Effect of pH on Schizochytrium limacinum Production Grown Using Crude Glycerol and Biogas Digestate Effluent
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganism, Activation and Seed Cultures
2.2. Growth Media Composition and Culture Conditions at Lab and Pilot Scale Experiments
2.2.1. Experiment at Lab Scale in Aerated Shake Flasks
2.2.2. Experiment at Pilot Scale in Open Pond Type Reactors
2.3. Measurements of Algal Biomass and Cell Content
2.4. Statistical Analysis
3. Results
3.1. Biomass Productivity
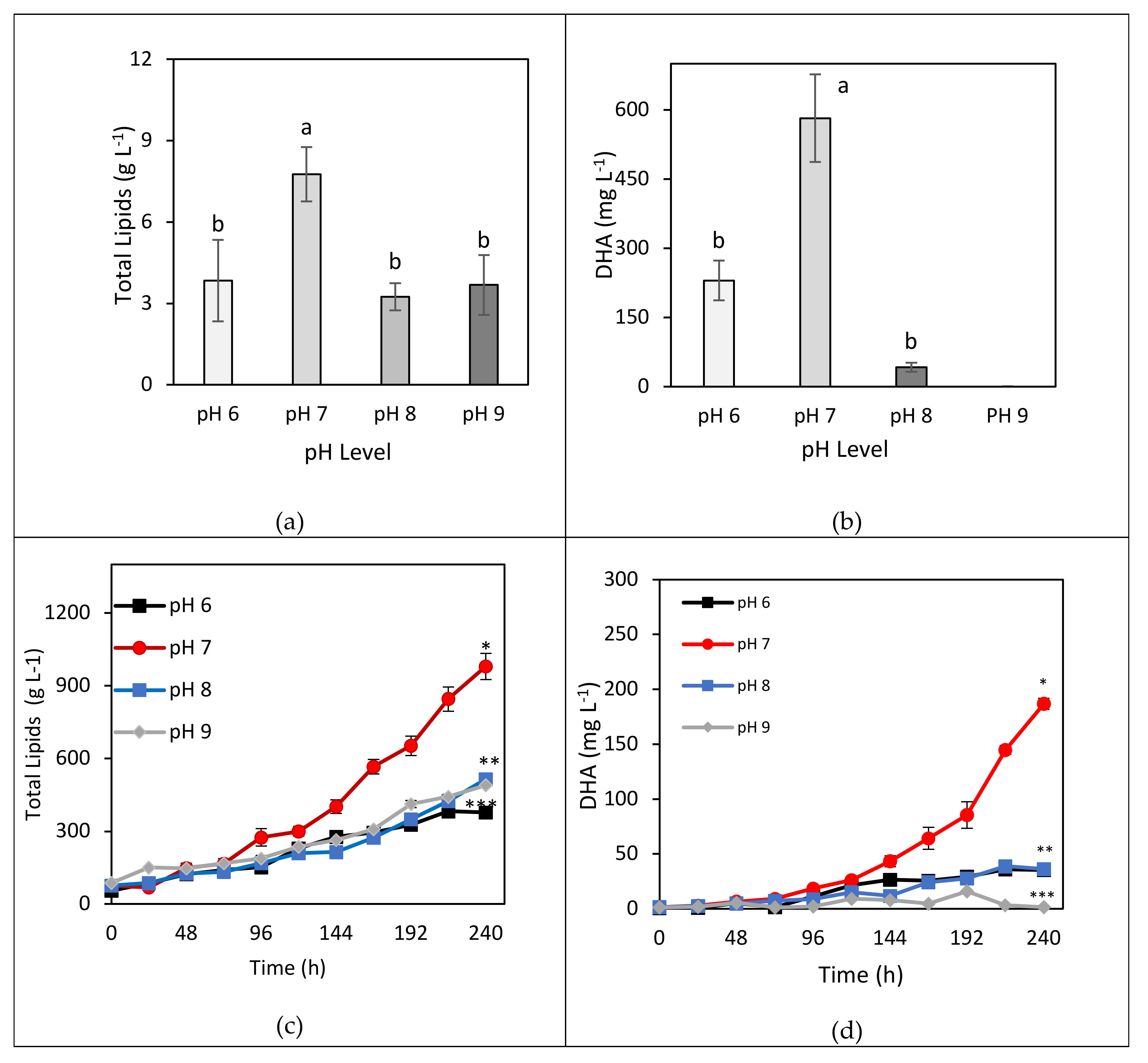
3.2. Total Lipids and DHA Yield
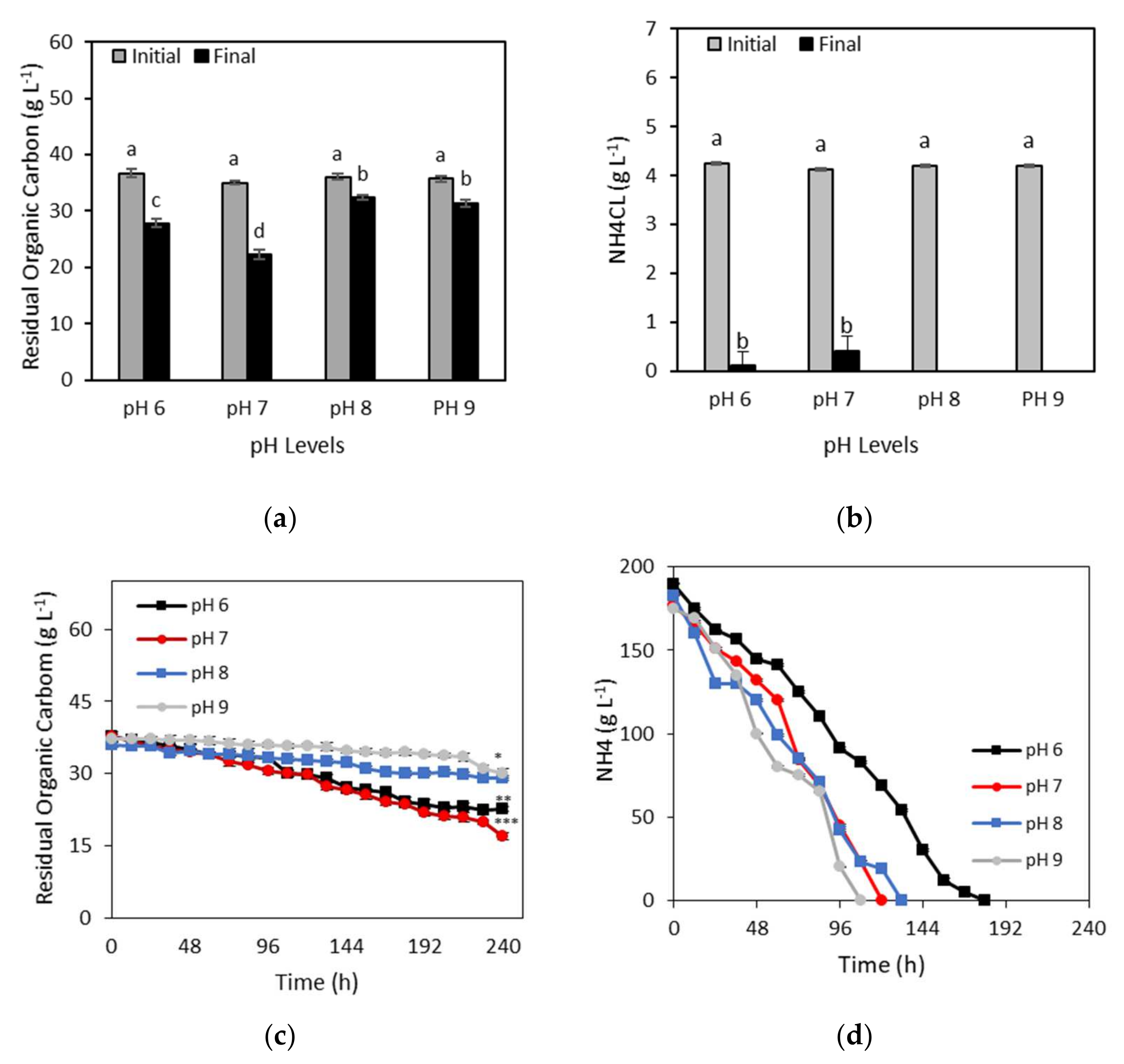
3.3. Carbon and Nitrogen Assimilation
3.4. Proximate Composition
4. Discussion
4.1. Effect of Varying pH Concentration on Biomass, Lipid Productivity, Carbon and Nitrogen Assimilation
4.2. Effect of Varying pH Concentrations on Proximate Composition
4.3. Effect of Varying pH Concentration on DHA Productivity
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kumar, R.P.; Bharathiraja, B.; Kataki, R.; Moholkar, V. Biomass Valorization to Bioenergy; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Guo, X.M.; Trably, E.; Latrille, E.; Carrere, H.; Steyer, J.-P. Hydrogen production from agricultural waste by dark fermentation: A review. Int. J. Hydrog. Energy 2010, 35, 10660–10673. [Google Scholar] [CrossRef]
- Holm-Nielsen, J.B.; Al Seadi, T.; Oleskowicz-Popiel, P. The future of anaerobic digestion and biogas utilization. Bioresour. Technol. 2009, 100, 5478–5484. [Google Scholar] [CrossRef]
- Pexas, G.; Mackenzie, S.; Wallace, M.; Kyriazakis, I. Environmental impacts of housing conditions and manure management in European pig production systems through a life cycle perspective: A case study in Denmark. J. Clean. Prod. 2020, 253, 120005. [Google Scholar] [CrossRef]
- Campbell, J.E.; Lobell, D.B.; Field, C.B. Greater Transportation Energy and GHG Offsets from Bioelectricity Than Ethanol. Science 2009, 324, 1055–1057. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, S.; Wentzel, S.; Eichler-Löbermann, B. Codigested dairy slurry as a phosphorus and nitrogen source for Zea mays L. and Amaranthus cruentus L. J. Plant Nutr. Soil Sci. 2011, 174, 908–915. [Google Scholar] [CrossRef]
- Dahiya, A.; Vasudevan, P. Biogas plant slurry as an alternative to chemical fertilizers. Biomass 1986, 9, 67–74. [Google Scholar] [CrossRef]
