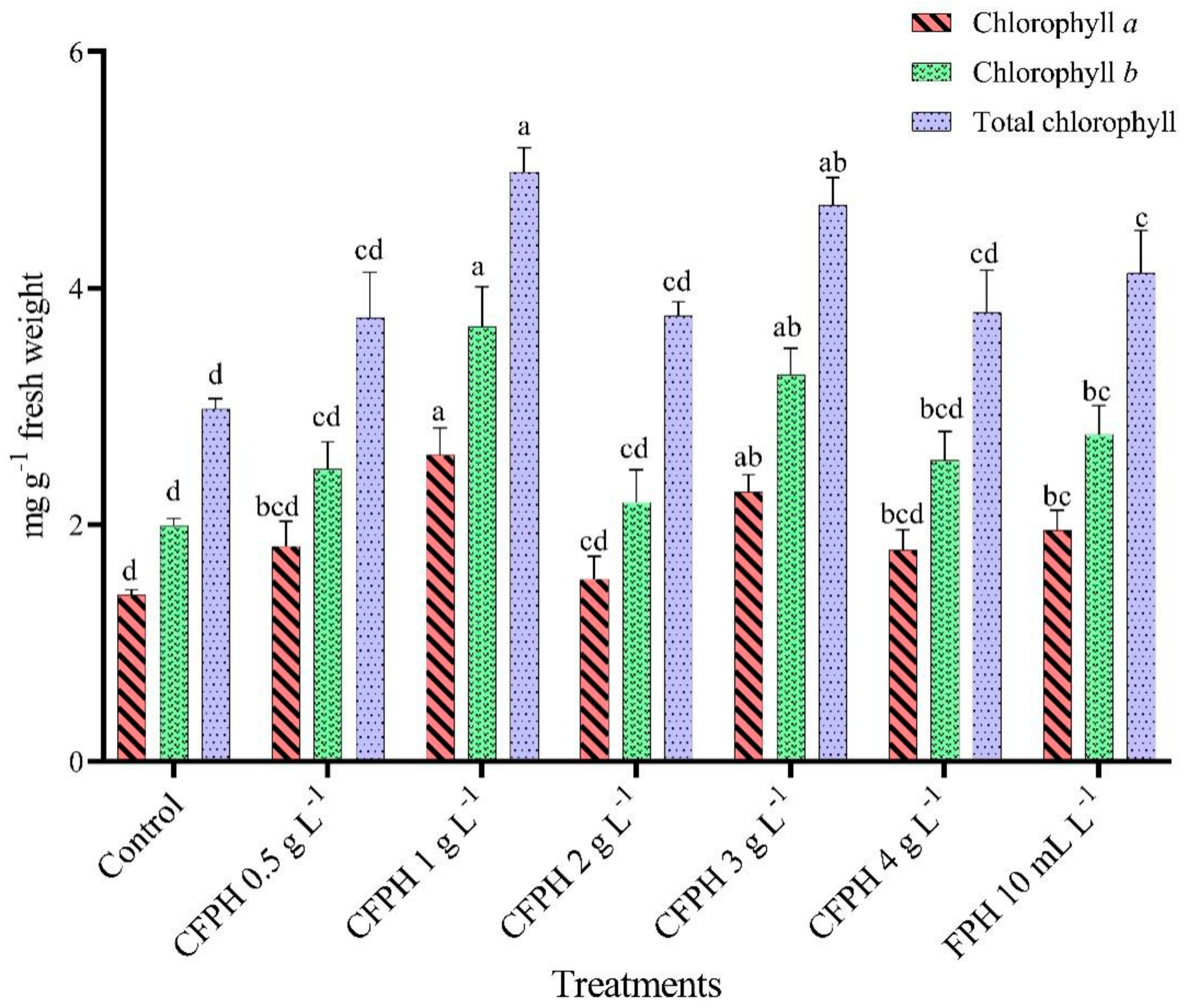
3.4. Chlorophyll Content
3.5. Leaf Gas Exchange
3.6. Leaf Nutrients
4. Discussion
4.1. Production and Characterization of CFPH
4.2. Shoot and Root Growth
4.3. Chlorophyll Content
4.4. Leaf Gas Exchange
4.5. Leaf Nutrients
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. FAO World Food and Agriculture-Statistical Yearbook 2020; FAO: Rome, Italy, 2020; ISBN 9789251333945. [Google Scholar]
- Gessesse, A.; Hatti-Kaul, R.; Gashe, B.A.; Mattiasson, B. Novel alkaline proteases from alkaliphilic bacteria grown on chicken feather. Enzym. Microb. Technol. 2003, 32, 519–524. [Google Scholar] [CrossRef]
- Prasanthi, N.; Bhargavi, S.; Machiraju, P.V.S.; Professor, A. Chicken Feather Waste-A Threat to the Environment. Int. J. Innov. Res. Sci. Eng. Technol. 2016, 5, 16759–16764. [Google Scholar] [CrossRef]
- Nurdiawati, A.; Nakhshiniev, B.; Zaini, I.N.; Saidov, N.; Takahashi, F.; Yoshikawa, K. Characterization of potential liquid fertilizers obtained by hydrothermal treatment of chicken feather. Environ. Prog. Sustain. Energy 2018, 37, 375–382. [Google Scholar] [CrossRef]
- Ben Hamad Bouhamed, S.; Kechaou, N. Kinetic study of sulphuric acid hydrolysis of protein feathers. Bioprocess Biosyst. Eng. 2017, 40, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Dudyński, M.; Kwiatkowski, K.; Bajer, K. From feathers to syngas-Technologies and devices. Waste Manag. 2012, 32, 685–691. [Google Scholar] [CrossRef]
- Gwyther, C.L.; Williams, A.P.; Golyshin, P.N.; Edwards-Jones, G.; Jones, D.L. The environmental and biosecurity characteristics of livestock carcass disposal methods: A review. Waste Manag. 2011, 31, 767–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baglieri, A.; Cadili, V.; Mozzetti, C.; Gennari, M.; Tabasso, S.; Montoneri, E.; Nardi, S.; Negre, M. Fertilization of bean plants with tomato plants hydrolysates. Effect on biomass production, chlorophyll content and N assimilation. Sci. Hortic. 2014, 176, 194–199. [Google Scholar] [CrossRef]
- Schaafsma, G. Safety of protein hydrolysates, fractions thereof and bioactive peptides in human nutrition. Eur. J. Clin. Nutr. 2009, 63, 1161–1168. [Google Scholar] [CrossRef] [Green Version]
- Olbrycht, M.; Kołodziej, M.; Bochenek, R.; Przywara, M.; Balawejder, M.; Matłok, N.; Antos, P.; Piątkowski, W.; Antos, D. Mechanism of nutrition activity of a microgranule fertilizer fortified with proteins. BMC Plant Biol. 2020, 20, 126. [Google Scholar] [CrossRef]
