Influence of Structure of Detrital Food Webs on Fusarium Head Blight of Winter Wheat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Plants, Grain, Straw, and Compost Parameters
- R—root rot development (%);
- a—the number of plants with root rot;
- b—the corresponding score;
- N—number of collected plants;
- K—the highest score on the categorical scale.
2.3. Identification and Counting of Fusarium Species in Soil and in Grain
2.4. Sampling and Identification of Invertebrates
2.5. Data Analysis
3. Results
3.1. FHB Related Study Endpoints
3.2. Dynamics of Plant Parameters and Fusarium Biomass
3.3. Dynamics of Key Groups of Invertebrates
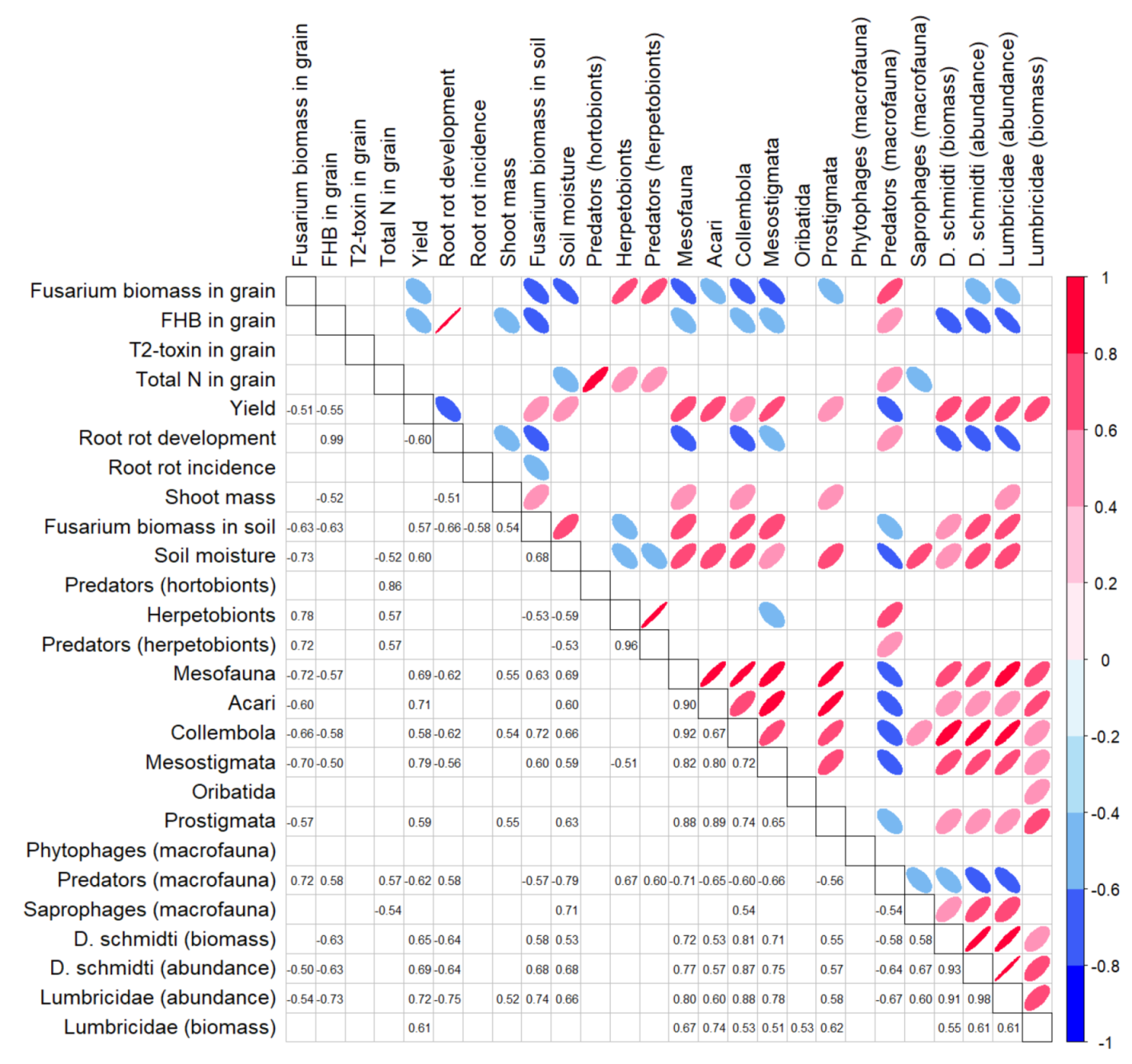
3.4. Relationship of FHB Related Study Endpoints with Other Ecosystem Parameters
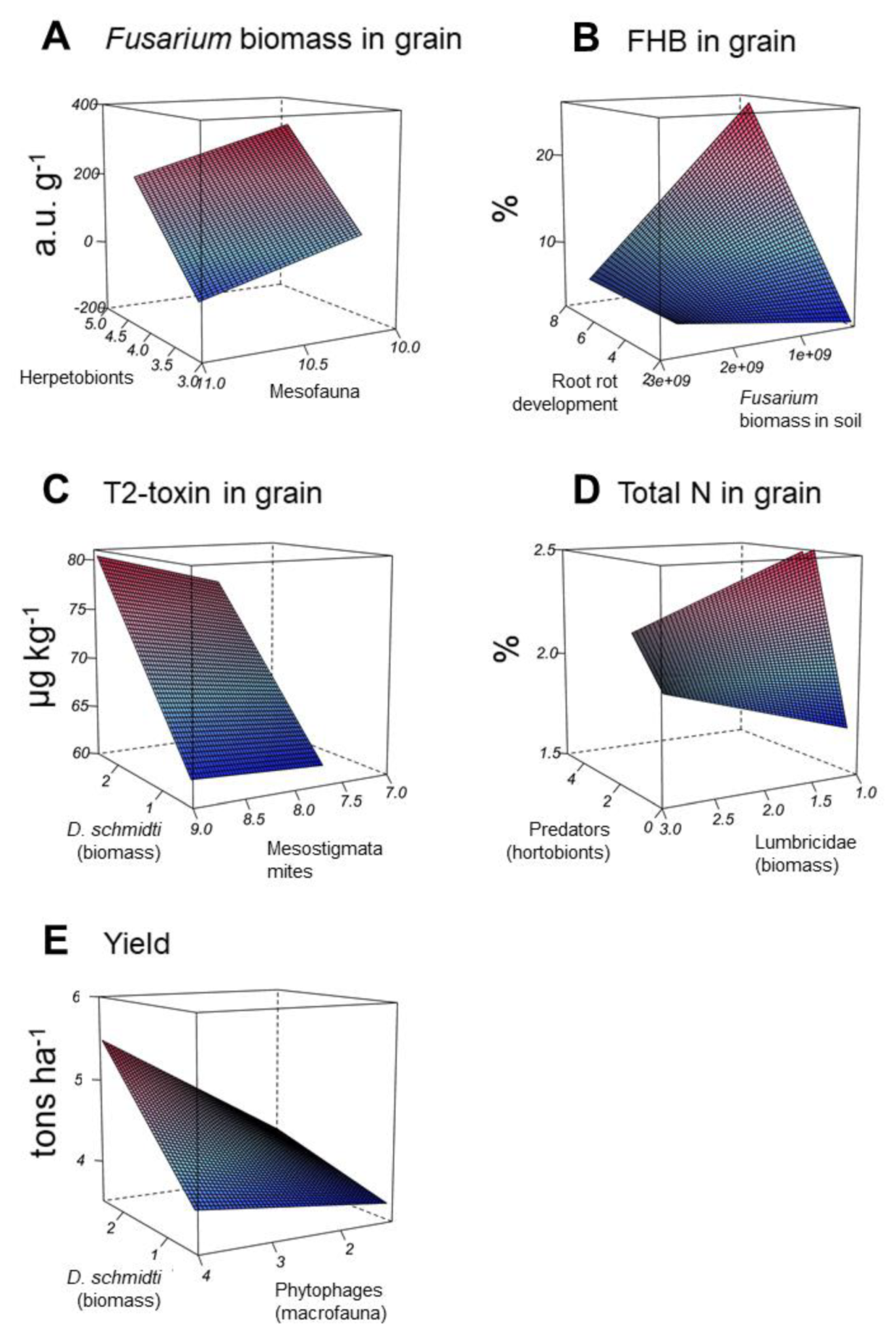
3.5. Linear Random-Effects Models for the Five FHB Related Study Endpoints
4. Discussion
4.1. Effect of Mulching on Soil Food Webs
4.2. FHB Transmission Routes Associated with Soil Food Webs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, S.N. An Overview of Ecological and Habitat Aspects in the Genus Fusarium with Special Emphasis on the Soil-Borne Pathogenic Forms. Plant Pathol. Bull. 2007, 16, 97–120. [Google Scholar]
- Bianchini, A.; Horsley, R.; Jack, M.M.; Kobielush, B.; Ryu, D.; Tittlemier, S.; Wilson, W.W.; Abbas, H.K.; Abel, S.; Harrison, G.; et al. DON Occurrence in Grains: A North American Perspective. Cereal Foods World 2015, 60, 32–56. [Google Scholar] [CrossRef] [Green Version]
- Mielniczuk, E.; Skwarylo-Bednarz, B. Fusarium Head Blight, Mycotoxins and Strategies for Their Reduction. Agronomy 2020, 10, 509. [Google Scholar] [CrossRef] [Green Version]
- Gagkaeva, T.Y.; Gavrilova, O.P.; Levitin, M.M.; Novozhilov, K.V. Head blight of cereal crops. Suppl. J. Zashchita Karantin Rastenij 2011, 5, 112. [Google Scholar]
- Karlsson, I.; Persson, P.; Friberg, H. Fusarium Head Blight from a Microbiome Perspective. Front. Microbiol. 2021, 12, 371. [Google Scholar] [CrossRef]
- Sutton, J.C. Epidemiology of Wheat Head Blight and Maize Ear Rot Caused by Fusarium Graminearum. Can. J. Plant Pathol. 1982, 4, 195–209. [Google Scholar] [CrossRef]
- Paul, P.A.; Bradley, C.A.; Madden, L.V.; Lana, F.D.; Bergstrom, G.C.; Dill-Macky, R.; Esker, P.D.; Wise, K.A.; McMullen, M.; Grybauskas, A. Meta-Analysis of the Effects of QoI and DMI Fungicide Combinations on Fusarium Head Blight and Deoxynivalenol in Wheat. Plant Dis. 2018, 102, 2602–2615. [Google Scholar] [CrossRef] [Green Version]
- Legrand, F.; Picot, A.; Cobo-Díaz, J.F.; Chen, W.; Le Floch, G. Challenges Facing the Biological Control Strategies for the Management of Fusarium Head Blight of Cereals Caused by F. Graminearum. Biol. Control 2017, 113, 26–38. [Google Scholar] [CrossRef]
- Riungu, G.M.; Muthomi, J.W.; Narla, R.D.; Wagacha, J.M.; Gathumbi, J.K. Management of Fusarium Head Blight of Wheat and Deoxynivalenol Accumulation Using Antagonistic Microorganisms. Plant Pathol. J. 2008, 7, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Brandt, S.C.; Brognaro, H.; Ali, A.; Ellinger, B.; Maibach, K.; Rühl, M.; Wrenger, C.; Schlüter, H.; Schäfer, W.; Betzel, C. Insights into the Genome and Secretome of Fusarium Metavorans DSM105788 by Cultivation on Agro-Residual Biomass and Synthetic Nutrient Sources. Biotechnol. Biofuels 2021, 14, 74. [Google Scholar] [CrossRef]
