Temporary Immersion Bioreactor System as an Efficient Method for Mass Production of In Vitro Plants in Horticulture and Medicinal Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Culture Conditions
2.2. Cytological and Histological Analysis
2.3. Chlorophyll and Carotenoid Content Measurement
2.4. Acclimatization of Harvested Plants from TIS
2.5. Statistical Analysis
3. Results and Discussion
3.1. Plant Growth in Different Culture Systems
3.2. Cytological and Histological Analysis
3.3. Chlorophyll and Carotenoid Contents
3.4. Acclimatization of Three Horticultural Species
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Diengngan, S.; Mahadevamma, M.; Murthy, B.N. Efficacy of in vitro propagation and crown sizes on the performance of strawberry (Fragaria × ananassa Duch) cv. Festival under field condition. J. Agric. Sci. Technol. 2016, 18, 255–264. [Google Scholar]
- Ghaderi, N.; Normohammadi, S.; Javadi, T. Morpho-physiological responses of strawberry (Fragaria × ananassa) to exogenous salicylic acid application under drought stress. J. Agric. Sci. Technol. 2015, 17, 167–178. [Google Scholar]
- Sowik, I.; Borkowska, B.; Markiewicz, M. The activity of mycorrhizal symbiosis in suppressing Verticillium wilt in susceptible and tolerant strawberry (Fragaria × ananassa Duch.) genotypes. Appl. Soil. Ecol. 2016, 101, 152–164. [Google Scholar] [CrossRef]
- Zhang, B.; Song, L.; Bekele, L.D.; Shi, J.; Jia, Q.; Zhang, B.; Chen, J. Optimizing factors affecting development and propagation of Bletilla striata in a temporary immersion bioreactor system. Sci. Hortic. 2018, 232, 121–126. [Google Scholar] [CrossRef]
- Kim, N.Y.; Hwang, H.D.; Kim, J.H.; Kwon, B.M.; Kim, D.; Park, S.Y. Efficient production of virus-free apple plantlets using the temporary immersion bioreactor system. Hortic. Environ. Biotechnol. 2020, 61, 779–785. [Google Scholar] [CrossRef]
- Alvard, D.; Cote, F.; Teisson, C. Comparison of methods of liquid medium culture for banana micropropagation: Effects of temporary immersion of explants. Plant Cell Tissue Organ Cult. 1993, 32, 55–60. [Google Scholar] [CrossRef]
- Businge, E.; Trifonova, A.; Schneider, C.; Rödel, P.; Egertsdotter, U. Evaluation of a new temporary immersion bioreactor system for micropropagation of cultivars of eucalyptus, birch and fir. Forests 2017, 8, 196. [Google Scholar] [CrossRef]
- Escalona, M.; Lorenzo, J.C.; González, B.; Daquinta, M.; González, J.L.; Desjardins, Y.; Borroto, C.G. Pineapple (Ananas comosus L. Merr) micropropagation in temporary immersion systems. Plant Cell Rep. 1999, 18, 743–748. [Google Scholar] [CrossRef]
- Jang, H.R.; Lee, H.J.; Shohael, A.M.; Park, B.J.; Paek, K.Y.; Park, S.Y. Production of biomass and bioactive compounds from soot cultures of Rosa rugosa using a bioreactor culture system. Hortic. Environ. Biotechnol. 2016, 57, 79–87. [Google Scholar] [CrossRef]
- Gianguzzi, V.; Inglese, P.; Barone, E.; Sottile, F. In vitro regeneration of Capparis spinosa L. by using a temporary immersion system. Plants 2019, 8, 177. [Google Scholar] [CrossRef] [Green Version]
- Posada-Pérez, L.; Montesinos, Y.P.; Guerra, D.G.; Daniels, D.; Gómez-Kosky, R. Complete germination of papaya (Carica papaya L. cv. MaradolRoja) somatic embryos using temporary immersion system type RITA® and phloroglucinol in semi-solid culture medium. In Vitro Cell Dev. Biol. Plant 2017, 53, 505–513. [Google Scholar] [CrossRef]
- Vervit SETIS™. Bioreactor Temporary Immersion Systems in Plant Micropropagation. 2021. Available online: http://www.setis-systems.be (accessed on 28 March 2021).