- Colla, G.; Nardi, S.; Cardarelli, M.; Ertani, A.; Lucini, L.; Canaguier, R.; Rouphael, Y. Protein hydrolysates as biostimulants in horticulture. Sci. Hortic. 2015, 196, 28–38. [Google Scholar] [CrossRef]
- Moreno-Hernández, J.M.; Benítez-García, I.; Mazorra-Manzano, M.A.; Ramírez-Suárez, J.C.; Sánchez, E. Strategies for production, characterization and application of protein-based biostimulants in agriculture: A review. Chil. J. Agric. Res. 2020, 80, 274–289. [Google Scholar] [CrossRef]
- Morales-Payan, J.P.; Stall, W.M. Papaya (Carica papaya) response to foliar treatments with organic complexes of peptides and amino acids. Proc. Fla. State Hortic. Soc. 2003, 116, 30–32. [Google Scholar]
- Cerdán, M.; Sánchez-Sánchez, A.; Oliver, M.; Juárez, M.; Sánchez-Andreu, J.J. Effect of foliar and root applications of amino acids on iron uptake by tomato plants. Acta Hortic. 2009, 830, 481–488. [Google Scholar] [CrossRef]
- Kauffman, G.L.; Kneivel, D.P.; Watschke, T.L. Effects of a biostimulant on the heat tolerance associated with photosynthetic capacity, membrane thermostability, and polyphenol production of perennial ryegrass. Crop Sci. 2007, 47, 261–267. [Google Scholar] [CrossRef]
- Kunicki, E.; Grabowska, A.; Sękara, A.; Wojciechowska, R. The effect of cultivar type, time of cultivation, and biostimulant treatment on the yield of spinach (Spinacia oleracea L.). Folia Hortic. 2010, 22, 9–13. [Google Scholar] [CrossRef] [Green Version]
- Ertani, A.; Pizzeghello, D.; Francioso, O.; Tinti, A.; Nardi, S. Biological activity of vegetal extracts containing phenols on plant metabolism. Molecules 2016, 21, 205. [Google Scholar] [CrossRef]
- du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Nardi, S.; Pizzeghello, D.; Schiavon, M.; Ertani, A. Plant biostimulants: Physiological responses induced by protein hydrolyzed-based products and humic substances in plant metabolism. Sci. Agric. 2016, 73, 18–23. [Google Scholar] [CrossRef] [Green Version]
- Colla, G.; Rouphael, Y.; Canaguier, R.; Svecova, E.; Cardarelli, M. Biostimulant action of a plant-derived protein hydrolysate produced through enzymatic hydrolysis. Front. Plant Sci. 2014, 5, 448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ertani, A.; Cavani, L.; Pizzeghello, D.; Brandellero, E.; Altissimo, A.; Ciavatta, C.; Nardi, S. Biostimulant activity of two protein hydrolyzates in the growth and nitrogen metabolism of maize seedlings. J. Plant Nutr. Soil Sci. 2009, 172, 237–244. [Google Scholar] [CrossRef]