- Suproniene, S.; Kadziene, G.; Irzykowski, W.; Sneideris, D.; Ivanauskas, A.; Sakalauskas, S.; Serbiak, P.; Svegzda, P.; Auskalniene, O.; Jedryczka, M. Weed Species within Cereal Crop Rotations Can Serve as Alternative Hosts for Fusarium Graminearum Causing Fusarium Head Blight of Wheat. Fungal Ecol. 2019, 37, 30–37. [Google Scholar] [CrossRef]
- Khan, M.K.; Pandey, A.; Athar, T.; Choudhary, S.; Deval, R.; Gezgin, S.; Hamurcu, M.; Topal, A.; Atmaca, E.; Santos, P.A.; et al. Fusarium Head Blight in Wheat: Contemporary Status and Molecular Approaches. 3 Biotech 2020, 10, 172. [Google Scholar] [CrossRef]
- Goncharov, A.A.; Glebova, A.A.; Tiunov, A.V. Trophic Interactions between Fusarium Species and Soil Fauna: A Meta-Analysis of Experimental Studies. Appl. Soil Ecol. 2020, 145, 103343. [Google Scholar] [CrossRef]
- Goncharov, A.A.; Gorbatova, A.S.; Sidorova, A.A.; Tiunov, A.V.; Bocharov, G.A. Mathematical Modelling of the Interaction of Winter Wheat (Triticum Aestivum) and Fusarium Species (Fusarium Spp.). Ecol. Model. 2022, 465, 109856. [Google Scholar] [CrossRef]
- Matarese, F.; Sarrocco, S.; Gruber, S.; Seidl-Seiboth, V.; Vannacci, G. Biocontrol of Fusarium Head Blight: Interactions between Trichoderma and Mycotoxigenic Fusarium. Microbiology 2012, 158, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Błaszczyk, L.; Basińska-Barczak, A.; Ćwiek-Kupczyńska, H.; Gromadzka, K.; Popiel, D.; Stępień, Ł. Suppressive Effect of Trichoderma Spp. on Toxigenic Fusarium Species. Pol. J. Microbiol. 2017, 66, 85–100. [Google Scholar] [CrossRef] [Green Version]
- Moody, S.A.; Piearce, T.G.; Dighton, J. Fate of Some Fungal Spores Associated with Wheat Straw Decomposition on Passage through the Guts of Lumbricus Terrestris and Aporrectodea Longa. Soil Biol. Biochem. 1996, 28, 533–537. [Google Scholar] [CrossRef]
- Sabatini, M.A.; Innocenti, G. Soil-Borne Plant Pathogenic Fungi in Relation to Some Collembolan Species under Laboratory Conditions. Mycol. Res. 2000, 104, 1197–1201. [Google Scholar] [CrossRef]
- Bonkowski, M.; Griffiths, B.S.; Ritz, K. Food Preferences of Earthworms for Soil Fungi. Pedobiologia 2000, 44, 666–676. [Google Scholar] [CrossRef]
- Wardle, D.A.; Bardgett, R.D.; Klironomos, J.N.; Setälä, H.; Van Der Putten, W.H.; Wall, D.H. Ecological Linkages between Aboveground and Belowground Biota. Science 2004, 304, 1629–1633. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.C.; McCann, K.; Setälä, H.; De Ruiter, P.C. Top-down Is Bottom-up: Does Predation in the Rhizosphere Regulate Aboveground Dynamics? Ecology 2003, 84, 846–857. [Google Scholar] [CrossRef]
- Riggi, L.G.; Bommarco, R. Subsidy Type and Quality Determine Direction and Strength of Trophic Cascades in Arthropod Food Webs in Agroecosystems. J. Appl. Ecol. 2019, 56, 1982–1991. [Google Scholar]
- Halaj, J.; Wise, D.H. Impact of a Detrital Subsidy on Trophic Cascades in a Terrestrial Grazing Food Web. Ecology 2002, 83, 3141–3151. [Google Scholar] [CrossRef]