- Wenke, L.; Lianfeng, D.; Qichang, Y. Biogas slurry added amino acids decreased nitrate concentrations of lettuce in sand culture. Acta Agric. Scand. Sect. B Soil Plant Sci. 2009, 59, 260–264. [Google Scholar] [CrossRef]
- Zhu, L.; Yan, C.; Li, Z. Microalgal cultivation with biogas slurry for biofuel production. Bioresour. Technol. 2016, 220, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Phang, S.-M.; Chu, W.-L.; Rabiei, R. Phycoremediation. In The Algae World; Springer: Berlin/Heidelberg, Germany, 2015; pp. 357–389. [Google Scholar]
- Kobayashi, N.; Noel, E.A.; Barnes, A.; Watson, A.; Rosenberg, J.N.; Erickson, G.; Oyler, G.A. Characterization of three Chlorella sorokiniana strains in anaerobic digested effluent from cattle manure. Bioresour. Technol. 2013, 150, 377–386. [Google Scholar] [CrossRef]
- Singh, M.; Reynolds, D.L.; Das, K.C. Microalgal system for treatment of effluent from poultry litter anaerobic digestion. Bioresour. Technol. 2011, 102, 10841–10848. [Google Scholar] [CrossRef]
- Ji, F.; Liu, Y.; Hao, R.; Li, G.; Zhou, Y.; Dong, R. Biomass production and nutrients removal by a new microalgae strain Desmodesmus sp. in anaerobic digestion wastewater. Bioresour. Technol. 2014, 161, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.; Guo, J.; Liu, X.; Zhang, X.; Wang, N.; Lu, Y.; Ng, I.-S. Impact of carbon and nitrogen feeding strategy on high production of biomass and docosahexaenoic acid (DHA) by Schizochytrium sp. LU310. Bioresour. Technol. 2015, 184, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Nematian, T.; Salehi, Z.; Shakeri, A. Conversion of bio-oil extracted from Chlorella vulgaris micro algae to biodiesel via modified superparamagnetic nano-biocatalyst. Renew. Energy 2020, 146, 1796–1804. [Google Scholar] [CrossRef]
- Thompson, J.C.; He, B.B. Characterization of crude glycerol from biodiesel production from multiple feedstocks. Appl. Eng. Agric. 2006, 22, 261–265. [Google Scholar] [CrossRef]
- Pyle, D.J.; Garcia, R.A.; Wen, Z. Producing Docosahexaenoic Acid (DHA)-Rich Algae from Biodiesel-Derived Crude Glycerol: Effects of Impurities on DHA Production and Algal Biomass Composition. J. Agric. Food Chem. 2008, 56, 3933–3939. [Google Scholar] [CrossRef]
- Guerfali, M.; Ayadi, I.; Sassi, H.-E.; Belhassen, A.; Gargouri, A.; Belghith, H. Biodiesel-derived crude glycerol as alternative feedstock for single cell oil production by the oleaginous yeast Candida viswanathii Y-E4. Ind. Crop. Prod. 2020, 145, 112103. [Google Scholar] [CrossRef]