- Halpern, M.; Bar-Tal, A.; Ofek, M.; Minz, D.; Muller, T.; Yermiyahu, U. The Use of Biostimulants for Enhancing Nutrient Uptake. Adv. Agron. 2015, 130, 141–174. [Google Scholar] [CrossRef]
- Colla, G.; Hoagland, L.; Ruzzi, M.; Cardarelli, M.; Bonini, P.; Canaguier, R.; Rouphael, Y. Biostimulant action of protein hydrolysates: Unraveling their effects on plant physiology and microbiome. Front. Plant Sci. 2017, 8, 2202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polo, J.; Barroso, R.; Ródenas, J.; Azcón-bieto, J.; Cáceres, R.; Marfà, O. Porcine Hemoglobin Hydrolysate as a Biostimulant for Lettuce Plants Subjected to Conditions of Thermal Stress. Horttechnology 2006, 16, 483–487. [Google Scholar] [CrossRef] [Green Version]
- Rouphael, Y.; Carillo, P.; Cristofano, F.; Cardarelli, M.; Colla, G. Effects of vegetal- versus animal-derived protein hydrolysate on sweet basil morpho-physiological and metabolic traits. Sci. Hortic. 2021, 284, 110123. [Google Scholar] [CrossRef]
- Ruiz, J.M.; Castilla, N.; Romero, L. Nitrogen metabolism in pepper plants applied with different bioregulators. J. Agric. Food Chem. 2000, 48, 2925–2929. [Google Scholar] [CrossRef] [PubMed]
- Lisiecka, J.; Mikołaj Knaflewski, T.S.; Frąszczak, B.; Alina Kałużewicz, W.K. The effect of animal protein hydrolysate on quantity and quality of strawberry daughter plants cv. ’Elsanta’. Acta Sci. Pol. Hortorum Cultus 2011, 10, 31–40. [Google Scholar]
- Moe, L.A. Amino acids in the rhizosphere: From plants to microbes. Am. J. Bot. 2013, 100, 1692–1705. [Google Scholar] [CrossRef]
- Corte, L.; Dell’Abate, M.T.; Magini, A.; Migliore, M.; Felici, B.; Roscini, L.; Sardella, R.; Tancini, B.; Emiliani, C.; Cardinali, G.; et al. Assessment of safety and efficiency of nitrogen organic fertilizers from animal-based protein hydrolysates-a laboratory multidisciplinary approach. J. Sci. Food Agric. 2014, 94, 235–245. [Google Scholar] [CrossRef]
- Nardi, S.; Carletti, P.; Pizzeghello, D.; Muscolo, A. Biological activities of humic substances. In Biophysico-Chemical Processes Involving Natural Nonliving Organic Matter in Environmental Systems; Senesi, N., Xing, B., Huang, P.M., Eds.; Wiley: Hoboken, NJ, USA, 2009; pp. 305–339. [Google Scholar]