- Wilschut, R.A.; Geisen, S. Nematodes as Drivers of Plant Performance in Natural Systems. Trends Plant Sci. 2021, 26, 237–247. [Google Scholar] [CrossRef]
- Wolfarth, F.; Schrader, S.; Oldenburg, E.; Weinert, J. Nematode–Collembolan-Interaction Promotes the Degradation of Fusarium Biomass and Deoxynivalenol According to Soil Texture. Soil Biol Biochem 2013, 57, 903–910. [Google Scholar] [CrossRef]
- Wolfarth, F.; Wedekind, S.; Schrader, S.; Oldenburg, E.; Brunotte, J. Regulation of the Mycotoxin Deoxynivalenol by Folsomia Candida (Collembola) and Aphelenchoides Saprophilus (Nematoda) in an on-Farm Experiment. Pedobiologia 2015, 58, 41–47. [Google Scholar] [CrossRef]
- Wolfarth, F.; Schrader, S.; Oldenburg, E.; Brunotte, J. Mycotoxin Contamination and Its Regulation by the Earthworm Species Lumbricus Terrestris in Presence of Other Soil Fauna in an Agroecosystem. Plant Soil 2016, 402, 331–342. [Google Scholar] [CrossRef]
- Tsurikov, M.N. Long-Term Dynamics of the Species Composition of Herpetobiont and Hortobiont Beetles (Coleoptera) in the Galichya Gora Nature Reserve. Entmol. Rev. 2016, 96, 191–198. [Google Scholar] [CrossRef]
- Afonina, V.M.; Tshernyshev, W.B.; Solovchenko, O.V. Which Biotopes Can Supply the Arable Field with Natural Enemies? IOBC Bull. 2008, 34, 1–4. [Google Scholar]
- Widstrom, N.W. The Role of Insects and Other Plant Pests in Aflatoxin Contamination of Corn, Cotton, and Peanuts—A Review. J. Environ. Qual. 1979, 8, 5–11. [Google Scholar] [CrossRef]
- Mann, R.S.; Ali, J.G.; Hermann, S.L.; Tiwari, S.; Pelz-Stelinski, K.S.; Alborn, H.T.; Stelinski, L.L. Induced Release of a Plant-Defense Volatile ‘Deceptively’ Attracts Insect Vectors to Plants Infected with a Bacterial Pathogen. PLoS Pathog. 2012, 8, e1002610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wardle, D.A. Impacts of Disturbance on Detritus Food Webs in Agro-Ecosystems of Contrasting Tillage and Weed Management Practices. In Advances in Ecological Research; Begon, M., Fitter, A.H., Eds.; Academic Press: Cambridge, MS, USA, 1995; Volume 26, pp. 105–185. [Google Scholar]
- Wardle, D.A.; Nicholson, K.S.; Yeates, K.W. Effect of Weed Management Strategies on Some Soil-Associated Arthropods in Maize and Asparagus Ecosystems. Pedobiologia 1993, 37, 257–269. [Google Scholar]
- Su, L.; Bai, T.; Qin, X.; Yu, H.; Wu, G.; Zhao, Q.; Tan, L. Organic Manure Induced Soil Food Web of Microbes and Nematodes Drive Soil Organic Matter under Jackfruit Planting. Appl. Soil Ecol. 2021, 166, 103994. [Google Scholar] [CrossRef]
- Goncharov, A.A.; Glebova, A.A.; Chernov, T.I.; Karpukhin, M.M.; Kuznetsova, N.A.; Leonov, V.D.; Makarova, O.L.; Maygurova, V.N.; Shesteperov, A.A.; Taranets, I.P. Detrital Subsidy Alters the Soil Invertebrate Community and Reduces Infection of Winter Wheat Seedlings by Fusarium Wilt. Appl. Soil Ecol. 2021, 163, 103914. [Google Scholar] [CrossRef]
- Jiang, M.; Yang, N.; Zhao, J.; Shaaban, M.; Hu, R. Crop Straw Incorporation Mediates the Impacts of Soil Aggregate Size on Greenhouse Gas Emissions. Geoderma 2021, 401, 115342. [Google Scholar] [CrossRef]
