The Use of Ecological Hydromulching Improves Growth in Escarole (Cichorium endivia L.) Plants Subjected to Drought Stress by Fine-Tuning Cytokinins and Abscisic Acid Balance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
2.2. Plant Growth-Related Determinations
2.3. Plant Water-Related Parameters
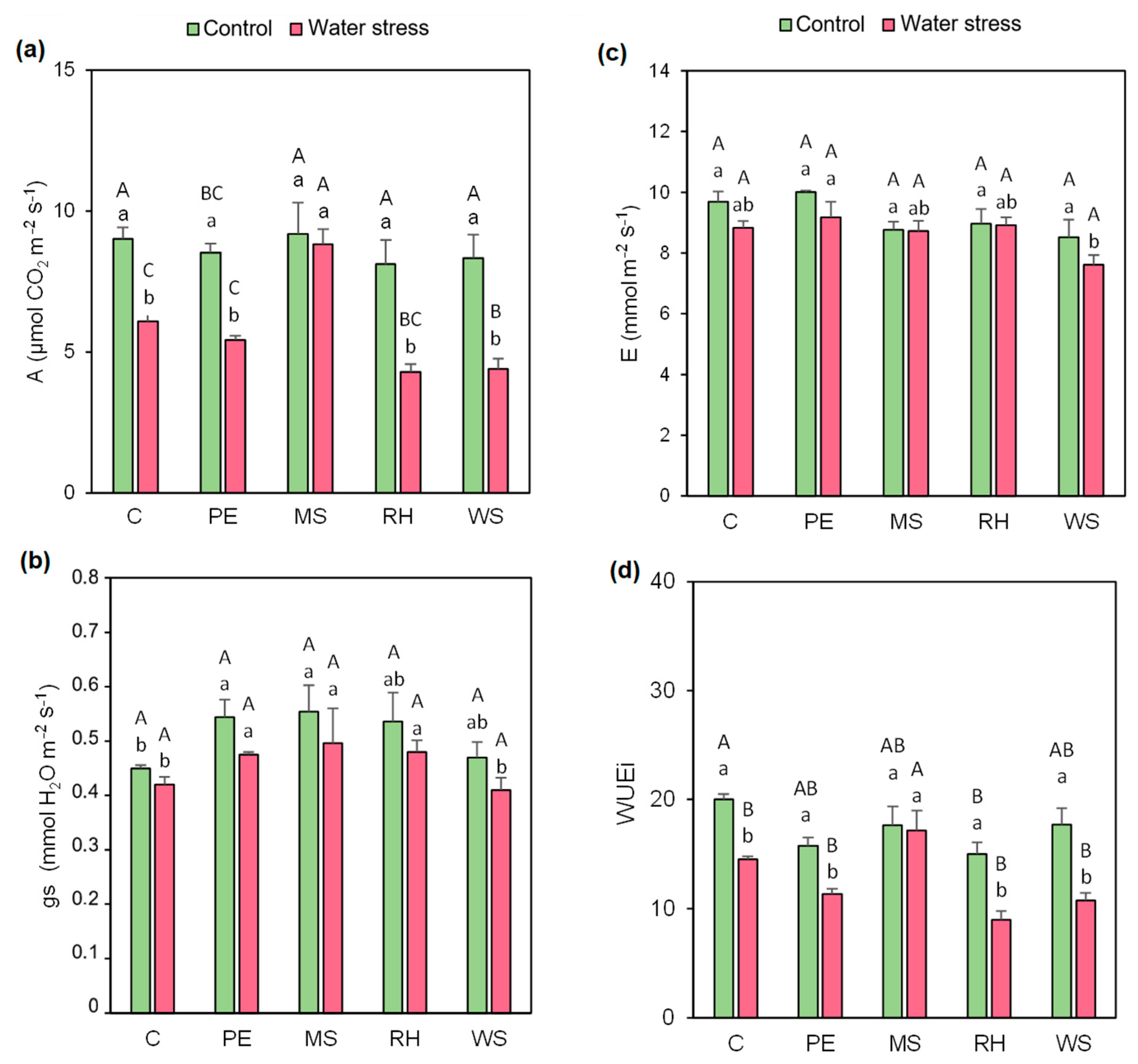
2.4. Gas Exchange Parameters
2.5. Chlorophyll Content and Fluorescence
2.6. Leaf Mineral Content
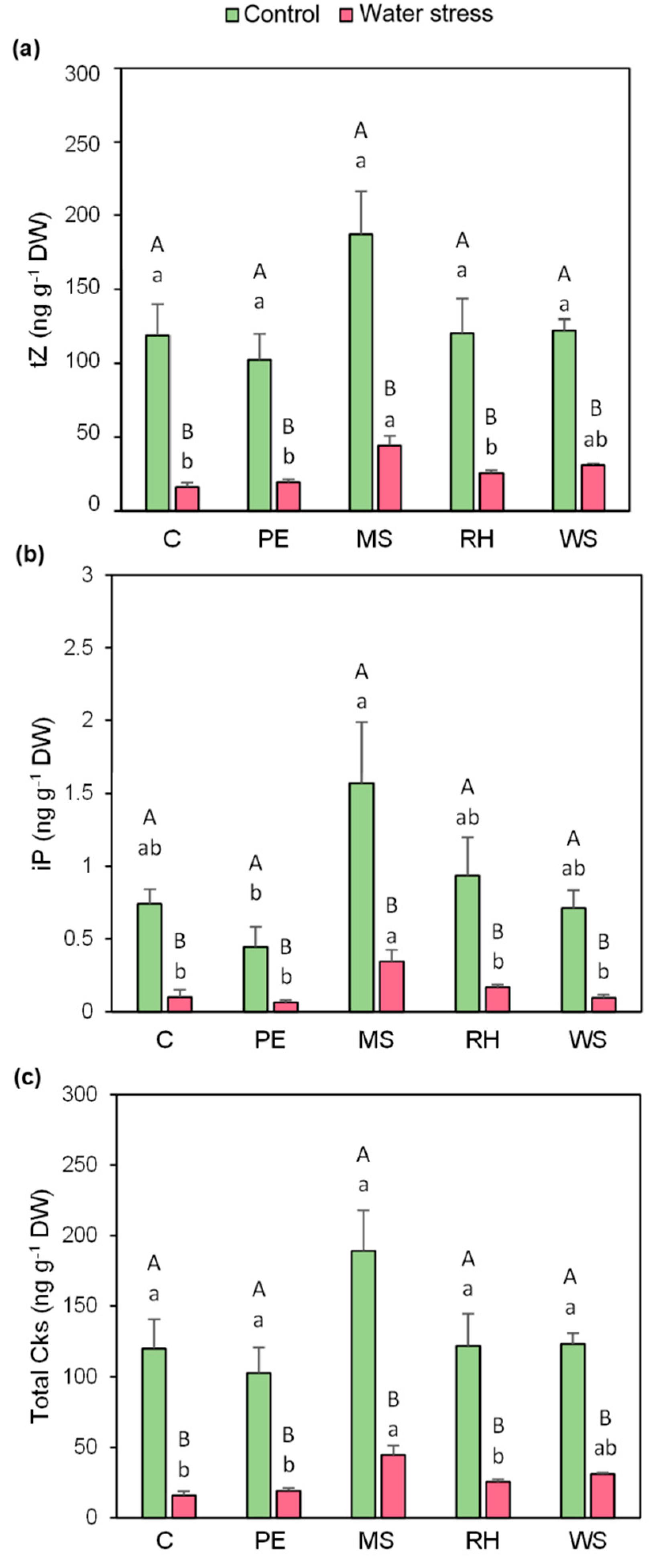
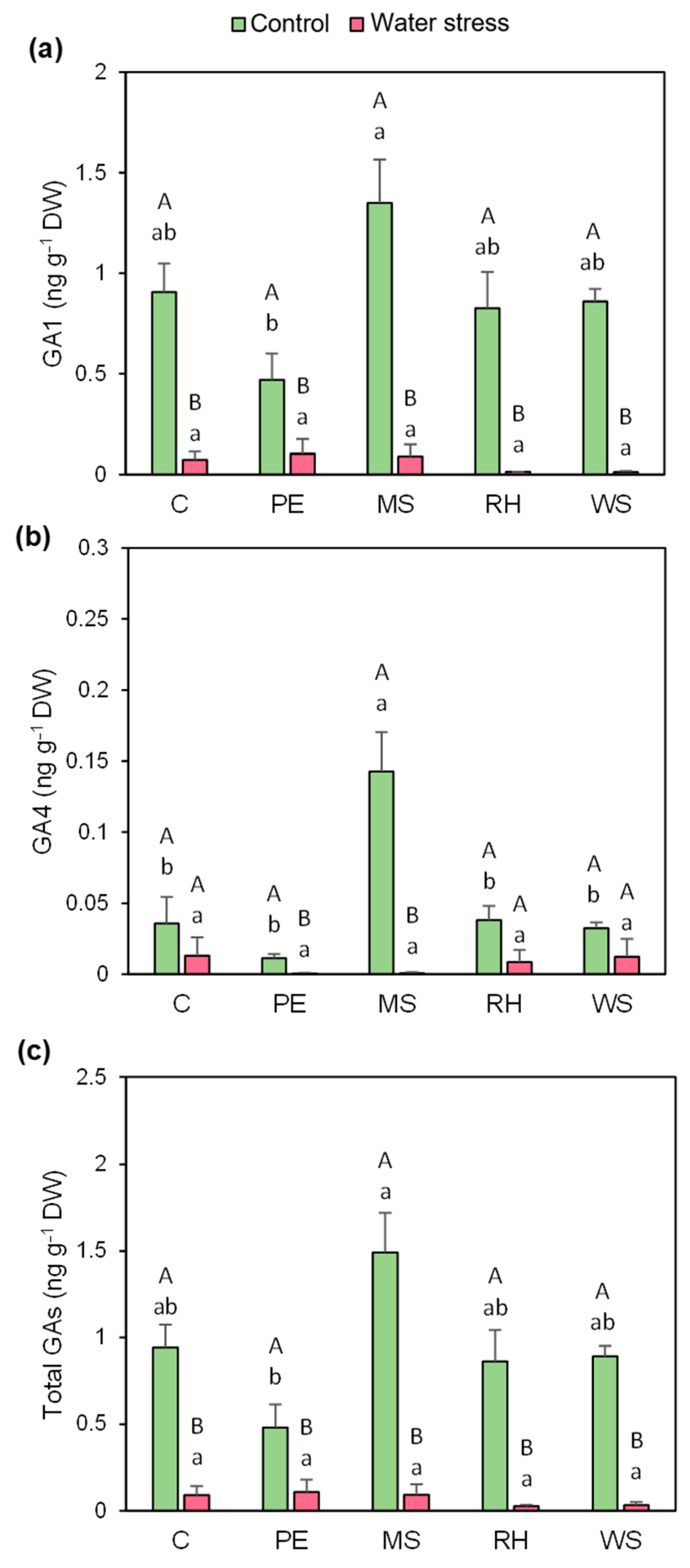
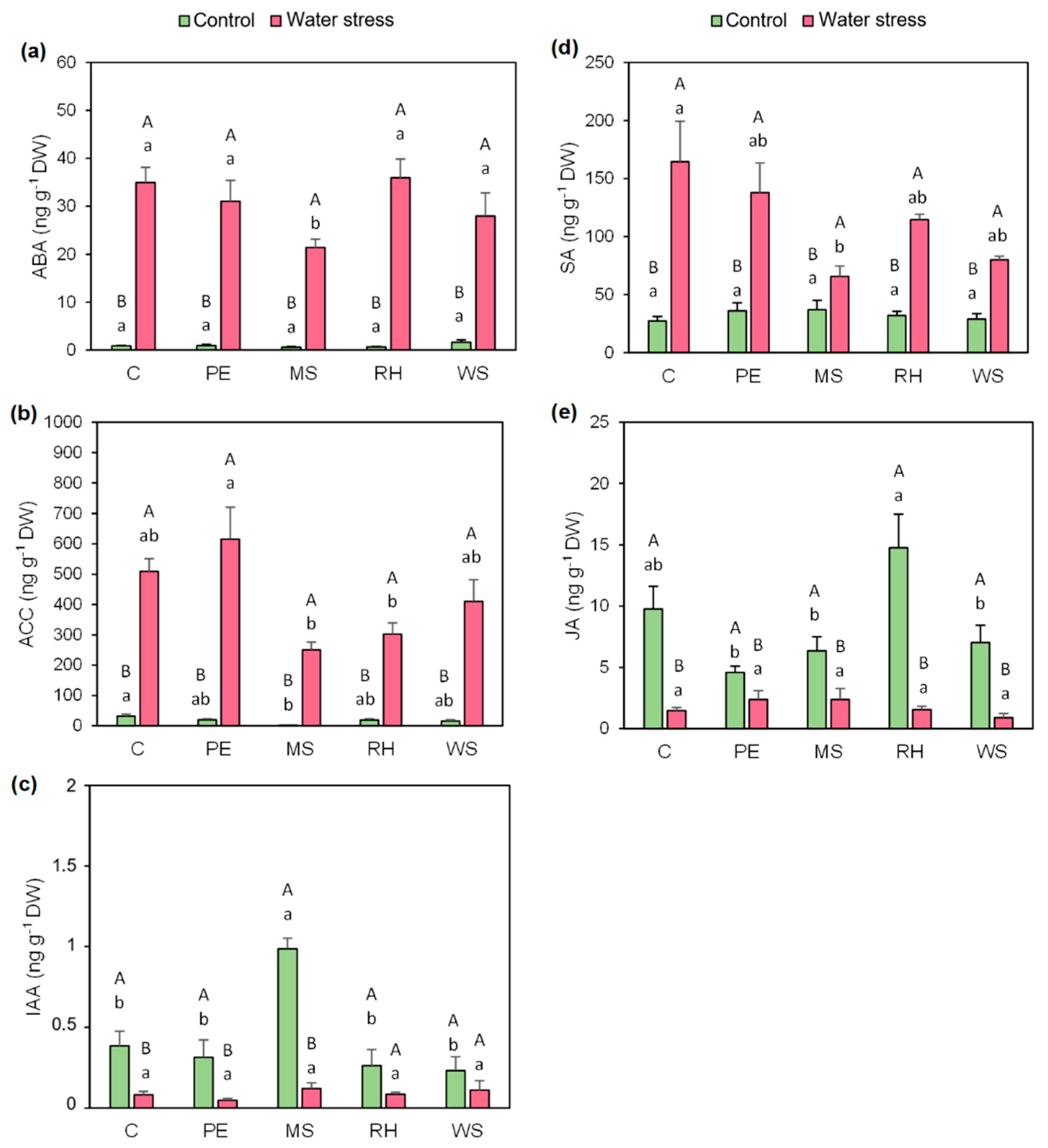
2.7. Hormone Extraction and Analysis
2.8. Statistical Analyses
3. Results
3.1. Plant Growth Parameters
3.2. Plant Water Relations
3.3. Gas Exchange Parameters
3.4. Chlorophyll Fluorescence and Content
3.5. Leaf Mineral Content
3.6. Hormonal Profiling
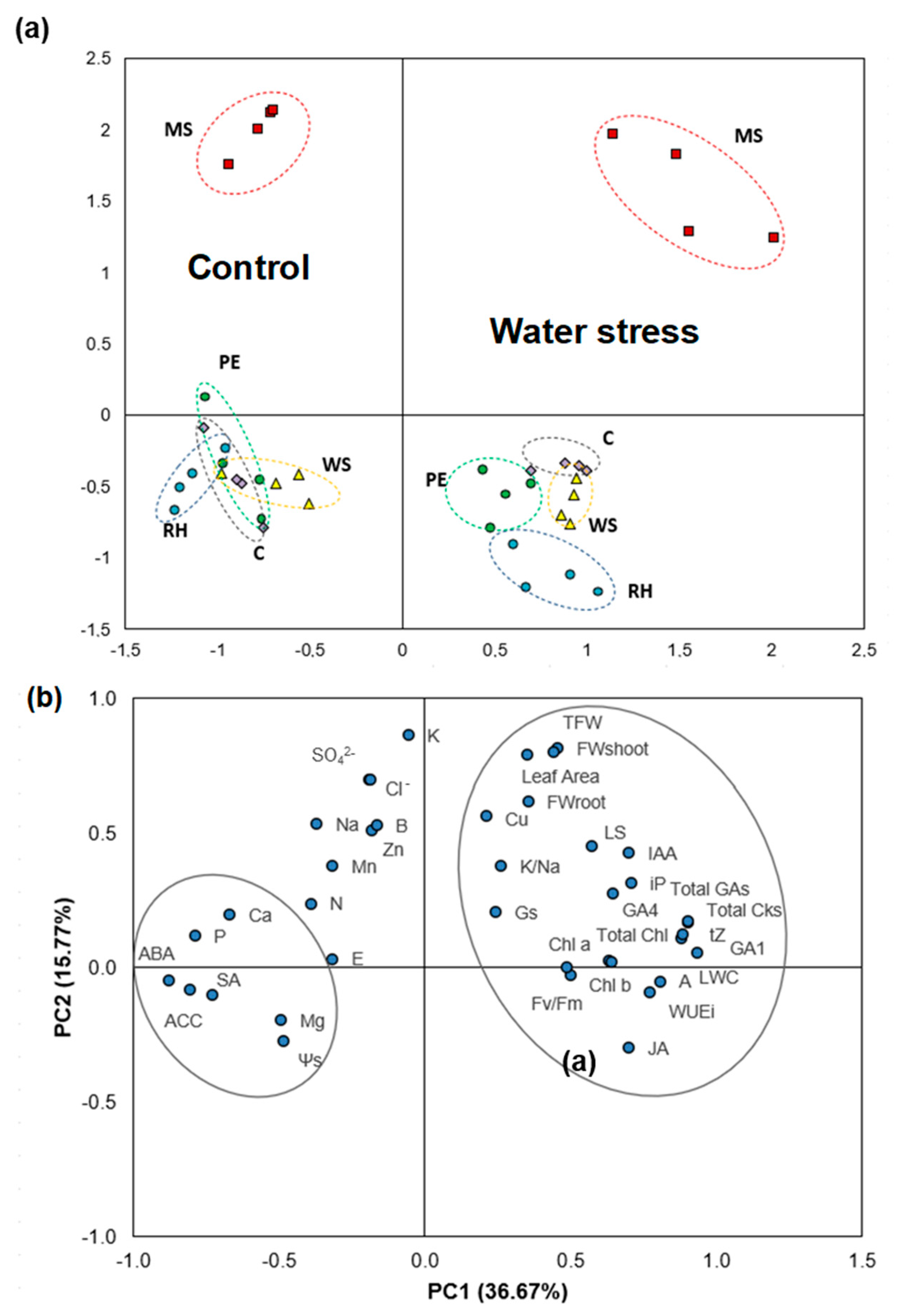
3.7. Principal Component Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, X.; You, S.; Tian, Y.; Li, J. Comparison of Plastic Film, Biodegradable Paper and Bio-Based Film Mulching for Summer Tomato Production: Soil Properties, Plant Growth, Fruit Yield and Fruit Quality. Sci. Hortic. 2019, 249, 38–48. [Google Scholar] [CrossRef]
- MAPA. Anuario de Estadística Agroalimentario Del Ministerio de Agricultura; Pesca y Alimentación: Madrid, Spain, 2020. [Google Scholar]
- Cook, B.I.; Anchukaitis, K.J.; Touchan, R.; Meko, D.M.; Cook, E.R. Spatiotemporal Drought Variability in the Mediterranean over the Last 900 Years. J. Geophys. Res. 2016, 121, 2060–2074. [Google Scholar] [CrossRef]
- Dubrovský, M.; Hayes, M.; Duce, P.; Trnka, M.; Svoboda, M.; Zara, P. Multi-GCM Projections of Future Drought and Climate Variability Indicators for the Mediterranean Region. Reg. Environ. Chang. 2014, 14, 1907–1919. [Google Scholar] [CrossRef]
- Kader, M.A.; Senge, M.; Mojid, M.A.; Ito, K. Recent Advances in Mulching Materials and Methods for Modifying Soil Environment. Soil Tillage Res. 2017, 168, 155–166. [Google Scholar] [CrossRef]
- Kannan, R. Uses of Mulching in Agriculture: A Review. In Current Research Soil Fertility; Lakhan, R., Ed.; AkiNik Publications: Delhi, India, 2020; Volume 2, pp. 1–32. [Google Scholar]
- Amiri Rodan, M.; Hassandokht, M.R.; Sadeghzadeh-Ahari, D.; Mousavi, A. Mitigation of Drought Stress in Eggplant by Date Straw and Plastic Mulches. J. Saudi Soc. Agric. Sci. 2020, 19, 492–498. [Google Scholar] [CrossRef]
- Sabatino, L.; Iapichino, G.; Vetrano, F.; Moncada, A.; Miceli, A.; de Pasquale, C.; D’Anna, F.; Giurgiulescu, L. Effects of Polyethylene and Biodegradable Starch-Based Mulching Films on Eggplant Production in a Mediterranean Area. Carpathian J. Food Sci. Technol. 2018, 10, 81–89. [Google Scholar]