- Bello-Bello, J.J.; Cruz-Cruz, C.A.; Pérez-Guerra, J.C. A new temporary immersion system for commercial micropropagation of banana (Musa AAA cv. Grand Naine). In Vitro Cell Dev. Biol. Plant 2019, 55, 313–320. [Google Scholar] [CrossRef]
- Aragón, C.E.; Sánchez, C.; Gonzalez-Olmedo, J.; Escalona, M.; Carvalho, L.; Amâncio, S. Comparison of plantain plantlets propagated in temporary immersion bioreactors y gelled medium during in vitro growth y acclimatization. Biol. Plant 2014, 58, 29–38. [Google Scholar] [CrossRef]
- Tisserat, B.; Vandercook, C.E. Development of an automated plant culture system. Plant Cell Tissue Organ Cult. 1985, 5, 107–117. [Google Scholar] [CrossRef]
- Ducos, J.P.; Labbe, G.; Lambot, C.; Pétiard, V. Pilot scale process for the production of pre-germinated somatic embryos of selected robusta (Coffea canephora) clones. In Vitro Cell Dev. Biol. Plant 2007, 43, 652–659. [Google Scholar] [CrossRef]
- Etienne, H.; Bertrand, B.; Georget, F.; Lartaud, M.; Montes, F.; Dechamp, E.; Verdeil, J.L.; Barry-Etienne, D. Development of coffee somatic and zygotic embryos to plants differs in the morphological, histochemical and hydration aspects. Tree Physiol. 2013, 33, 640–653. [Google Scholar] [CrossRef] [Green Version]