- Tian, G.; Kang, B.T.; Brussaard, L. Effect of Mulch Quality on Earthworm Activity and Nutrient Supply in the Humid Tropics. Soil Biol. Biochem. 1997, 29, 369–373. [Google Scholar] [CrossRef]
- Sun, L.; Wu, J. Combined Application of Animal Manure and Straw Benefit Soil Fauna Community in Dryland Farming. SOIL Discuss. 2021, 1–26. [Google Scholar] [CrossRef]
- Li, X.; Zhu, W.; Xu, F.; Du, J.; Tian, X.; Shi, J.; Wei, G. Organic Amendments Affect Soil Organic Carbon Sequestration and Fractions in Fields with Long-Term Contrasting Nitrogen Applications. Agr. Ecosyst. Environ. 2021, 322, 107643. [Google Scholar] [CrossRef]
- Zhang, N.; He, X.; Zhang, J.; Raza, W.; Yang, X.-M.; Ruan, Y.-Z.; Shen, Q.-R.; Huang, Q.-W. Suppression of Fusarium Wilt of Banana with Application of Bio-Organic Fertilizers. Pedosphere 2014, 24, 613–624. [Google Scholar] [CrossRef]
- WRB, IUSS Working Group. WRB World Reference Base for Soil Resources 2014: International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; Food and Agriculture Organization: Rome, Italy, 2014; ISBN 978-92-5-108369-7. [Google Scholar]
- Lawlor, D.W. Photosynthesis, Productivity and Environment. J. Exp. Bot. 1995, 46, 1449–1461. [Google Scholar] [CrossRef]
- Sychev, V.G. (Ed.) Guidelines for Conducting Registration Tests of Agrochemicals in Agriculture; FGBNU “Rosinformagrotech”: Moscow, Russia, 2018; ISBN 978-5-7367-1459-9. (In Russian) [Google Scholar]
- Dolzhenko, V.I. (Ed.) Methodological Guidelines for Registration Tests of Fungicides in Agriculture; VIZR: Saint Petersburg, Russia, 2009; ISBN 978-5-7367-1473-5. (In Russian) [Google Scholar]
- Gagkaeva, T.; Orina, A.; Gavrilova, O. Fusarium Head Blight in the Russian Far East: 140 Years after Description of the “drunken Bread” Problem. Peer J. 2021, 9, e12346. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, A.E.; Shutova, A.S.; Gannesen, A.V.; Lebedin, Y.S.; Eremin, S.A. Determination of the Mycelium and Antigens of a Number of Micromycetes in Soil Extracts via Enzyme-Linked Immunosorbent Assay. Appl. Biochem. Microbiol. 2020, 56, 72–77. [Google Scholar] [CrossRef]
- Gongalsky, K.B.; Wikars, L.-O.; Persson, T. Dynamics of Pyrophilous Carabids in a Burned Pine Forest in Central Sweden. Balt. J. Col. 2003, 3, 107–111. [Google Scholar]
- Zar, J.H. Biostatistical Analysis; Pearson: London, UK; Prentice-Hall: Hoboken, NJ, USA, 2010. [Google Scholar]
- Bonate, P.L. Pharmacokinetic-Pharmacodynamic Modeling and Simulation, 2nd ed.; Springer: New York, NY, USA, 2011; ISBN 978-1-4419-9484-4. [Google Scholar]
- Goncharov, A.A.; Gongalsky, K.B.; Yazrikova, T.E.; Kostina, N.V.; Korobushkin, D.I.; Makarov, M.I.; Zaitsev, A.S. Greenhouse Gas-Producing Soil Biological Activity in Burned and Unburned Forests along a Transect in European Russia. Appl. Soil. Ecol. 2020, 148, 103491. [Google Scholar] [CrossRef]
- Wei, T.; Simko, V. R Package “Corrplot”: Visualization of a Correlation Matrix. 2021. Available online: https://github.com/taiyun/corrplot (accessed on 15 December 2021).
- Bates, D.; Kliegl, R.; Vasishth, S.; Baayen, H. Parsimonious Mixed Models. arXiv 2015, arXiv:1506.04967. Available online: https://arxiv.org/abs/1506.04967 (accessed on 15 December 2021).