- Li, F.M.; Wang, P.; Wang, J.; Xu, J.Z. Effects of Irrigation before Sowing and Plastic Film Mulching on Yield and Water Uptake of Spring Wheat in Semiarid Loess Plateau of China. Agric. Water Manag. 2004, 67, 77–88. [Google Scholar] [CrossRef]
- Gangaiah, B.; babu, M.B.B.P.; Latha, P.C.; Singh, T.V.; Rao, P.R. Rice Cultivation Using Plastic Mulch under Saturated Moisture Regime and Its Implications on Weed Management, Water Saving, Productivity and Profitability. Indian J. Weed Sci. 2019, 51, 198. [Google Scholar] [CrossRef]
- Qi, Y.; Ossowicki, A.; Yang, X.; Lwanga, E.H.; Dini-Andreote, F.; Geissen, V.; Garbeva, P. Effects of Plastic Mulch Film Residues on Wheat Rhizosphere and Soil Properties. J. Hazard. Mater. 2020, 387, 121711. [Google Scholar] [CrossRef]
- Sekara, A.; Pokluda, R.; Cozzolino, E.; del Piano, L.; Cuciniello, A.; Caruso, G. Plant Growth, Yield, and Fruit Quality of Tomato Affected by Biodegradable and Non-Degradable Mulches. Hortic. Sci. 2019, 46, 138–145. [Google Scholar] [CrossRef]
- López-Marín, J.; Abrusci, C.; González, A.; Fernández, J.A. Study of Degradable Materials for Soil Mulching in Greenhouse-Grown Lettuce. Acta Hortic. 2012, 952, 393–398. [Google Scholar] [CrossRef]
- Romero, M.; Gálvez, A.; Del Amor, F.M.; López-Marín, J. Evaluación Preliminar Del Comportamiento Agronómico de Un Cultivo de Alcachofa Con Hidromulch. In Actas de Horticultura No 83; Sánchez-Manzanera, C.A., Calvo, Y.S., Izquierdo, R.V., Mínguez, J.J.M., García, I.L., Soria, C.B., Eds.; Sociedad Española de Ciencias Hortícolas: Valladolid, Spain, 2019; pp. 152–156. ISBN 978-84-09-11754-3. [Google Scholar]
- López-Marín, J.; Romero, M.; Gálvez, A.; del Amor, F.M.; Piñero, M.C.; Brotons-Martínez, J.M. The Use of Hydromulching as an Alternative to Plastic Films in an Artichoke (Cynara Cardunculus Cv. Symphony) Crop: A Study of the Economic Viability. Sustainability 2021, 13, 5313. [Google Scholar] [CrossRef]
- Claramunt, J.; Mas, M.T.; Pardo, G.; Cirujeda, A.; Verdú, A.M.C. Mechanical Characterization of Blends Containing Recycled Paper Pulp and Other Lignocellulosic Materials to Develop Hydromulches for Weed Control. Biosyst. Eng. 2020, 191, 35–47. [Google Scholar] [CrossRef]
- Verdú, A.M.C.; Mas, M.T.; Josa, R.; Ginovart, M. The Effect of a Prototype Hydromulch on Soil Water Evaporation under Controlled Laboratory Conditions. J. Hydrol. Hydromech. 2020, 68, 404–410. [Google Scholar] [CrossRef]
- Jordán, A.; Zavala, L.M.; Gil, J. Effects of Mulching on Soil Physical Properties and Runoff under Semi-Arid Conditions in Southern Spain. Catena 2010, 81, 77–85. [Google Scholar] [CrossRef]