- Godoy, S.; Tapia, E.; Seit, P.; Andrade, D.; Sánchez, E.; Andrade, P.; Prieto, H. Temporary immersion systems for the mass propagation of sweet cherry cultivars and cherry rootstocks: Development of a micropropagation procedure and effect of culture conditions on plant quality. In Vitro Cell Dev. Biol. Plant 2017, 53, 494–504. [Google Scholar] [CrossRef]
- Arano-Avalos, S.; Gómez-Merino, F.C.; Mancilla-Álvarez, E.; Sánchez-Páez, R.; Bello-Bello, J.J. An efficient protocol for commercial micropropagation of malanga (Colocasia esculenta L. Schott) using temporary immersion. Sci. Hortic. 2020, 261, 108998. [Google Scholar] [CrossRef]
- Song, J.Y.; Mattson, N.S.; Jeong, B.R. Efficiency of shoot regeneration from leaf, stem, petiole and petal explants of six cultivars of Chrysanthemum morifolium. Plant Cell Tissue Organ Cult. 2011, 107, 295. [Google Scholar] [CrossRef]
- Akbar Mozafari, A.; Havas, F.; Ghaderi, N. Application of iron nanoparticles and salicylic acid in in vitro culture of strawberries (Fragaria × ananassa Duch.) to cope with drought stress. Plant Cell Tissue Organ Cult. 2018, 132, 511–523. [Google Scholar] [CrossRef]
- Lee, C.Y.; Kim, Y.K.; Kim, Y.S.; Suh, S.Y.; Lee, S.Y.; Park, S.U. Somatic embryogenesis and plant regeneration in Cnidium officinale Makino. J. Med. Plants Res. 2009, 3, 96–100. [Google Scholar]
- Kim, D.H.; Park, J.M.; Kang, S.M.; Lee, S.M.; Seo, C.W.; Lee, I.Y.; Lee, I.J. Distribution characteristics of weeds and vegetation types in Cnidium officinale field. Weed Turf. Sci. 2015, 4, 279–287. [Google Scholar] [CrossRef] [Green Version]