- Goncharov, A.A.; Tiunov, A.V. Trophic Chains in the Soil. Biol. Bull. Rev. 2014, 4, 393–403. [Google Scholar] [CrossRef]
- Moore, J.C.; Hunt, H.W. Resource Compartmentation and the Stability of Real Ecosystems. Nature 1988, 333, 261–263. [Google Scholar] [CrossRef]
- Pollierer, M.M.; Langel, R.; Scheu, S.; Maraun, M. Compartmentalization of the Soil Animal Food Web as Indicated by Dual Analysis of Stable Isotope Ratios (15N/14N and 13C/12C). Soil Biol. Biochem. 2009, 41, 1221–1226. [Google Scholar] [CrossRef]
- Matalin, A.V.; Makarov, K.V. Using Demographic Data to Better Interpret Pitfall Trap Catches. ZooKeys 2011, 100, 223. [Google Scholar] [CrossRef]
- Zalewski, M.; Dudek-Godeau, D.; Tiunov, A.; Godeau, J.-F.; Okuzaki, Y.; Ikeda, H.; Sienkiewicz, P.; Ulrich, W. Wing Morphology is Linked to Stable Isotope Composition of Nitrogen and Carbon in Ground Beetles (Coleoptera: Carabidae). Eur. J. Entomol. 2015, 112, 810–817. [Google Scholar] [CrossRef] [Green Version]
- Palmu, E.; Birkhofer, K.; Hanson, H.I.; Hedlund, K. Can ΔD and Δ18O Stable Isotopes Be Used to Detect Long-Range Dispersal among Carabid Beetles? Appl. Soil Ecol. 2017, 119, 99–103. [Google Scholar] [CrossRef]
- Schallhart, N.; Wallinger, C.; Juen, A.; Traugott, M. Dispersal Abilities of Adult Click Beetles in Arable Land Revealed by Analysis of Carbon Stable Isotopes. Agr. For. Entomol. 2009, 11, 333–339. [Google Scholar] [CrossRef]
- Schallhart, N.; Tusch, M.J.; Staudacher, K.; Wallinger, C.; Traugott, M. Stable Isotope Analysis Reveals Whether Soil-Living Elaterid Larvae Move between Agricultural Crops. Soil Biol. Biochem. 2011, 43, 1612–1614. [Google Scholar] [CrossRef] [Green Version]
- Rapoport, I.B. Ecology and Chorology of Earthworms (Oligochaeta, Lumbricidae) of the Kabardino-Balkarian High-Mountain Reserve (Central Caucasus). Bull. Soc. Nat. Moscou 2012, 117, 65–71. [Google Scholar]
- Edwards, C.A.; Bohlen, P.J. Biology and Ecology of Earthworms; Springer Science & Business Media: Berlin, Germany, 1996; Volume 3. [Google Scholar]
- Krantz, G.W. A Manual of Acarology; Oregon State University Book Stores: Corvallis, OR, USA, 1978. [Google Scholar]
- Walter, D.E.; Proctor, H.C. Mites: Ecology, Evolution, and Behaviour; Springer: Dordrecht, The Netherlands, 1999. [Google Scholar]
- Gerson, U.; Smiley, R.L.; Ochoa, R. Mites (Acari) for Pest Control; John Wiley & Sons: Hoboken, NJ, USA, 2008; ISBN 978-1-4051-5097-2. [Google Scholar]
- Kasuga, S.; Honda, K. Suitability of Organic Matter, Fungi and Vegetables as Food for Tyrophagus Similis (Acari: Acaridae). Appl. Entomol. Zool. 2006, 41, 227–231. [Google Scholar] [CrossRef] [Green Version]
- Hedlund, K.; Öhrn, M.S. Tritrophic Interactions in a Soil Community Enhance Decomposition Rates. Oikos 2000, 88, 585–591. [Google Scholar] [CrossRef]
- Usher, M.B. Seasonal and Vertical Distribution of a Population of Soil Arthropods: Collembola. Pedobiologia 1970, 10, 224–236. [Google Scholar]
- Farrow, R.A.; Greenslade, P. A Vertical Migration of Collembola. Entomologist 1992, 111, 38–45. [Google Scholar]