- Dhargalkar, V.; Verlecar, X. Southern Ocean seaweeds: A resource for exploration in food and drugs. Aquaculture 2009, 287, 229–242. [Google Scholar] [CrossRef]
- Humphrey, A. Chlorophyll as a Color and Functional Ingredient. J. Food Sci. 2004, 69, C422–C425. [Google Scholar] [CrossRef]
- Kumar, M.; Sharma, M.K.; Kumar, A. Spirulina fusiformis: A Food Supplement against Mercury Induced Hepatic Toxicity. J. Health Sci. 2005, 51, 424–430. [Google Scholar] [CrossRef]
- Stiles, W.A.; Styles, D.; Chapman, S.P.; Esteves, S.; Bywater, A.; Melville, L.; Silkina, A.; Lupatsch, I.; Grünewald, C.F.; Lovitt, R.; et al. Using microalgae in the circular economy to valorise anaerobic digestate: Challenges and opportunities. Bioresour. Technol. 2018, 267, 732–742. [Google Scholar] [CrossRef]
- Leyland, B.; Leu, S.; Boussiba, S. Are thraustochytrids algae? Fungal Biol. 2017, 121, 835–840. [Google Scholar] [CrossRef]
- Yokochi, T.; Honda, D.; Higashihara, T.; Nakahara, T. Optimization of docosahexaenoic acid production by Schizochytrium limacinum SR21. Appl. Microbiol. Biotechnol. 1998, 49, 72–76. [Google Scholar] [CrossRef]
- Santigosa, E.; Brambilla, F.; Milanese, L. Microalgae Oil as an Effective Alternative Source of EPA and DHA for Gilthead Seabream (Sparus aurata) Aquaculture. Animals 2021, 11, 971. [Google Scholar] [CrossRef] [PubMed]
- Tacon, A.G.J.; Metian, M. Global overview on the use of fish meal and fish oil in industrially compounded aquafeeds: Trends and future prospects. Aquaculture 2008, 285, 146–158. [Google Scholar] [CrossRef]
- Zhao, B.; Li, Y.; Mbifile, M.D.; Li, C.; Yang, H.; Wang, W. Improvement of docosahexaenoic acid fermentation from Schizochytrium sp. AB-610 by staged pH control based on cell morphological changes. Eng. Life Sci. 2017, 17, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Hammond, B.G.; Mayhew, D.A.; Naylor, M.W.; Ruecker, F.A.; Mast, R.W.; Sander, W.J. Safety assessment of DHA-rich microalgae from Schizochytrium Sp.: I. Subchronic rat feeding study. Regul. Toxicol. Pharmacol. 2001, 33, 192–204. [Google Scholar] [CrossRef]
- Chen, G. The Study of Biomass Yield and Macromolecular Content of Microalgae Change as a Function of Physiological State and Nutrient Supply Conditions; University of Kansas: Lawrence, KS, USA, 2013. [Google Scholar]