- Hanhineva, K.; Kokko, H.; Kärenlampi, S. Shoot regeneration from leaf explants of five strawberry (Fragaria × ananassa) cultivars in temporary immersion bioreactor system. In Vitro Cell Dev. Biol. Plant 2005, 41, 826–831. [Google Scholar] [CrossRef]
- Paek, K.Y.; Chakrabarty, D.; Hahn, E.J. Application of bioreactor systems for large scale production of horticultural and medicinal plants. Plant Cell Tissue Organ Cult. 2005, 81, 287–300. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Yeung, E.C. The use of histology in the study of plant tissue culture systems-some practical comments. In Vitro Cell Dev. Biol. Plant 1999, 35, 137–143. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Martínez-Estrada, E.; Islas-Luna, B.; Pérez-Sato, J.A.; Bello-Bello, J.J. Temporary immersion improves in vitro multiplication and acclimatization of Anthurium andreanum Lind. Sci. Hortic. 2019, 249, 185–191. [Google Scholar] [CrossRef]
- Etienne, H.; Berthouly, M. Temporary immersion systems in plant micropropagation. Plant Cell Tissue Organ Cult. 2002, 69, 215–231. [Google Scholar] [CrossRef]
- Thi, L.T.; Park, Y.G.; Jeong, B.R. Growth and development of carnation ‘Dream Yul’ plantlets in a temporary immersion system and comparisons with conventional solid culture methods. In Vitro Cell Dev. Biol. Plant 2019, 55, 539–548. [Google Scholar] [CrossRef]