- Luo, H.H.; Zhang, Y.L.; Zhang, W.F. Effects of Water Stress and Rewatering on Photosynthesis, Root Activity, and Yield of Cotton with Drip Irrigation under Mulch. Photosynthetica 2016, 54, 65–73. [Google Scholar] [CrossRef]
- Cline, J.; Neilsen, G.; Hogue, E.; Kuchta, S.; Neilsen, D. Spray-on-Mulch Technology for Intensively Grown Irrigated Apple Orchards: Influence on Tree Establishment, Early Yields, and Soil Physical Properties. HortTechnology 2011, 21, 398–411. [Google Scholar] [CrossRef] [Green Version]
- Mostafaei, E.; Zehtab-Salmasi, S.; Salehi-Lisar, Y.; Ghassemi-Golezani, K. Changes in Photosynthetic Pigments, Osmolytes and Antioxidants of Indian Mustard by Drought and Exogenous Polyamines. Acta Biol. Hung. 2018, 69, 313–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, Y.; Khan, S.; Ma, X. Climate Change Impacts on Crop Yield, Crop Water Productivity and Food Security—A Review. Prog. Nat. Sci. 2009, 19, 1665–1674. [Google Scholar] [CrossRef]
- Davis, R.F.; Earl, H.J.; Timper, P. Effect of Simultaneous Water Deficit Stress and Meloidogyne Incognita Infection on Cotton Yield and Fiber Quality. J. Nematol. 2014, 46, 108–118. [Google Scholar] [PubMed]
- Ullah, A.; Manghwar, H.; Shaban, M.; Khan, A.H.; Akbar, A.; Ali, U.; Ali, E.; Fahad, S. Phytohormones Enhanced Drought Tolerance in Plants: A Coping Strategy. Environ. Sci. Pollut. Res. 2018, 25, 33103–33118. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Pandey, D.M.; Goswami, C.L.; Jain, S. Effect of Growth Regulators on Photosynthesis, Transpiration and Related Parameters in Water Stressed Cotton. Biol. Plant. 2001, 44, 475–478. [Google Scholar] [CrossRef]
- Llorach, R.; Martínez-Sánchez, A.; Tomás-Barberán, F.A.; Gil, M.I.; Ferreres, F. Characterisation of Polyphenols and Antioxidant Properties of Five Lettuce Varieties and Escarole. Food Chem. 2008, 108, 1028–1038. [Google Scholar] [CrossRef] [PubMed]
- Maroto, J.V.; Miguel, A.; Baixauli, C. La Lechuga y La Escarola; Mundi-Prensa, Ed.; Fundación Caja Rural Valencia: Valencia, Spain, 2000. [Google Scholar]
- Sorrentino, M.; Colla, G.; Rouphael, Y.; Panzarová, K.; Trtílek, M. Lettuce Reaction to Drought Stress: Automated High-Throughput Phenotyping of Plant Growth and Photosynthetic Performance. Acta Hortic. 2020, 1268, 133–141. [Google Scholar] [CrossRef]
- Morgan, J.M. Osmoregulation and Water Stress in Higher Plants. Annu. Rev. Plant Physiol. 1984, 35, 299–319. [Google Scholar] [CrossRef]
- Basu, S.; Ramegowda, V.; Kumar, A.; Pereira, A. Plant Adaptation to Drought Stress. F1000Research 2016, 5, 1554. [Google Scholar] [CrossRef]
- Figueiredo-Lima, K.V.; Falcão, H.M.; Melo-de-Pinna, G.F.; Albacete, A.; Dodd, I.C.; Lima, A.L.; Santos, M.G. Leaf Phytohormone Levels and Stomatal Control in an Evergreen Woody Species under Semiarid Environment in a Brazilian Seasonally Dry Tropical Forest. Plant Growth Regul. 2018, 85, 437–445. [Google Scholar] [CrossRef]