- McAuley, P.J.; Dorling, M.; Hodge, H. Effect of maltose release on uptake and assimilation of ammonium by symbiotic chlorella (chlorophyta) 1. J. Phycol. 1996, 32, 839–846. [Google Scholar] [CrossRef]
- Begum, H.; Yusoff, F.M.; Banerjee, S.; Khatoon, H.; Shariff, M. Availability and Utilization of Pigments from Microalgae. Crit. Rev. Food Sci. Nutr. 2016, 56, 2209–2222. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lin, Y.; Chang, M.; Jin, Q.; Wang, X. Efficient production of arachidonic acid by Mortierella alpina through integrating fed-batch culture with a two-stage pH control strategy. Bioresour. Technol. 2015, 181, 275–282. [Google Scholar] [CrossRef]
- Dorling, M.; McAuley, P.; Hodge, H. Effect of pH on growth and carbon metabolism of maltose-releasing Chlorella (Chlorophyta). Eur. J. Phycol. 1997, 32, 19–24. [Google Scholar] [CrossRef][Green Version]
- Gerloff-Elias, A.; Spijkerman, E.; Proschold, T. Effect of external pH on the growth, photosynthesis and photosynthetic electron transport of Chlamydomonas acidophila Negoro, isolated from an extremely acidic lake (pH 2.6). Plant Cell Environ. 2005, 28, 1218–1229. [Google Scholar] [CrossRef]
- Raven, J.A.; Beardall, J. Carbohydrate Metabolism and Respiration in Algae. In Photosynthesis in Algae; Larkum, A.W.D., Douglas, S.E., Raven, J.A., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2003; pp. 205–224. [Google Scholar]
- Bouras, S.; Katsoulas, N.; Antoniadis, D.; Karapanagiotidis, I.T. Use of Biofuel Industry Wastes as Alternative Nutrient Sources for DHA-Yielding Schizochytrium limacinum Production. Appl. Sci. 2020, 10, 4398. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, J.; Zhao, J.; Gao, Z.; Zhang, C.; Chen, M. Regulation of lipid accumulation in Schizochytrium sp. ATCC 20888 in response to different nitrogen sources. Eur. J. Lipid Sci. Technol. 2017, 119, 1700025. [Google Scholar] [CrossRef]
- Chi, Z.; Pyle, D.; Wen, Z.; Frear, C.; Chen, S. A laboratory study of producing docosahexaenoic acid from biodiesel-waste glycerol by microalgal fermentation. Process Biochem. 2007, 42, 1537–1545. [Google Scholar] [CrossRef]
- Nakahara, T.; Yokochi, T.; Higashihara, T.; Tanaka, S.; Yaguchi, T.; Honda, D. Production of docosahexaenoic and docosapentaenoic acids by Schizochytrium sp. isolated from Yap Islands. J. Am. Oil Chem. Soc. 1996, 73, 1421–1426. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, X.; Ren, X.; Zhu, Q. Effects of culture conditions on growth and docosahexaenoic acid production from Schizochytrium limacinum. J. Ocean Univ. China 2008, 7, 83–88. [Google Scholar] [CrossRef]