- Debergh, P.; Aitken-Christie, J.; Cohen, D.; Grout, B.; Von Arnold, S.; Zimmerman, R.; Ziv, M. Reconsideration of the term ‘vitrification’ as used in micropropagation. Plant Cell Tissue Organ Cult. 1992, 30, 135–140. [Google Scholar] [CrossRef]
- Picoli, E.A.; Otoni, W.C.; Figueira, M.L.; Carolino, S.M.; Almeida, R.S.; Silva, E.A.; Fontes, E.P. Hyperhydricity in in vitro eggplant regenerated plants: Structural characteristics and involvement of BiP (binding protein). Plant Sci. 2001, 160, 857–868. [Google Scholar] [CrossRef]
- Matuszkiewicz, M.; Koter, M.D.; Filipecki, M. Limited ventilation causes stress and changes in Arabidopsis morphological, physiological and molecular phenotype during in vitro growth. Plant Physiol. Biochem. 2019, 135, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Zobayed, S.M.A. Ventilation in micropropagation. In Photoautotrophic (Sugar-Free Medium) Micropropagation as a New Micropropagation and Transplant Production System; Springer: Dordrecht, The Netherlands, 2005; pp. 147–186. [Google Scholar]
- Martínez, M.T.; Corredoira, E.; Vieitez, A.M.; Cernadas, M.J.; Montenegro, R.; Ballester, A.; San José, M.C. Micropropagation of mature Quercus ilex L. trees by axillary budding. Plant Cell Tissue Organ. Cult. 2017, 131, 499–512. [Google Scholar] [CrossRef]