- Albacete, A.; Martínez-Andújar, C.; Ghanem, M.E.; Acosta, M.; SÁnchez-Bravo, J.; Asins, M.J.; Cuartero, J.; Lutts, S.; Dodd, I.C.; PÉrez-Alfocea, F. Rootstock-Mediated Changes in Xylem Ionic and Hormonal Status Are Correlated with Delayed Leaf Senescence, and Increased Leaf Area and Crop Productivity in Salinized Tomato. Plant Cell Environ. 2009, 32, 928–938. [Google Scholar] [CrossRef]
- Zargar, S.M.; Zargar, M.Y. Abiotic Stress-Mediated Sensing and Signaling in Plants: An Omics Perspective; Springer: Singapore, 2018; ISBN 9789811074790. [Google Scholar]
- Pandey, G.K.; Varshney, R.K.; Nick, P.; Kohli, A.; Riemann, M.; Dhakarey, R.; Hazman, M.; Miro, B. Exploring Jasmonates in the Hormonal Network of Drought and Salinity Responses. Front. Plant Sci. 2015, 6, 1077. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Xu, Z.-S.; Lu, P.-P.; Hu, D.; Chen, M.; Li, L.-C.; Ma, Y.-Z. A Wheat PI4K Gene Whose Product Possesses Threonine Autophophorylation Activity Confers Tolerance to Drought and Salt in Arabidopsis. J. Exp. Bot. 2013, 64, 2915–2927. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.; Lee, D.K.; do Choi, Y.; Kim, J.K. OsIAA6, a Member of the Rice Aux/IAA Gene Family, Is Involved in Drought Tolerance and Tiller Outgrowth. Plant Sci. 2015, 236, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, T. Expansion and Stress Responses of the AP2/EREBP Superfamily in Cotton. BMC Genom. 2017, 18, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Herrera-Estrella, L.; Tran, L.S.P. The Yin-Yang of Cytokinin Homeostasis and Drought Acclimation/Adaptation. Trends Plant Sci. 2016, 21, 548–550. [Google Scholar] [CrossRef] [PubMed]
- Lubovská, Z.; Dobrá, J.; Štorchová, H.; Wilhelmová, N.; Vanková, R. Cytokinin Oxidase/Dehydrogenase Overexpression Modifies Antioxidant Defense against Heat, Drought and Their Combination in Nicotiana Tabacum Plants. J. Plant Physiol. 2014, 171, 1625–1633. [Google Scholar] [CrossRef]
- Jiroutova, P.; Oklestkova, J.; Strnad, M. Molecular Sciences Crosstalk between Brassinosteroids and Ethylene during Plant Growth and under Abiotic Stress Conditions. Int. J. Mol. Sci. 2018, 19, 3283. [Google Scholar] [CrossRef] [Green Version]
- Mantovani, A. A Method to Improve Leaf Succulence Quantification. Braz. Arch. Biol. Technol. 1999, 42, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Nagata, M.; Yamashita, I. Simple Method for Simultaneous Determination of Chlorophyll and Carotenoids in Tomato Fruit. Nippon. Shokuhin Kogyo Gakkaishi 1992, 39, 925–928. [Google Scholar] [CrossRef] [Green Version]