- Shafiq, M.; Zeb, L.; Cui, G.; Jawad, M.; Chi, Z. High-Density pH-Auxostat Fed-Batch Culture of Schizochytrium limacinum SR21 with Acetic Acid as a Carbon Source. Appl. Biochem. Biotechnol. 2020, 192, 1163–1175. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Min, M.; Li, Y.; Chen, P.; Chen, Y.; Liu, Y.; Wang, Y.; Ruan, R. Cultivation of Green Algae Chlorella sp. in Different Wastewaters from Municipal Wastewater Treatment Plant. Appl. Biochem. Biotechnol. 2010, 162, 1174–1186. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-Q.; Zhang, Y.; Chen, X. Ammonia removal mechanism by the combination of air stripping and ultrasound as the function of pH. IOP Conf. Series Earth Environ. Sci. 2019, 344, 012051. [Google Scholar] [CrossRef]
- Ratledge, C.; Wynn, J.P. The Biochemistry and Molecular Biology of Lipid Accumulation in Oleaginous Microorganisms. Adv. Appl. Microbiol. 2002, 51, 1–52. [Google Scholar] [CrossRef]
- Gancedo, C.; Gancedo, J.M.; Sols, A. Glycerol Metabolism in Yeasts. Pathways of Utilization and Production. JBIC J. Biol. Inorg. Chem. 1968, 5, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Ludevese-Pascual, G.; Dela Peña, M.; Tornalejo, J. Biomass production, proximate composition and fatty acid profile of the local marine thraustochytrid isolate, Schizochytrium sp. LEY 7 using low-cost substrates at optimum culture conditions. Aquac. Res. 2016, 47, 318–328. [Google Scholar] [CrossRef]
- Hardy, R.W.; Halver, J. Fish Nutrition; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Aasen, I.M.; Ertesvåg, H.; Heggeset, T.M.B.; Liu, B.; Brautaset, T.; Vadstein, O.; Ellingsen, T.E. Thraustochytrids as production organisms for docosahexaenoic acid (DHA), squalene, and carotenoids. Appl. Microbiol. Biotechnol. 2016, 100, 4309–4321. [Google Scholar] [CrossRef] [PubMed]
- Jennings, D.H. Some aspects of the physiology and biochemistry of marine fungi. Biol. Rev. 1983, 58, 423–459. [Google Scholar] [CrossRef]
- Driver, T.; Trivedi, D.K.; McIntosh, O.A.; Dean, A.P.; Goodacre, R.; Pittman, J.K. Two glycerol-3-phosphate dehydrogenases from Chlamydomonas have distinct roles in lipid metabolism. Plant Physiol. 2017, 174, 2083–2097. [Google Scholar] [CrossRef]
- Wu, S.-T.; Yu, S.-T.; Lin, L.-P. Effect of culture conditions on docosahexaenoic acid production by Schizochytrium sp. S31. Process Biochem. 2005, 40, 3103–3108. [Google Scholar] [CrossRef]