- Paul, T.; Halder, S.K.; Das, A.; Bera, S.; Maity, C.; Mandal, A.; Das, P.S.; Das Mohapatra, P.K.; Pati, B.R.; Mondal, K.C. Exploitation of chicken feather waste as a plant growth promoting agent using keratinase producing novel isolate Paenibacillus woosongensis TKB2. Biocatal. Agrocult. Biotechnol. 2013, 2, 50–57. [Google Scholar] [CrossRef]
- Genc, E.; Atici, Ö. Chicken feather protein hydrolysate as a biostimulant improves the growth of wheat seedlings by affecting biochemical and physiological parameters. Turk. J. Bot. 2019, 43, 67–79. [Google Scholar] [CrossRef] [Green Version]
- Taskin, M.; Ozkan, B.; Atici, O.; Aydogan, M.N. Utilization of chicken feather hydrolysate as a novel fermentation substrate for production of exopolysaccharide and mycelial biomass from edible mushroom Morchella esculenta. Int. J. Food Sci. Nutr. 2012, 63, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Coombs, J.; Hind, G.; Leegood, R.C.; Tieszen, L.L.; Vonshak, A. Analytical Techniques. In Techniques in Bioproductivity and Photosynthesis, 2nd ed.; Pergamon Press: Oxford, UK, 1987. [Google Scholar]
- Bremner, J. Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; Wiley: Hoboken, NJ, USA, 1965; Volume 9, pp. 1149–1178. [Google Scholar]
- Lee, Y.S.; Phang, L.Y.; Ahmad, S.A.; Ooi, P.T. Microwave-Alkali Treatment of Chicken Feathers for Protein Hydrolysate Production. Waste Biomass Valorization 2016, 7, 1147–1157. [Google Scholar] [CrossRef]
- Cheong, C.W.; Lee, Y.S.; Ahmad, S.A.; Ooi, P.T.; Phang, L.Y. Chicken feather valorization by thermal alkaline pretreatment followed by enzymatic hydrolysis for protein-rich hydrolysate production. Waste Manag. 2018, 79, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.M.; Sun, Y.Y.; Ye, X.Y.; Li, Z.G. Signaling Role of Glutamate in Plants. Front. Plant Sci. 2020, 10, 1743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Buono, D. Can biostimulants be used to mitigate the effect of anthropogenic climate change on agriculture? It is time to respond. Sci. Total Environ. 2021, 751, 141763. [Google Scholar] [CrossRef] [PubMed]
- Bellagamba, F.; Caprino, F.; Mentasti, T.; Vasconi, M.; Moretti, V.M. The impact of processing on amino acid racemization and protein quality in processed animal proteins of poultry origin. Ital. J. Anim. Sci. 2015, 14, 3770. [Google Scholar] [CrossRef] [Green Version]
- Joardar, J.C.; Rahman, M.M. Poultry feather waste management and effects on plant growth. Int. J. Recycl. Org. Waste Agric. 2018, 7, 183–188. [Google Scholar] [CrossRef] [Green Version]