- Großkinsky, D.K.; Albacete, A.; Jammer, A.; Krbez, P.; van der Graaff, E.; Pfeifhofer, H.; Roitsch, T. A Rapid Phytohormone and Phytoalexin Screening Method for Physiological Phenotyping. Mol. Plant 2014, 7, 1053–1056. [Google Scholar] [CrossRef] [Green Version]
- McLaughlin, D.; Kinzelbach, W. Food Security and Sustainable Resource Management. Water Resour. Res. 2015, 51, 4966–4985. [Google Scholar] [CrossRef]
- Banerjee, H.; Puste, A.M.; Ray, K.; Sarkar, S.; Chakraborty, A.; Rana, L. Influence of Irrigation Levels and Mulching on Growth, Water Use, Yield, Economics and Quality of Potato (Solanum Tuberosum) under New Alluvial Soil of West Bengal. Indian J. Agron. 2016, 61, 377–383. [Google Scholar]
- Chakraborty, D.; Nagarajan, S.; Aggarwal, P.; Gupta, V.K.; Tomar, R.K.; Garg, R.N.; Sahoo, R.N.; Sarkar, A.; Chopra, U.K.; Sarma, K.S.S.; et al. Effect of Mulching on Soil and Plant Water Status, and the Growth and Yield of Wheat (Triticum aestivum L.) in a Semi-Arid Environment. Agric. Water Manag. 2008, 95, 1323–1334. [Google Scholar] [CrossRef]
- Mukherjee, A.; Kundu, M.; Sarkar, S. Role of Irrigation and Mulch on Yield, Evapotranspiration Rate and Water Use Pattern of Tomato (Lycopersicon esculentum L.). Agric. Water Manag. 2010, 98, 182–189. [Google Scholar] [CrossRef]
- Albacete, A.; Martínez-Andújar, C.; Martínez-Pérez, A.; Thompson, A.J.; Dodd, I.C.; Pérez-Alfocea, F. Unravelling Rootstock×scion Interactions to Improve Food Security. J. Exp. Bot. 2015, 66, 2211–2226. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Zhan, A.; Chen, H.; Luo, S.; Bu, L.; Chen, X.; Li, S. Response of Nitrogen Use Efficiency and Soil Nitrate Dynamics to Soil Mulching in Dryland Maize (Zea mays L.) Fields. Nutr. Cycl. Agroecosystems 2015, 101, 271–283. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Fisiología vegetal; Taiz, L., Zeiger, E., Eds.; Universitat Jaume I: Castellón, Spain, 2006; Volume 2, pp. 1129–1188. [Google Scholar]
- Song, D.; Li, M.; Wang, Q. Evaluation of Models of the Photosynthetic Light Response of Spring Wheat under Different Mulching Treatments in a Desert Oasis in Northwestern China. Crop Sci. 2020, 61, 2036–2047. [Google Scholar] [CrossRef]
- Yang, Y.; Ding, J.; Zhang, Y.; Wu, J.; Zhang, J.; Pan, X.; Gao, C.; Wang, Y.; He, F. Effects of Tillage and Mulching Measures on Soil Moisture and Temperature, Photosynthetic Characteristics and Yield of Winter Wheat. Agric. Water Manag. 2018, 201, 299–308. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.; Li, Y.; Zhang, S. Improving Water-Use Efficiency by Decreasing Stomatal Conductance and Transpiration Rate to Maintain Higher Ear Photosynthetic Rate in Drought-Resistant Wheat. Crop J. 2017, 5, 231–239. [Google Scholar] [CrossRef]