- Gonçalves, S.; Martins, N.; Romano, A. Physiological traits and oxidative stress markers during acclimatization of micropropagated plants from two endangered Plantago species: P. algarbiensis Samp. and P. almogravensis Franco. In Vitro Cell Dev. Biol. Plant 2017, 53, 249–255. [Google Scholar] [CrossRef]
- Yang, S.H.; Yeh, D.M. In vitro leaf anatomy, ex vitro photosynthetic behaviors and growth of Calathea orbifolia (Linden) Kennedy plants obtained from semi-solid medium and temporary immersion systems. Plant Cell Tissue Organ Cult. 2008, 93, 201–207. [Google Scholar] [CrossRef]

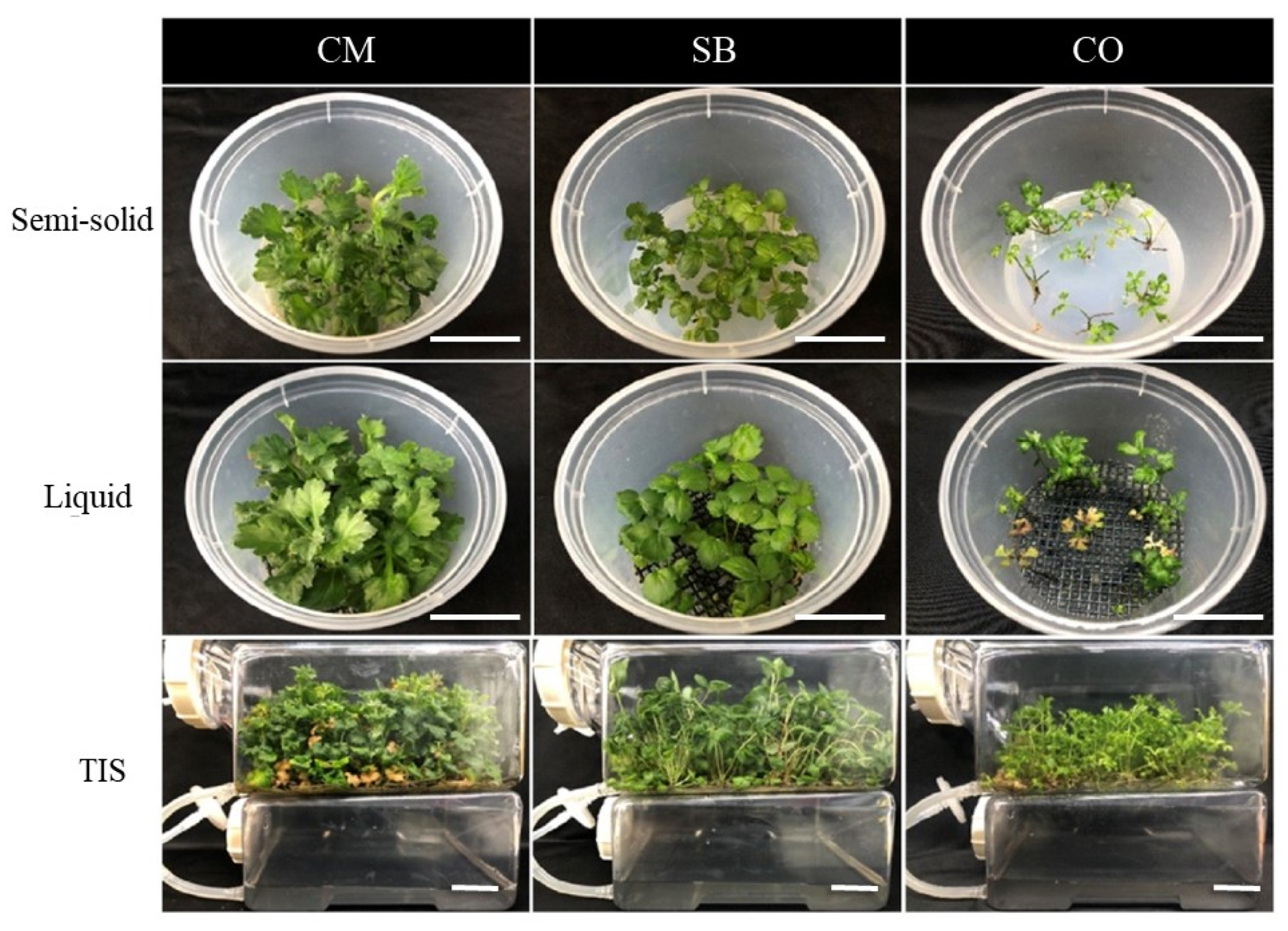

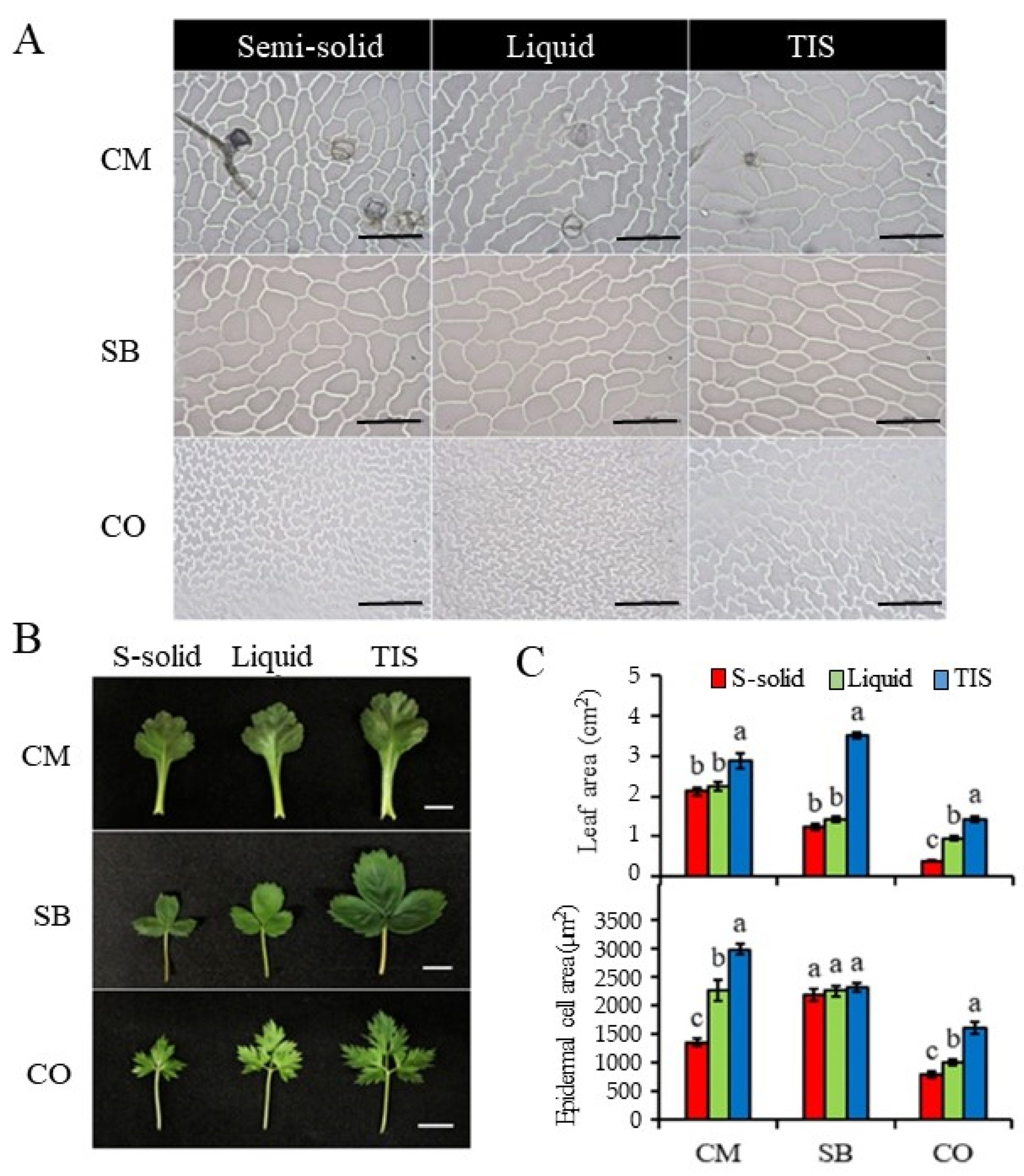
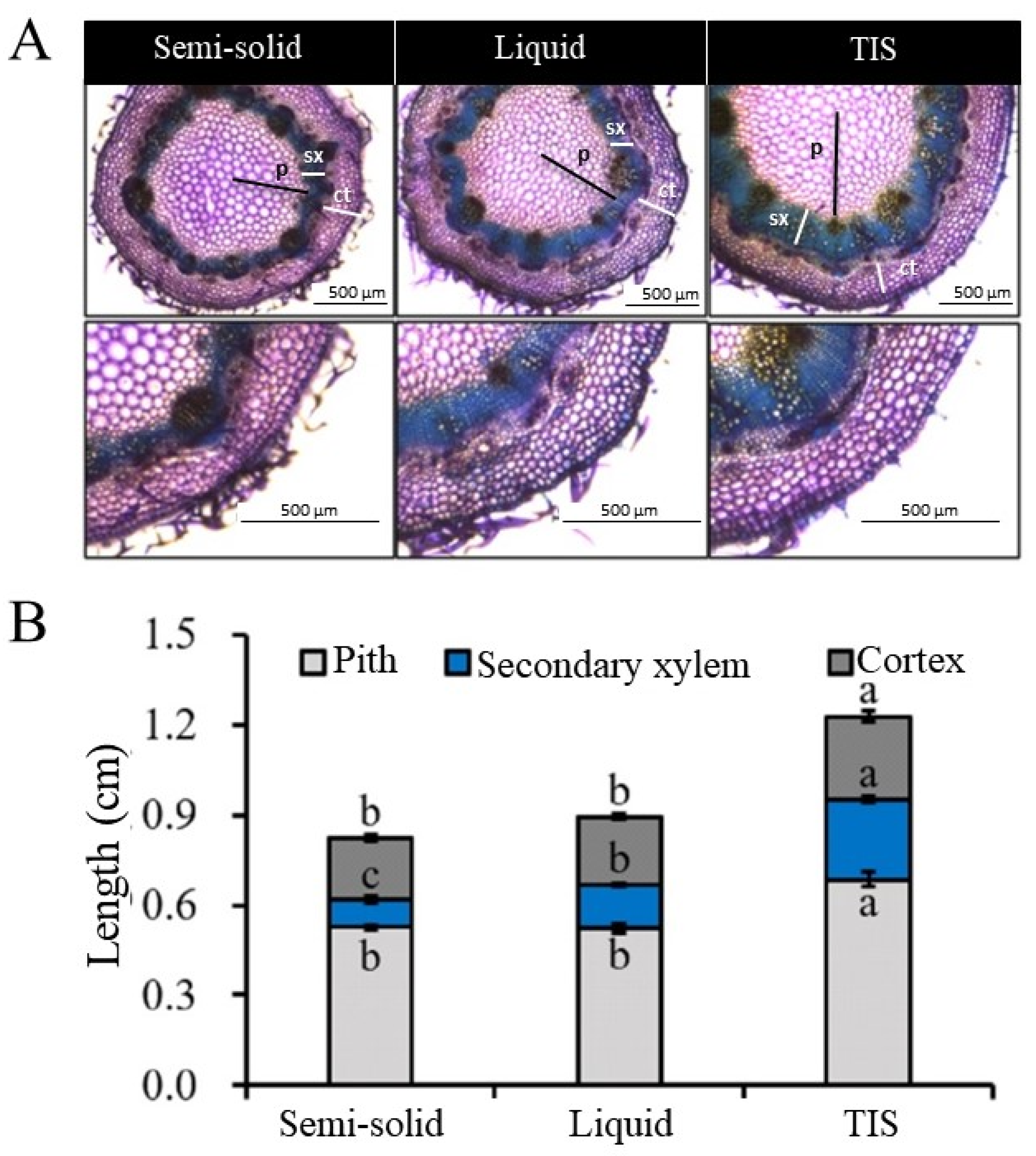
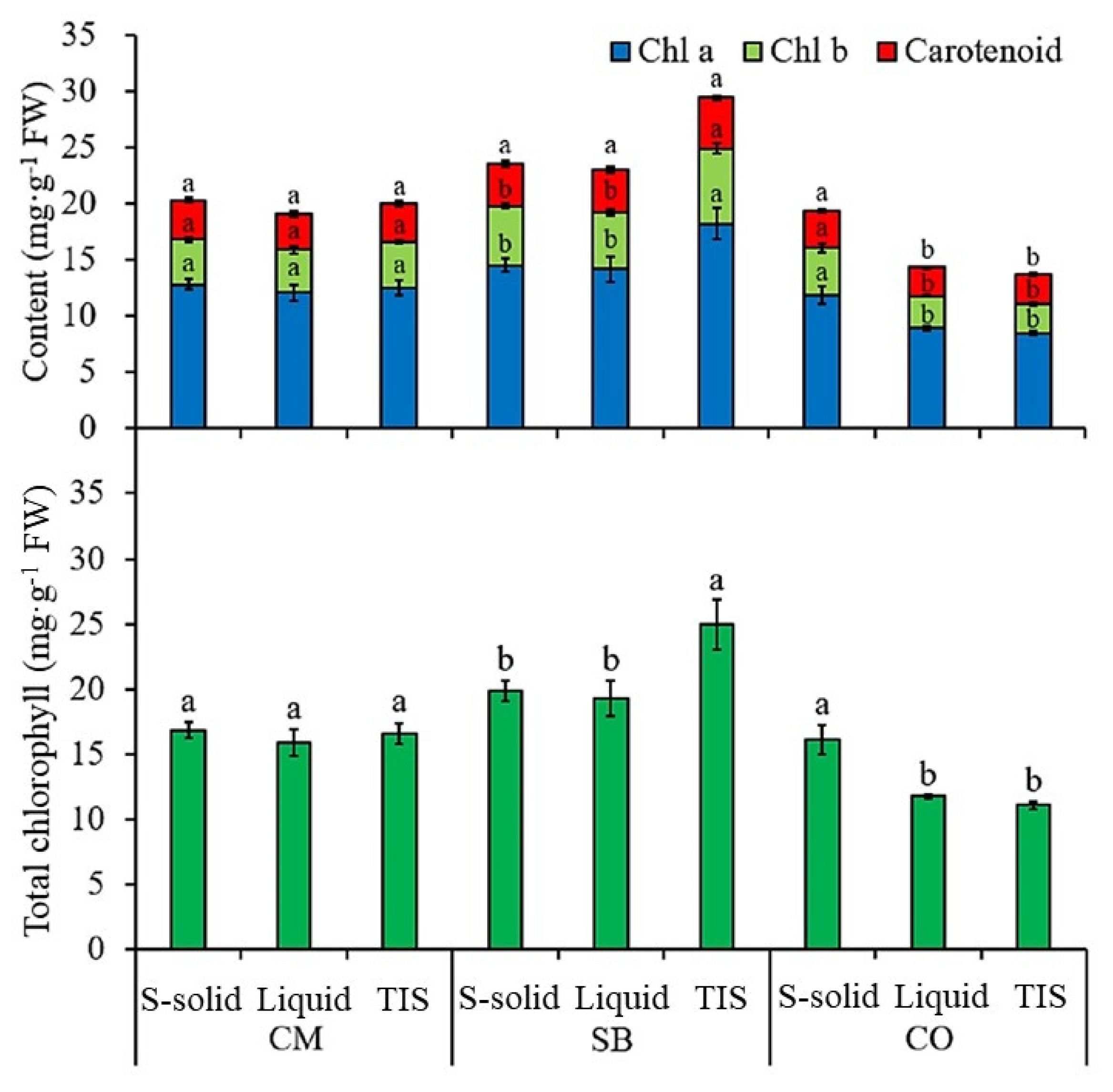

| Species | Culture | Fresh Weight (g) | Dry Weight (g) | Shoot Length (cm) | No. of Leaves/Plantlet | Root Length (cm) | No. of Roots/Plantlet | Survival Rate (%) |
|---|---|---|---|---|---|---|---|---|
| Semi-solid | 6.82 b z | 0.69 b | 5.03 b | 10.80 b | 5.30 a | 19.70 a | 100 a | |
| CM | Liquid | 6.11 c | 0.62 c | 4.99 b | 10.00 b | 4.53 b | 14.30 b | 100 a |
| TIS | 7.84 a | 0.91 a | 5.79 a | 14.20 a | 5.13 a | 14.10 b | 100 a | |
| Semi-solid | 3.01 c | 0.67 c | 4.87 c | 8.80 a | 4.62 a | 8.20 b | 100 a | |
| SB | Liquid | 4.32 b | 0.79 b | 6.83 b | 7.50 b | 3.77 a | 8.60 b | 100 a |
| TIS | 7.11 a | 0.14 a | 8.65 a | 8.30 a | 4.36 a | 10.50 a | 100 a | |
| Semi-solid | 1.75 b | 0.27 b | 5.32 b | 5.20 b | 2.33 a | 6.80 a | 89.0 b | |