| pH Level | Crude Lipid (%) | Crude Protein (%) | Crude Carbohydrates (%) | Ash Content (%) | Moisture Content (%) | Gross Energy (MJ kg−1) |
|---|---|---|---|---|---|---|
| 6 | 16.8 ± 0.6 a | 44.0 ± 3.70 a | 30.83 ± 3.1 a | 8.0 ± 0.3 a | 0.37 ± 0.03 a | 15.0 ± 0.1 a |
| 7 | 17.0 ± 3.5 a | 25.0 ± 0.04 b | 27.0 ± 2.8 b | 30.8 ± 7.1 b | 0.30 ± 0.08 a | 15.8 ± 1.2 b |
| 8 | 28.0 ± 5.3 c | 36.5 ± 0.78 c | 8.57 ± 1.3 c | 26.5 ± 0.2 c | 0.27 ± 0.01 a | 18.8 ± 2.5 c |
| 9 | 31.5 ± 2.0 c | 36.0 ± 0.19 c | 3.37 ± 0.5 d | 29.0 ± 1.0 d | 0.51 ± 0.05 a | 19.4 ± 2.1 c |
| pH Level | Crude Lipid (%) | Crude Protein (%) | Crude Carbohydrates (%) | Ash Content (%) | Moisture Content (%) | Gross Energy (MJ kg−1) |
|---|---|---|---|---|---|---|
| 6 | 9.0 ± 1.9 b | 17.0 ± 0.8 a | 61.9 ± 0.3 c | 9.0 ± 0.5 a | 3.1 ± 0.4 a | 16.6 ± 0.5 c |
| 7 | 10.0 ± 0.8 b | 13.1 ± 0.9 c | 69.3 ± 2.7 a | 5.6 ± 0.7 c | 2.0 ± 0.6 b | 17.7 ± 0.4 b |
| 8 | 13.0 ± 0.1 a | 16.0 ± 0.7 b | 59.6 ± 0.9 c | 8.0 ± 0.8 b | 3.4 ± 0.5 a | 18.1 ± 0.9 a |
| 9 | 14.0 ± 1.4 a | 12.0 ± 0.12 d | 63.3 ± 1.0 b | 7.8 ± 0.3 b | 2.9 ± 0.33 a | 18.9 ± 1.0 a |
| Time (h) | Crude Lipid (%) | Crude Protein (%) | Crude Carbohydrates (%) | Ash Content (%) | Moisture Content (%) | Gross Energy (MJ kg−1) |
|---|---|---|---|---|---|---|
| 0 | 3.1 ± 0.5 f | 26 ± 1.43 a | 60.8 ± 2.96 b | 7 ± 0.27 d | 3.1 ± 0.35 c | 15.4 ± 0.5 b |
| 24 | 4.5 ± 0.48 e | 25.5 ± 1.39 a | 59.3 ± 1.03 b | 7.9 ± 0.97 b | 2.8 ± 0.15 b | 16.4 ± 1.60 a |
| 48 | 5.6 ± 1.06 f | 24 ± 1.74 b | 60.1 ± 2.68 b | 7.8 ± 0.81 c | 2.5 ± 0.67 b | 15.5 ± 0.72 c |
| 72 | 6.7 ± 1.25 f | 24.9 ± 1.3 b | 58.2 ± 1.18 c | 7.4 ± 0.09 c | 2.8 ± 0.1 b | 16.5 ± 1.97 a |
| 96 | 6.5 ± 0.65 f | 23.8 ± 1.42 b | 59.1 ± 1.21 b | 7.7 ± 0.67 c | 2.9 ± 1.03 b | 15.9 ± 0.8 b |
| 120 | 6.8 ± 0.18 e | 21 ± 1.69 c | 61.8 ± 1.14 a | 8.2 ± 0.58 b | 2.2 ± 0.08 c | 16 ± 1.33 a |
| 144 | 7.3 ± 1.14 d | 20 ± 1.89 c | 60.4 ± 1.05 ab | 8.9 ± 0.83 b | 3.4 ± 1.08 b | 16.7 ± 1.24 a |
| 168 | 7.9 ± 0.37 c | 19.1± 1.98 c | 59.9 ± 2.77 b | 9.4 ± 1.25 | 3.7 ± 1.20 a | 15.8 ± 1.11 a |
| 192 | 8.5 ± 0.68 b | 18.8 ± 1.40 c | 59.5 ± 1.37 b | 9.6 ± 0.49 a | 3.6 ± 0.88 a | 16.4 ± 2 a |
| 216 | 8.9 ± 0.9 a | 17.9 ± 1.33 d | 60.4 ± 2.85 ab | 9.7 ± 0.89 a | 3.1 ± 0.37 b | 16.1 ± 0.78 b |
| 240 | 9 ± 1.93 a | 17 ± 1.83 d | 61.9 ± 0.3 a | 9 ± 0.47 a | 3.1 ± 0.44 b | 16.6 ±0.53 a |