- Mulet, J.M.; Campos, F.; Yenush, L. Editorial: Ion Homeostasis in Plant Stress and Development. Front. Plant Sci. 2020, 11, 10–12. [Google Scholar] [CrossRef]
- Gálvez, A.; Albacete, A.; Martínez-Andújar, C.; del Amor, F.M.; López-Marín, J. Contrasting Rootstock-Mediated Growth and Yield Responses in Salinized Pepper Plants (Capsicum annuum L.) Are Associated with Changes in the Hormonal Balance. Int. J. Mol. Sci. 2021, 22, 3297. [Google Scholar] [CrossRef]
- Conversa, G.; Bonasia, A.; Lazzizera, C.; La Rotonda, P.; Elia, A. Reduction of Nitrate Content in Baby-Leaf Lettuce and Cichorium Endivia Through the Soilless Cultivation System, Electrical Conductivity and Management of Nutrient Solution. Front. Plant Sci. 2021, 12, 645671. [Google Scholar] [CrossRef]
- Colmenero-Flores, J.M.; Franco-Navarro, J.D.; Cubero-Font, P.; Peinado-Torrubia, P.; Rosales, M.A. Chloride as a Beneficial Macronutrient in Higher Plants: New Roles and Regulation. Int. J. Mol. Sci. 2019, 20, 4686. [Google Scholar] [CrossRef] [Green Version]
- Franco-Navarro, J.D.; Brumós, J.; Rosales, M.A.; Cubero-Font, P.; Talón, M.; Colmenero-Flores, J.M. Chloride Regulates Leaf Cell Size and Water Relations in Tobacco Plants. J. Exp. Bot. 2016, 67, 873–891. [Google Scholar] [CrossRef] [Green Version]
- Franco-Navarro, J.D.; Díaz-Rueda, P.; Rivero-Núñez, C.M.; Brumós, J.; Rubio-Casal, A.E.; de Cires, A.; Col-menero-Flores, J.M.; Rosales, M.A. Chloride Nutrition Improves Drought Resistance by Enhancing Water De- ficit Avoidance and Tolerance Mechanisms. J. Exp. Bot. 2021, 72, 5246–5261. [Google Scholar] [CrossRef]
- Pietrini, F.; Carnevale, M.; Beni, C.; Zacchini, M.; Gallucci, F.; Santangelo, E. Effect of Different Copper Levels on Growth and Morpho-Physiological Parameters in Giant Reed (Arundo donax L.) in Semi-Hydroponic Mesocosm Experiment. Water 2019, 11, 1837. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Wu, L.; Chai, T.; Zhang, Y.; Tan, J.; Ma, S. The Effects of Copper, Manganese and Zinc on Plant Growth and Elemental Accumulation in the Manganese-Hyperaccumulator Phytolacca Americana. J. Plant Physiol. 2012, 169, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Sarwat, M.; Tuteja, N. Hormonal Signaling to Control Stomatal Movement during Drought Stress. Plant Gene 2017, 11, 143–153. [Google Scholar] [CrossRef]
- Takahashi, F.; Kuromori, T.; Urano, K.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Drought Stress Responses and Resistance in Plants: From Cellular Responses to Long-Distance Intercellular Communication. Front. Plant Sci. 2020, 11, 147. [Google Scholar] [CrossRef]