- Nurdiawati, A.; Suherman, C.; Maxiselly, Y.; Akbar, M.A.; Purwoko, B.A.; Prawisudha, P.; Yoshikawa, K. Liquid feather protein hydrolysate as a potential fertilizer to increase growth and yield of patchouli (Pogostemon cablin Benth) and mung bean (Vigna radiata). Int. J. Recycl. Org. Waste Agric. 2019, 8, 221–232. [Google Scholar] [CrossRef] [Green Version]
- Cristiano, G.; Pallozzi, E.; Conversa, G.; Tufarelli, V.; De Lucia, B. Effects of an animal-derived biostimulant on the growth and physiological parameters of potted snapdragon (Antirrhinummajus L.). Front. Plant Sci. 2018, 9, 861. [Google Scholar] [CrossRef]
- Zhang, X.; Ervin, E.H.; Schmidt, R.E. Physiological effects of liquid applications of a seaweed extract and a humic acid on creeping bentgrass. J. Am. Soc. Hortic. Sci. 2003, 128, 492–496. [Google Scholar] [CrossRef] [Green Version]
- Gousterova, A.; Nustorova, M.; Paskaleva, D.; Naydenov, M.; Neshev, G.; Vasileva-Tonkova, E. Assessment of feather hydrolysate from thermophilic actinomycetes for soil amendment and biological control application. Int. J. Environ. Res. 2011, 5, 1065–1070. [Google Scholar] [CrossRef]
- Ertani, A.; Pizzeghello, D.; Francioso, O.; Sambo, P.; Sanchez-Cortes, S.; Nardi, S. Capsicum chinensis L. growth and nutraceutical properties are enhanced by biostimulants in a long-term period: Chemical and metabolomic approaches. Front. Plant Sci. 2014, 5, 375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurbanoglu, E.B.; Atici, O.; Algur, O.F. Effect of ram horn hydrolyzate on the growth of bean (Phaseolus vulgaris cv. aziziye-94). Biol. Agric. Hortic. 2004, 22, 121–131. [Google Scholar] [CrossRef]
- Horii, A.; McCue, P.; Shetty, K. Seed vigour studies in corn, soybean and tomato in response to fish protein hydrolysates and consequences on phenolic-linked responses. Bioresour. Technol. 2007, 98, 2170–2177. [Google Scholar] [CrossRef]
- Gough, S.P.; Westergren, T.; Hansson, M. Chlorophyll biosynthesis in higher plants. Regulatory aspects of 5-aminolevulinate formation. J. Plant Biol. 2003, 46, 135–160. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G.; Giordano, M.; El-Nakhel, C.; Kyriacou, M.C.; De Pascale, S. Foliar applications of a legume-derived protein hydrolysate elicit dose-dependent increases of growth, leaf mineral composition, yield and fruit quality in two greenhouse tomato cultivars. Sci. Hortic. 2017, 226, 353–360. [Google Scholar] [CrossRef]