| Time (h) | Crude Lipid (%) | Crude Protein (%) | Crude Carbohydrates (%) | Ash Content (%) | Moisture Content (%) | Gross Energy (MJ kg−1) |
|---|---|---|---|---|---|---|
| 0 | 3 ±0.5 d | 30 ± 1.47 a | 59.5 ± 1.66 c | 6 ± 0.78 a | 1.5 ± 0.41 b | 15.8 ± 0.89 c |
| 24 | 3 ± 0.92 d | 25 ± 2.85 b | 63.5 ± 2.83 b | 6.7 ± 0.40 a | 1.8 ± 0.88 a | 16.3 ± 0.82 b |
| 48 | 4 ± 0.94 d | 23.4 ± 1.29 b | 64.1 ± 1.97 b | 6.8 ± 0.34 a | 1.7 ± 0.28 a | 16.4 ± 0.62 b |
| 72 | 4.5 ± 0.73 d | 20.5 ± 0.89 b | 68.6 ± 2.56 c | 5 ± 0.5 b | 1.4 ± 0.65 b | 16.4 ± 0.53 b |
| 96 | 5.5 ± 0.91 d | 17.3 ± 2.73 c | 69.6 ± 1.72 c | 5.8 ± 0.9 b | 1.8 ± 0.41 b | 16.8 ± 0.73 a |
| 120 | 5 ± 0.55 d | 18 ± 1.69 c | 69.4 ± 2.17 c | 5.7 ± 0.6 b | 1.9 ± 0.77 a | 16 ± 0.64 b |
| 144 | 6 ± 0.69 c | 15.3 ± 0.89 d | 71.1 ± 2.62 c | 5.5 ± 0.26 b | 2.1 ± 0.25 a | 16.4 ± 0.46 b |
| 168 | 6.3 ± 0.23 c | 14 ± 1.26 d | 71.3 ± 2.32 c | 5.4 ± 0.85 c | 2.4 ± 0.38 a | 16.5 ± 1.18 a |
| 192 | 7.5 ± 0.76 b | 14.25 ± 0.79 d | 70.95 ± 1.52 c | 5.3 ± 0.41 c | 2 ± 0.51 b | 16.8 ± 0.71 a |
| 216 | 9.5 ± 0.54 a | 13.8 ± 1.73 d | 68 ± 1.86 c | 5.7 ± 0.43 b | 3 ± 0.91 a | 17.3 ± 0.83 a |
| 240 | 10 ± 0.8 a | 13.11 ± 0.92 d | 69.3 ± 2.72 c | 5.6 ± 0.75 b | 2 ±0.59 b | 17.7 ± 0.42 a |
| Time (h) | Crude Lipid (%) | Crude Protein (%) | Crude Carbohydrates (%) | Ash Content (%) | Moisture Content (%) | Gross Energy (MJ kg−1) |
|---|---|---|---|---|---|---|
| 0 | 4 ± 0.08 g | 29 ± 2.48 a | 58.2 ± 2.31 c | 5.9 ± 1.42 c | 2.9 ± 0.74 b | 16.2 ± 0.87 d |
| 24 | 4.8 ± 0.41 f | 25 ± 2.51 b | 61.1 ± 2.26 b | 6.3 ± 1.54 c | 2.8 ± 0.19 b | 16.3 ± 0.64 d |
| 48 | 6 ± 0.80 e | 23 ± 2.84 b | 61.5 ± 1.98 b | 6.8 ±1.08 b | 2.7 ± 0.97 b | 16.7 ± 0.36 c |
| 72 | 6.7 ± 0.74 e | 26 ± 2.54 b | 58.5 ± 1.25 c | 6.4 ± 0.81 b | 2.4 ± 0.34 c | 16.7 ± 0.26 c |
| 96 | 8 ± 0.12 d | 24 ± 2.61 b | 58.2 ± 2.1 c | 7.2 ± 0.34 b | 2.6 ± 0.12 c | 16.8 ± 0.26 c |
| 120 | 8.4 ± 0.83 c | 24 ± 1.42 b | 57.5 ± 1.17 c | 7.3 ± 1.01 b | 2.8 ± 0.28 b | 16.9 ± 0.16 c |
| 144 | 8 ± 0.18 c | 23 ± 1.59 b | 59.8 ± 1.13 c | 7.4 ± 0.29 b | 1.8 ± 0.59 d | 17.2 ± 0.45 b |
| 168 | 9.1 ± 1.07 c | 20 ± 1.86 c | 60.4 ±2.37 c | 7.8 ± 0.97 a | 2.7 ± 0.94 b | 17.2 ± 0.65 b |
| 192 | 10 ± 0.29 c | 17 ± 0.79 d | 62.7 ± 2.4 b | 7.7 ± 0.12 a | 2.6 ± 0.91 b | 17.6 ± 0.59 b |
| 216 | 11.5 ± 0.42 b | 14 ± 1.48 e | 63 ± 0.99 a | 7.8 ± 0.9 a | 3.7 ± 0.18 a | 17.3 ± 0.92 b |
| 240 | 13 ±0.12 a | 16 ± 0.66 d | 59.6 ± 0.88 c | 8 ± 0.78 a | 3.4 ± 0.46 a | 18.1 ± 0.88 a |