- Du, H.; Liu, H.; Xiong, L.; Pollmann, S. Endogenous Auxin and Jasmonic Acid Levels Are Differentially Modulated by Abiotic Stresses in Rice. Front. Plant Sci. 2013, 4, 397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Z.; Kong, X.; Zhang, Y.; Li, W.; Zhang, D.; Dai, J.; Fang, S.; Chu, J.; Dong, H. Leaf-Derived Jasmonate Mediates Water Uptake FromHydrated Cotton Roots under PartialRoot-Zone Irrigation. Plant Physiol. 2019, 180, 1660–1676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.-W.; Li, C.-H.; Cao, J.; Zhang, Y.-C.; Zhang, S.-Q.; Xia, Y.-F.; Sun, D.-Y.; Sun, Y. Altered Architecture and Enhanced Drought Tolerance in Rice via the Down-Regulation of Indole-3-Acetic Acid by TLD1/OsGH3.13 Activation 1. Plant Physiol. 2009, 151, 1889–1901. [Google Scholar] [CrossRef] [Green Version]
- Prerostova, S.; Dobrev, P.I.; Gaudinova, A.; Knirsch, V.; Körber, N.; Pieruschka, R.; Fiorani, F.; Brzobohatý, B.; Spichal, L.; Humplik, J.; et al. Cytokinins: Their Impact on Molecular and Growth Responses to Drought Stress and Recovery in Arabidopsis. Front. Plant Sci. 2018, 9, 655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghanem, M.E.; Albacete, A.; Smigocki, A.C.; Fré Bort, I.; Pospíš Ilová, H.; Martínez-Andú Jar, C.; Acosta, M.; Sánchez-Bravo, J.; Lutts, S.; Dodd, I.C.; et al. Root-Synthesized Cytokinins Improve Shoot Growth and Fruit Yield in Salinized Tomato (Solanum lycopersicum L.) Plants. J. Exp. Bot. 2011, 62, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Cantero-Navarro, E.; Romero-Aranda, R.; Fernández-Muñoz, R.; Martínez-Andújar, C.; Pérez-Alfocea, F.; Albacete, A. Improving Agronomic Water Use Efficiency in Tomato by Rootstock-Mediated Hormonal Regulation of Leaf Biomass. Plant Sci. 2016, 251, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Fotopoulos, V.; Anjum, N.A.; Iqbal, M.; Khan, R.; Fatma, M.; Per, T.S.; Khan, N.A. Salicylic Acid-Induced Abiotic Stress Tolerance and Underlying Mechanisms in Plants. Front. Plant Sci. 2015, 6, 462. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, W.; Imran, M.; Yaseen, M.; Ul Haq, T.; Jamshaid, M.U.; Rukh, S.; Ikram, R.M.; Ali, M.; Ali, A.; Maqbool, M.; et al. Role of Salicylic Acid in Regulating Ethylene and Physiological Characteristics for Alleviating Salinity Stress on Germination, Growth and Yield of Sweet Pepper. PeerJ 2020, 8, e8475. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, J.; Liu, H.; Qiu, R.; Gao, Y.; Duan, A. Role of Hydraulic Signal and ABA in Decrease of Leaf Stomatal and Mesophyll Conductance in Soil Drought-Stressed Tomato. Front. Plant Sci. 2021, 1, 653186. [Google Scholar] [CrossRef]