| CO | Liquid | 1.92 b | 0.30 b | 6.32 b | 5.60 b | 2.52 a | 6.20 a | 86.0 b |
| TIS | 4.42 a | 0.74 a | 8.14 a | 7.70 a | 2.56 a | 8.60 a | 97.0 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, H.-D.; Kwon, S.-H.; Murthy, H.N.; Yun, S.-W.; Pyo, S.-S.; Park, S.-Y. Temporary Immersion Bioreactor System as an Efficient Method for Mass Production of In Vitro Plants in Horticulture and Medicinal Plants. Agronomy 2022, 12, 346. https://doi.org/10.3390/agronomy12020346
Hwang H-D, Kwon S-H, Murthy HN, Yun S-W, Pyo S-S, Park S-Y. Temporary Immersion Bioreactor System as an Efficient Method for Mass Production of In Vitro Plants in Horticulture and Medicinal Plants. Agronomy. 2022; 12(2):346. https://doi.org/10.3390/agronomy12020346
Chicago/Turabian StyleHwang, Ho-Dong, Suk-Hyun Kwon, Hosakatte Niranjana Murthy, Seung-Won Yun, Sung-Soo Pyo, and So-Young Park. 2022. "Temporary Immersion Bioreactor System as an Efficient Method for Mass Production of In Vitro Plants in Horticulture and Medicinal Plants" Agronomy 12, no. 2: 346. https://doi.org/10.3390/agronomy12020346
APA StyleHwang, H.-D., Kwon, S.-H., Murthy, H. N., Yun, S.-W., Pyo, S.-S., & Park, S.-Y. (2022). Temporary Immersion Bioreactor System as an Efficient Method for Mass Production of In Vitro Plants in Horticulture and Medicinal Plants. Agronomy, 12(2), 346. https://doi.org/10.3390/agronomy12020346