- Kromp, B. Carabid Beetles in Sustainable Agriculture: A Review on Pest Control Efficacy, Cultivation Impacts and Enhancement. Agr. Ecosyst. Environ. 1999, 74, 187–228. [Google Scholar] [CrossRef]
- Sasakawa, K. Field Observations of Climbing Behavior and Seed Predation by Adult Ground Beetles (Coleoptera: Carabidae) in a Lowland Area of the Temperate Zone. Environ. Entomol. 2010, 39, 1554–1560. [Google Scholar] [CrossRef]
- Goss, J.A. Development, Physiology, and Biochemistry of Corn and Wheat Pollen. Bot. Rev. 1968, 34, 333–359. [Google Scholar] [CrossRef]
- Vogel, P.; Liberato da Silva, G.; Esswein, I.Z.; Dallazen, M.C.; Heidrich, D.; Pagani, D.M.; Hoehne, L.; Scroferneker, M.L.; Valente, P.; Ferla, N.J. Effects of Infestations of the Storage Mite Tyrophagus Putrescentiae (Acaridae) on the Presence of Fungal Species and Mycotoxin Production in Stored Products. J. Stored Prod. Res. 2021, 94, 101883. [Google Scholar] [CrossRef]
- Bonkowski, M.; Villenave, C.; Griffiths, B. Rhizosphere Fauna: The Functional and Structural Diversity of Intimate Interactions of Soil Fauna with Plant Roots. Plant Soil 2009, 321, 213–233. [Google Scholar] [CrossRef]
- Biere, A.; Goverse, A. Plant-Mediated Systemic Interactions between Pathogens, Parasitic Nematodes, and Herbivores above-and Belowground. Annu. Rev. Phytopathol. 2016, 54, 499–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayer, Z.; Juhász, Á.; Posta, K. Mycorrhizal Root Exudates Induce Changes in the Growth and Fumonisin Gene (FUM1) Expression of Fusarium Proliferatum. Agronomy 2019, 9, 291. [Google Scholar] [CrossRef] [Green Version]

| N-Poor Mulch | N-Medium Mulch | N-Rich Mulch | ||
|---|---|---|---|---|
| C dosage, g C m−2 | Total | 215.2 | 240.3 | 290.6 |
| From straw | 180.0 | 180.0 | 180.0 | |
| From compost | 35.2 | 60.3 | 110.6 | |
| N dosage, g N m−2 | Total | 5.2 | 7.4 | 11.9 |
| From straw | 2.1 | 2.1 | 2.1 | |
| From compost | 3.1 | 5.3 | 9.8 | |
| C:N ratio in mulch | 41 | 32 | 25 | |
| Measurement | Control | N-Poor Mulch | N-Medium Mulch | N-Rich Mulch | |
|---|---|---|---|---|---|
| FHB related study endpoints | |||||
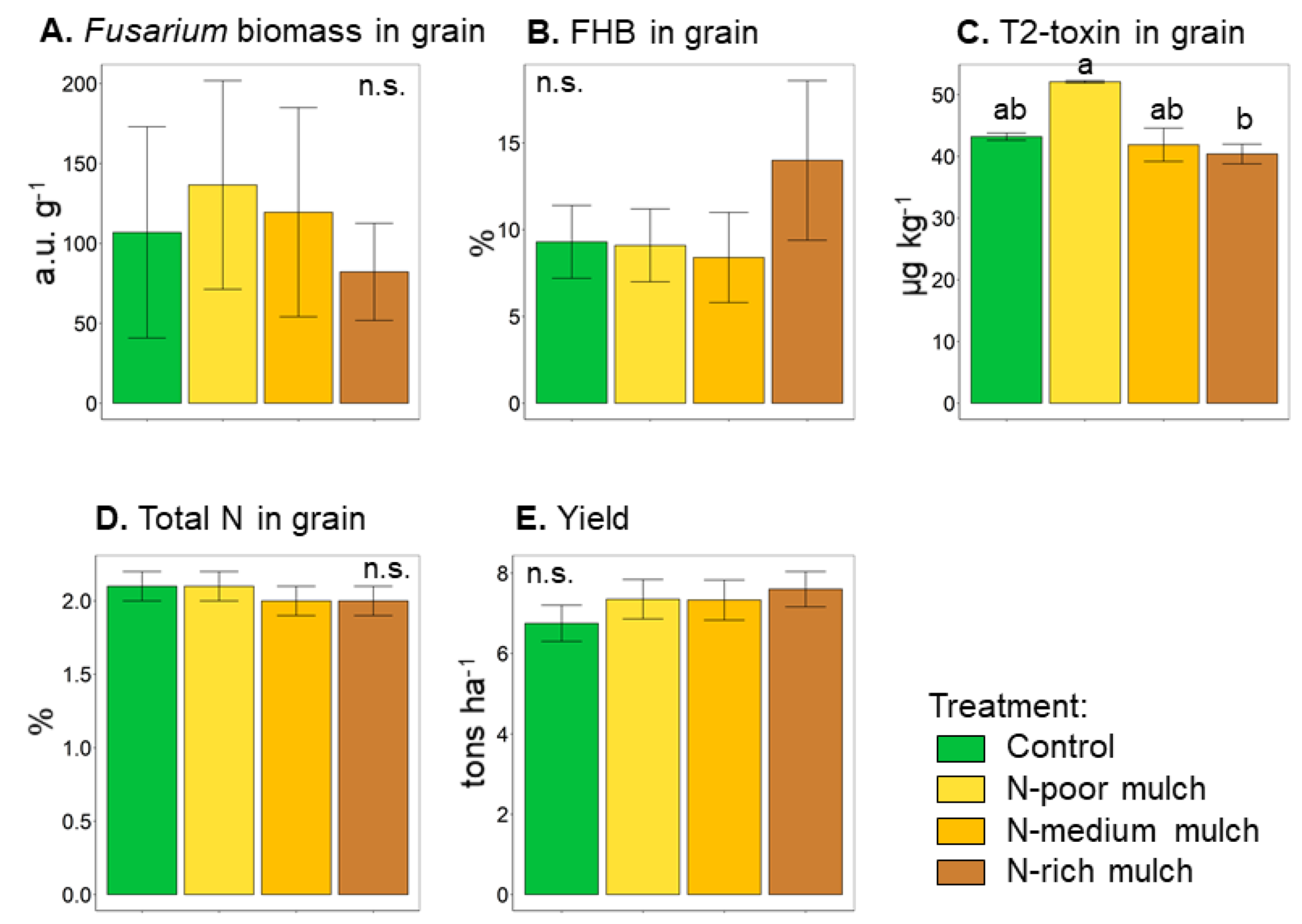
| Fusarium biomass in grain, a.u. g−1 | 106.9 ± 66.1 | 136.6 ± 65.3 | 119.5 ± 65.4 | 82.2 ± 30.4 | n.s. |
| Incidence of FHB in grain, % | 9.3 ± 2.1 | 9.1 ± 2.1 | 8.4 ± 2.6 | 14 ± 4.6 | n.s. |
| T2-toxin in grain, μg kg−1 | 43.2 ± 0.6 ab | 52.1 ± 0.2 a | 41.9 ± 2.7 ab | 40.4 ± 1.6 b | |
| Total N in grain, % | 2.1 ± 0.1 | 2.1 ± 0.1 | 2.0 ± 0.1 | 2.0 ± 0.1 | n.s. |
| Yield, t ha−1 | 6.8 ± 0.5 | 7.4 ± 0.5 | 7.3 ± 0.5 | 7.6 ± 0.4 | n.s. |
| Ecosystem parameters | |||||
| Acari, ind. × 103 m−2 | 24.4 ± 2.3 | 33.2 ± 4.0 | 29.4 ± 4.3 | 35.6 ± 3.1 | n.s. |
| Collembola, ind. × 103 m−2 | 14.1 ± 5.1 a | 21.9 ± 6.4 a | 23.4 ± 8.3 a | 19.7 ± 6.6 a | n.s. |
| D. schmidti (biomass), g m−2 | 3.7 ± 2.1 a | 9.0 ± 1.5 b | 6.8 ± 1.0 ab | 5.6 ± 1.9 ab | |
| D. schmidti (abundance), ind. m−2 | 72.3 ± 43.9 | 97.7 ± 43.9 | 86.5 ± 25.8 | 65.6 ± 27.5 | n.s. |
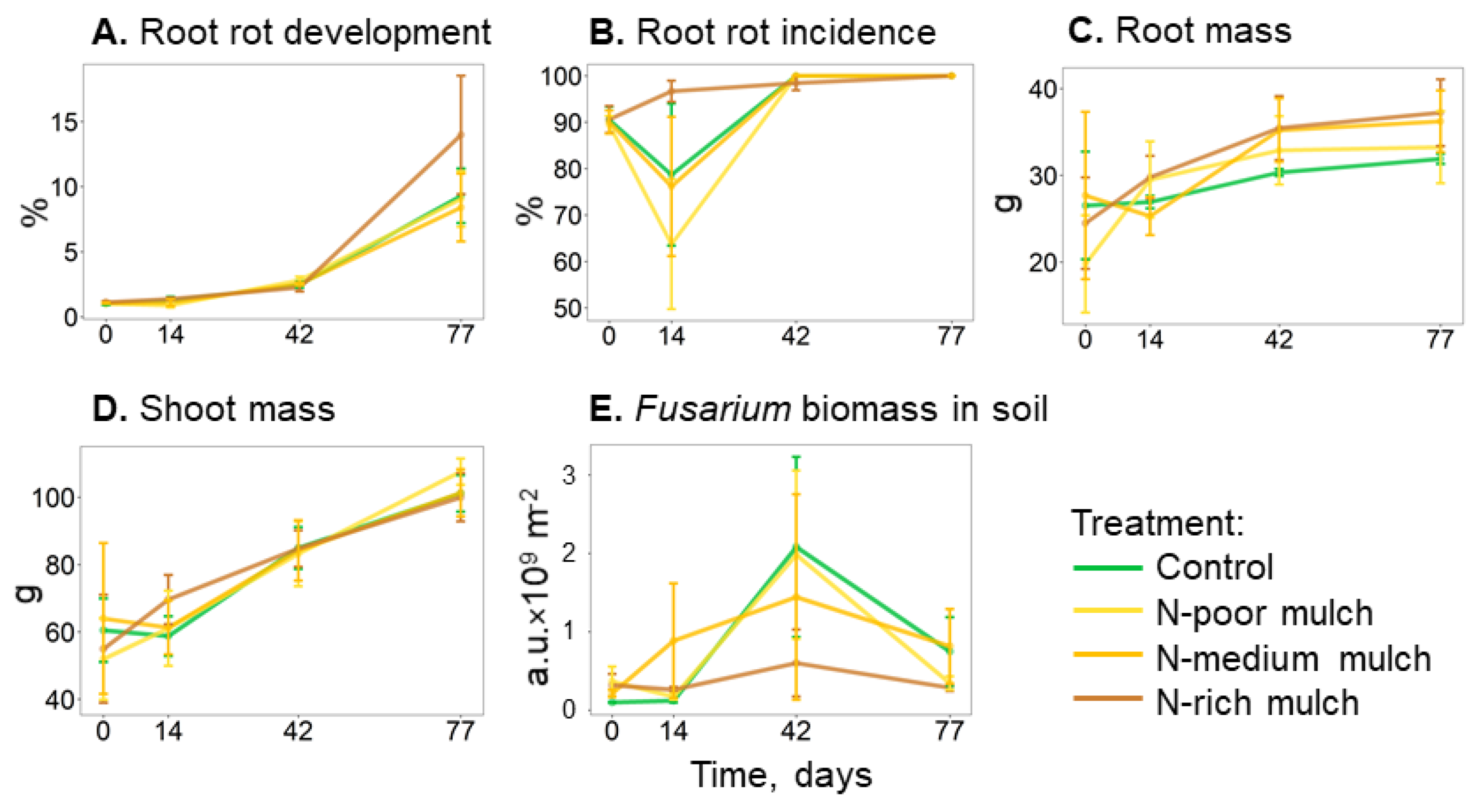
| Fusarium biomass in soil, a.u. × 109 m−2 | 1.1 ± 0.5 | 1.0 ± 0.4 | 1.0 ± 0.6 | 0.4 ± 0.2 | n.s. |