| Time (h) | Crude Lipid (%) | Crude Protein (%) | Crude Carbohydrates (%) | Ash Content (%) | Moisture Content (%) | Gross Energy (MJ kg−1) |
|---|---|---|---|---|---|---|
| 0 | 3.8 ± 0.62 f | 31 ± 1.76 a | 55.2 ± 0.96 b | 6.5 ± 1.61 d | 3.5 ± 0.86 a | 15.7 ± 0.48 c |
| 24 | 6 ± 1.60 e | 27 ± 2.5 b | 56.9 ± 0.82 b | 6.8 ± 1.54 c | 3.3 ± 0.62 a | 16 ± 0.18 c |
| 48 | 6.7 ± 0.27 e | 21 ± 2.28 c | 62.5 ± 0.94 a | 6.7 ± 0.41 c | 3.1 ± 0.99 a | 17.4 ± 0.25 b |
| 72 | 8 ± 0.38 d | 19 ± 2.34 c | 63.9 ± 1.54 b | 6.4 ± 0.08 c | 2.7 ± 0.73 b | 17.5 ± 0.17 b |
| 96 | 9.5 ± 1.5 c | 19.5 ± 1.7 c | 62.1 ± 1.3 a | 6.8 ± 0.38 c | 2.1 ± 0.55 c | 17.3 ± 0.21 b |
| 120 | 10.3 ± 1.3 c | 17 ± 1.95 d | 63.5 ± 1.1 a | 6.7 ± 1.69 c | 2.5 ± 0.27 b | 17.8 ± 0.89 b |
| 144 | 10.6 ± 1.38 c | 16 ± 2.51 d | 64.5 ± 0.9 a | 7.3 ± 1.54 c | 1.6 ± 0.73 d | 18.3 ± 0.26 a |
| 168 | 11 ± 1.36 b | 15.8 ± 2.14 d | 63.8 ± 0.68 | 7.5 ± 0.32 b | 1.9 ± 0.33 d | 18.4 ± 1.07 a |
| 192 | 12.5 ± 1.5 b | 14 ± 1.74 e | 62.2 ± 1.52 a | 7.8 ± 1.69 a | 3.5 ± 0.95 a | 18.7 ± 0.55 a |
| 216 | 13 ± 0.41 a | 13.8 ± 1.82 e | 62.4 ± 0.57 a | 7.7 ± 1.57 a | 3.1 ± 0.45 a | 18.6 ± 0.06 a |
| 240 | 14 ± 0.44 a | 12 ± 0.12 f | 63.3 ± 0.98 a | 7.8 ± 0.31 a | 2.9 ± 0.33 a | 18.9 ± 0.97 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouras, S.; Antoniadis, D.; Kountrias, G.; Karapanagiotidis, I.T.; Katsoulas, N. Effect of pH on Schizochytrium limacinum Production Grown Using Crude Glycerol and Biogas Digestate Effluent. Agronomy 2022, 12, 364. https://doi.org/10.3390/agronomy12020364
Bouras S, Antoniadis D, Kountrias G, Karapanagiotidis IT, Katsoulas N. Effect of pH on Schizochytrium limacinum Production Grown Using Crude Glycerol and Biogas Digestate Effluent. Agronomy. 2022; 12(2):364. https://doi.org/10.3390/agronomy12020364
Chicago/Turabian StyleBouras, Sofoklis, Dimitrios Antoniadis, Georgios Kountrias, Ioannis T. Karapanagiotidis, and Nikolaos Katsoulas. 2022. "Effect of pH on Schizochytrium limacinum Production Grown Using Crude Glycerol and Biogas Digestate Effluent" Agronomy 12, no. 2: 364. https://doi.org/10.3390/agronomy12020364
APA StyleBouras, S., Antoniadis, D., Kountrias, G., Karapanagiotidis, I. T., & Katsoulas, N. (2022). Effect of pH on Schizochytrium limacinum Production Grown Using Crude Glycerol and Biogas Digestate Effluent. Agronomy, 12(2), 364. https://doi.org/10.3390/agronomy12020364