- Xu, C.; Mou, B. Drench application of fish-derived protein hydrolysates affects lettuce growth, chlorophyll content, and gas exchange. Horttechnology 2017, 27, 539–543. [Google Scholar] [CrossRef]
- Gurav, R.G.; Jadhav, J.P. A novel source of biofertilizer from feather biomass for banana cultivation. Environ. Sci. Pollut. Res. 2013, 20, 4532–4539. [Google Scholar] [CrossRef]
- Yang, C.; Yang, L.; Yang, Y.; Ouyang, Z. Rice root growth and nutrient uptake as influenced by organic manure in continuously and alternately flooded paddy soils. Agric. Water Manag. 2004, 70, 67–81. [Google Scholar] [CrossRef]
- Cristiano, G.; De Lucia, B. Petunia Performance under Application of Animal-Based Protein Hydrolysates: Effects on Visual Quality, Biomass, Nutrient Content, Root Morphology, and Gas Exchange. Front. Plant Sci. 2021, 12, 608. [Google Scholar] [CrossRef] [PubMed]
- Ryser, P. The mysterious root length. Plant Soil 2006, 286, 1–6. [Google Scholar] [CrossRef]


| Elements | Chicken Feather | CFPH |
|---|---|---|
| Total C (%) | 47.09 | 9.42 |
| Total N (%) | 13.54 | 3.06 |
| C/N ratio | 2.81 | 2.31 |
| P (%) | 0.12 | 17.13 |
| K (%) | 0.29 | 25.41 |
| Ca (%) | 0.31 | 0.074 |
| Mg (%) | 0.03 | 0.007 |
| S (%) | 1.61 | 0.44 |
| Zn (mg kg−1) | 0.02 | <0.01 |
| Mn (mg kg−1) | <0.01 | <0.01 |
| Cu (mg kg−1) | <0.01 | <0.01 |
| Fe (mg kg−1) | 0.04 | <0.01 |
| Amino Acids | Amino Acids (%) | Chicken Feather (mg g−1) |
|---|---|---|
| Alanine | 6.21 | 10.27 |
| Arginine | 6.61 | 10.94 |
| Aspartic acid | 8.03 | 13.29 |
| Cysteine | 0.79 | 1.30 |
| Glutamic acid | 13.36 | 22.11 |
| Glycine | 7.28 | 12.04 |
| Histidine | ND | 0.00 |
| Isoleucine | 6.28 | 10.40 |
| Leucine | 9.42 | 15.59 |
| Lysine | 2.73 | 4.53 |
| Methionine | 0.75 | 1.25 |
| Phenylalanine | 4.77 | 7.90 |
| Proline | 11.75 | 19.45 |
| Serine | 8.11 | 13.43 |
| Threonine | 2.42 | 3.71 |
| Tyrosine | 2.56 | 4.24 |
| Valine | 9.06 | 14.99 |
| Hydroxyproline | ND | 0.00 |
| Total | 100 | 165.50 |
| Treatment | Plant Height (cm) | Leaf Number (n plant−1) | Shoot Fresh Weight (g plant−1) | Shoot Dry Weight (g plant−1) |
|---|---|---|---|---|
| Control | 22.7 ± 1.4 e | 7.0 ± 0.6 d | 6.6 ± 0.4 e | 2.13 ± 0.17 e |
| CFPH 0.5 g L−1 | 27.2 ± 1.0 d | 8.0 ± 0.6 cd | 8.5 ± 0.5 d | 2.76 ± 0.23 de |
| CFPH 1 g L−1 | 39.6 ± 0.7 b | 10.0 ± 0.6 ab | 12.5 ± 0.6 ab | 4.23 ± 0.43 ab |
| CFPH 2 g L−1 | 45.0 ± 1.1 a | 11.3 ± 0.3 a | 13.7 ± 0.7 a | 4.87 ± 0.27 a |
| CFPH 3 g L−1 | 37.7 ± 0.6 bc | 8.6 ± 0.3 bc | 12.2 ± 0.6 abc | 4.03 ± 0.32 bc |