| Herpetobionts, ind. 100 trap days−1 | 44.2 ± 12.5 | 48.0 ± 5.0 | 42.5 ± 4.4 | 44.4 ± 5.9 | n.s. |
| Hortobionts, ind. sampling−1 | 32.5 ± 2.8 a | 28.7 ± 2.7 ab | 18.8 ± 3.0 b | 26.3 ± 3.3 ab | |
| Lumbricidae (abundance), ind. m−2 | 80.5 ± 46.9 | 104.1 ± 43.2 | 92.2 ± 26.2 | 69.9 ± 28.2 | n.s. |
| Lumbricidae (biomass), g m−2 | 5.6 ± 1.7 | 11.1 ± 0.8 | 7.9 ± 1.1 | 13.2 ± 4 | n.s. |
| Macrofauna, ind. × 103 m−2 | 4.5 ± 0.8 | 4.6 ± 0.5 | 6.0 ± 0.6 | 5.0 ± 0.4 | n.s. |
| Mesofauna, ind. × 103 m−2 | 38.5 ± 6.5 a | 55.0 ± 10.3 b | 52.7 ± 12.3 ab | 55.3 ± 8.9 b | |
| Mesostigmata mites, ind. × 103 m−2 | 4.5 ± 1.0 | 6.0 ± 1.3 | 4.4 ± 0.8 | 6.4 ± 1.1 | n.s. |
| Oribatida mites, ind. ×103 m−2 | 8.0 ± 1.7 | 9.3 ± 0.8 | 6.9 ± 0.9 | 10.1 ± 1.8 | n.s. |
| Phytophages (herpetobionts), ind. 100 trap days−1 | 8.7 ± 2.9 | 7 ± 1.2 | 7.2 ± 1.5 | 6.6 ± 1.4 | n.s. |
| Phytophages (hortobionts), ind. sampling−1 | 19.9 ± 2.9 a | 16.4 ± 1.7 ab | 9.0 ± 1.8 b | 16.3 ± 3.2 ab | |
| Phytophages (macrofauna), ind. m−2 | 10.1 ± 10.1 | 35.8 ± 8 | 2.5 ± 1.6 | 8.9 ± 4.5 | n.s. |
| Predators (herpetobionts), ind. 100 trap days−1 | 29.3 ± 8.7 | 33 ± 3.3 | 28.6 ± 3 | 31.1 ± 4.6 | n.s. |
| Predators (hortobionts), ind. sampling−1 | 2.0 ± 0.7 | 2.1 ± 0.4 | 1.4 ± 0.3 | 1.6 ± 0.7 | n.s. |
| Predators (macrofauna), ind. × 103 m−2 | 1.0 ± 0.5 | 0.8 ± 0.1 | 0.6 ± 0.2 | 0.7 ± 0.1 | n.s. |
| Prostigmata mites, ind. × 103 m−2 | 10.3 ± 3.3a | 17.7 ± 3.2b | 17.1 ± 3.1b | 17.9 ± 2.3b | |
| Root mass, g | 29.4 ± 0.7 | 30.8 ± 4.0 | 32.0 ± 1.5 | 33.3 ± 2.6 | n.s. |
| Root rot development, % | 3.6 ± 0.6 | 3.5 ± 0.6 | 3.3 ± 0.6 | 4.6 ± 1.1 | n.s. |
| Root rot incidence, % | 93.3 ± 4.4 | 89.2 ± 4 | 92.6 ± 4 | 97.6 ± 0.4 | n.s. |
| Saprophages (macrofauna), ind. × 103 m−2 | 1.7 ± 0.6 | 1.7 ± 0.4 | 3.3 ± 0.6 | 2.0 ± 0.3 | n.s. |
| Shoot mass, g | 79.3 ± 5.6 | 79.9 ± 8.6 | 80.0 ± 7.7 | 81.4 ± 6.8 | n.s. |
| Soil moisture, % | 17.5 ± 0.9 | 17.9 ± 0.6 | 19.0 ± 0.7 | 18.5 ± 0.2 | n.s. |
| Ecosystem Parameter | Fusarium Biomass in Grain † | FHB in Grain | T2-Toxin in Grain | Total N in Grain | Yield |
|---|---|---|---|---|---|
| Root rot development | n.s. | 0.98; <0.001 | n.s. | n.s. | 0.38; 0.008 |
| D. schmidti (biomass) | 0.12; 0.001 ‡ | 0.45; 0.003 | 0.31; 0.031 | n.s. § | 0.31; 0.029 |
| Lumbricidae (abundance) | n.s. | 0.56; 0.001 | n.s. | n.s. | 0.53; 0.001 |
| D. schmidti (abundance) | n.s. | 0.36; 0.022 | n.s. | 0.36; 0.013 | 0.61; <0.001 |
| Mesostigmata mites | n.s. | n.s. | n.s. | 0.25; 0.041 | 0.64; <0.001 |
| Acari | n.s. | n.s. | n.s. | n.s. | 0.52; 0.001 |
| Mesofauna | n.s. | n.s. | n.s. | n.s. | 0.49; 0.002 |
| Predators (macrofauna) | n.s. | n.s. | n.s. | 0.34; 0.014 | 0.40; 0.006 |
| Phytophages (macrofauna) | n.s. | n.s. | 0.36; 0.048 | n.s. | n.s. |
| Prostigmata mites | n.s. | n.s. | n.s. | n.s. | 0.36; 0.010 |
| Collembola | n.s. | n.s. | n.s. | n.s. | 0.35; 0.012 |
| Soil moisture | n.s. | n.s. | n.s. | 0.29; 0.026 | 0.34; 0.018 |
| Fusarium biomass in soil | n.s. | n.s. | n.s. | n.s. | 0.34; 0.014 |
| Lumbricidae (biomass) | n.s. | n.s. | n.s. | n.s. | 0.33; 0.016 |
| Root rot incidence | n.s. | n.s. | 0.24; 0.046 | n.s. | n.s. |
| Herpetobionts | 0.19; 0.044 | n.s. | n.s. | 0.34; 0.013 | n.s. |
| Oribatida mites | n.s. | n.s. | n.s. | n.s. | 0.14; 0.050 |
| Predators (herpetobionts) | n.s. | n.s. | n.s. | 0.33; 0.014 | n.s. |
| Predators (hortobionts) | n.s. | n.s. | n.s. | 0.79; <0.001 | n.s. |
| Saprophages (macrofauna) | n.s. | n.s. | n.s. | 0.30; 0.021 | n.s. |
| Shoot mass | n.s. | n.s. | n.s. | 0.26; 0.030 | n.s. |
| Total C in mulch | n.s. | n.s. | n.s. | n.s. | n.s. |
| Total N in mulch | n.s. | n.s. | n.s. | n.s. | n.s. |
| C:N ratio in mulch | n.s. | n.s. | n.s. | n.s. | n.s. |
| Phytophages (herpetobionts) | n.s. | n.s. | n.s. | n.s. | n.s. |
| Hortobionts | n.s. | n.s. | n.s. | n.s. | n.s. |
| Phytophages (hortobionts) | n.s. | n.s. | n.s. | n.s. | n.s. |
| Macrofauna | n.s. | n.s. | n.s. | n.s. | n.s. |
| Root mass | n.s. | n.s. | n.s. | n.s. | n.s. |
| Dependent Variables | Regressor Parameters | Model Parameters | ||||
|---|---|---|---|---|---|---|
| Name | Estimate | SE, % | p | R2 Marginal | R2 Conditional | |
| Fusarium biomass in grain | Intercept | 903.75 | 45.9 | 0.044 | 66.1 | 84.2 |
| Herpetobionts | 212.12 | 18.4 | <0.001 | |||
| Mesofauna | −147.72 | 21.3 | <0.001 | |||
| FHB in grain | Intercept | −6.47 | 10.0 | <0.001 | 65.9 | 99.2 |
| Root rot development | 4.46 | 3.3 | <0.001 | |||
| Fusarium biomass in soil | 4.44 × 109 | 20.0 | <0.001 | |||
| Fusarium biomass in soil × Root rot development | −1.61 × 109 | 20.7 | <0.001 | |||
| T2-toxin in grain | Intercept | 35.79 | 6.4 | <0.001 | 46.7 | 70.8 |
| D. schmidti (biomass) × Phytophagous macrofauna | 1.92 | 21.4 | 0.001 | |||
| Total N in grain | Intercept | 1.57 | 5.3 | <0.001 | 51.2 | 94.6 |
| Predatory herpetobionts | 0.82 | 10.8 | <0.001 | |||
| Lumbricidae (biomass) | 0.14 | 29.3 | <0.001 | |||
| Predatory herpetobionts × Lumbricidae (biomass) | −0.22 | 18.1 | 0.005 | |||
| Yield | Intercept | 5.79 | 10.3 | <0.001 | 29.5 | 38.1 |
| D. schmidti (biomass) × Mesostigmata mites | 0.11 | 35.3 | 0.013 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goncharov, A.A.; Kiseleva, A.D.; Mashkov, K.A.; Sotnikov, I.V.; Izvarina, A.G.; Ladatko, V.A.; Maygurova, V.N.; Miroshnichenko, O.O.; Rozanova, O.L.; Rapoport, I.B.; et al. Influence of Structure of Detrital Food Webs on Fusarium Head Blight of Winter Wheat. Agronomy 2022, 12, 393. https://doi.org/10.3390/agronomy12020393
Goncharov AA, Kiseleva AD, Mashkov KA, Sotnikov IV, Izvarina AG, Ladatko VA, Maygurova VN, Miroshnichenko OO, Rozanova OL, Rapoport IB, et al. Influence of Structure of Detrital Food Webs on Fusarium Head Blight of Winter Wheat. Agronomy. 2022; 12(2):393. https://doi.org/10.3390/agronomy12020393
Chicago/Turabian StyleGoncharov, Anton A., Arina D. Kiseleva, Kirill A. Mashkov, Ivan V. Sotnikov, Anastasia G. Izvarina, Valery A. Ladatko, Valentina N. Maygurova, Olesya O. Miroshnichenko, Oksana L. Rozanova, Irina B. Rapoport, and et al. 2022. "Influence of Structure of Detrital Food Webs on Fusarium Head Blight of Winter Wheat" Agronomy 12, no. 2: 393. https://doi.org/10.3390/agronomy12020393