| CFPH 4 g L−1 | 34.3 ± 1.3 c | 8.6± 0.3 bc | 10.6 ± 0.5 c | 2.73 ± 0.06 de |
| FPH 10 mL L−1 | 37.0 ± 1.5 bc | 8.6 ± 0.3 bc | 11.5 ± 0.5 bc | 3.31 ± 0.13 cd |
| Treatment | Pn | Gs | Ci | Tr |
|---|---|---|---|---|
| Control | 10.88 ± 0.21 b | 0.22 ± 0.02 a | 290 ± 12 a | 6.17 ± 0.36 bc |
| CFPH 0.5 g L−1 | 11.15 ± 0.20 ab | 0.26 ± 0.01 a | 292 ± 10 a | 6.99 ± 0.13 ab |
| CFPH 1 g L−1 | 10.59 ± 0.20 b | 0.21 ± 0.03 a | 283 ± 07 a | 5.78 ± 0.09 c |
| CFPH 2 g L−1 | 10.47 ± 0.26 b | 0.23 ± 0.04 a | 289 ± 04 a | 6.54 ± 0.46 bc |
| CFPH 3 g L−1 | 11.93 ± 0.42 a | 0.25 ± 0.02 a | 287 ± 03 a | 7.81 ± 0.18 a |
| CFPH 4 g L−1 | 10.65 ± 0.24 b | 0.26 ± 0.01 a | 295 ± 07 a | 7.03 ± 0.44 ab |
| FPH 10 mL L−1 | 11.20 ± 0.35 ab | 0.24 ± 0.02 a | 289 ± 03 a | 6.77 ± 0.23 b |
| N (g kg−1) | P (g kg−1) | Mn (mg kg−1) | Cu (mg kg−1) | |
|---|---|---|---|---|
| Control | 38.4 ± 0.4 b | 2.20 ± 0.07 b | 49.7± 0.6 d | 6.23 ± 0.36 c |
| CFPH 0.5 g L−1 | 37.7 ± 1.8 b | 2.15 ± 0.03 b | 54.0 ± 1.4 cd | 6.31 ± 0.51 bc |
| CFPH 1 g L−1 | 38.9 ± 1.2 b | 2.18 ± 0.07 b | 59.6 ± 1.2 bc | 7.38 ± 0.54 abc |
| CFPH 2 g L−1 | 43.7 ± 1.9 a | 2.63 ± 0.19 a | 64.3 ± 3.3 ab | 7.95 ± 0.53 a |
| CFPH 3 g L−1 | 45.4 ± 0.7 a | 2.53 ± 0.12 a | 67.2 ± 1.3 a | 7.56 ± 0.16 ab |
| CFPH 4 g L−1 | 44.0 ± 1.1 a | 2.63 ± 0.04 a | 66.8 ± 2.7 a | 8.36 ± 0.17 a |
| FPH 10 mL L−1 | 44.4 ± 0.7 a | 2.56 ± 0.03 a | 65.5 ± 1.1 a | 7.85 ± 0.55 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raguraj, S.; Kasim, S.; Md Jaafar, N.; Nazli, M.H. Growth of Tea Nursery Plants as Influenced by Different Rates of Protein Hydrolysate Derived from Chicken Feathers. Agronomy 2022, 12, 299. https://doi.org/10.3390/agronomy12020299
Raguraj S, Kasim S, Md Jaafar N, Nazli MH. Growth of Tea Nursery Plants as Influenced by Different Rates of Protein Hydrolysate Derived from Chicken Feathers. Agronomy. 2022; 12(2):299. https://doi.org/10.3390/agronomy12020299
Chicago/Turabian StyleRaguraj, Sriharan, Susilawati Kasim, Noraini Md Jaafar, and Muhamad Hazim Nazli. 2022. "Growth of Tea Nursery Plants as Influenced by Different Rates of Protein Hydrolysate Derived from Chicken Feathers" Agronomy 12, no. 2: 299. https://doi.org/10.3390/agronomy12020299
APA StyleRaguraj, S., Kasim, S., Md Jaafar, N., & Nazli, M. H. (2022). Growth of Tea Nursery Plants as Influenced by Different Rates of Protein Hydrolysate Derived from Chicken Feathers. Agronomy, 12(2), 299. https://doi.org/10.3390/agronomy12020299