- Saradadevi, R.; Palta, J.A.; Siddique, K.H.M. ABA-Mediated Stomatal Response in Regulating Water Use during the Development of Terminal Drought in Wheat. Front. Plant Sci. 2017, 8, 1251. [Google Scholar] [CrossRef]
- Huang, X.; Hou, L.; Meng, J.; You, H.; Li, Z.; Gong, Z.; Yang, S.; Shi, Y. The Antagonistic Action of Abscisic Acid and Cytokinin Signaling Mediates Drought Stress Response in Arabidopsis. Mol. Plant 2018, 11, 970–982. [Google Scholar] [CrossRef] [Green Version]
- Kambhampati, S.; Kurepin, L.V.; Kisiala, A.B.; Bruce, K.E.; Cober, E.R.; Morrison, M.J.; Emery, R.J.N. Yield Associated Traits Correlate with Cytokinin Profiles in Developing Pods and Seeds of Field-Grown Soybean Cultivars. Field Crops Res. 2017, 214, 175–184. [Google Scholar] [CrossRef]
- Wilkinson, S.; Kudoyarova, G.R.; Veselov, D.S.; Arkhipova, T.N.; Davies, W.J. Plant Hormone Interactions: Innovative Targets for Crop Breeding and Management. J. Exp. Bot. 2012, 63, 3499–3509. [Google Scholar] [CrossRef]
- Gálvez, A.; Albacete, A.; del Amor, F.M.; López-Marín, J. The Use of Red Shade Nets Improves Growth in Salinized Pepper (Capsicum annuum L.) Plants by Regulating Their Ion Homeostasis and Hormone Balance. Agronomy 2020, 10, 1766. [Google Scholar] [CrossRef]
- Nishiyama, R.; Watanabe, Y.; Fujita, Y.; Le, D.T.; Kojima, M.; Werner, T.; Vankova, R.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Kakimoto, T.; et al. Analysis of Cytokinin Mutants and Regulation of Cytokinin Metabolic Genes Reveals Important Regulatory Roles of Cytokinins in Drought, Salt and Abscisic Acid Responses, and Abscisic Acid Biosynthesis. Plant Cell 2011, 23, 2169–2183. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Irrigation | Mulch | Ψs (MPa) | LWC (%) | LS (g H2O cm−2) |
|---|---|---|---|---|
| Control | C | −0.97 ± 0.05 A a | 91.72 ± 0.24 A a | 0.05 ± 0.002 A bc |
| PE | −1.04 ± 0.05 A a | 90.13 ± 0.25 A a | 0.05 ± 0.002 A bc | |
| MS | −1.11 ± 0.07 A a | 92.19 ± 0.42 A a | 0.07 ± 0.003 A a | |
| RH | −0.93 ± 0.01 A a | 88.94 ± 0.16 A b | 0.04 ± 0.001 A c | |
| WS | −1.08 ± 0.07 A a | 91.09 ± 0.10 A a | 0.06 ± 0.002 A b | |
| Stress | C | −1.27 ± 0.06 B b | 84.69 ± 0.07 B c | 0.04 ± 0.001 B a |
| PE | −1.08 ± 0.03 A ab | 85.52 ± 0.14 B bc | 0.05 ± 0.003 A a | |
| MS | −1.26 ± 0.07 A b | 85.94 ± 0.33 Ba | 0.05 ± 0.002 B a | |
| RH | −1.01 ± 0.07 A a | 85.50 ± 0.39 B bc | 0.04 ± 0.002 A a | |
| WS | −1.02 ± 0.01 A a | 85.57 ± 1.13 B b | 0.05 ± 0.004 A a |
| Irrigation | Mulch | N (mg g−1 DW) | P (mg g−1 DW) | K (mg g−1 DW) | Mg (mg g−1 DW) | Ca (mg g−1 DW) | SO4−2 (mg g−1 DW) |
|---|---|---|---|---|---|---|---|
| Control | C | 14.89 A a | 8.70 A a | 23.60 B b | 2.61 A a | 7.57 A a | 2.54 A a |
| PE | 16.94 A ab | 8.63 B a | 31.33 A ab | 2.39 A a | 7.38 A a | 2.90 A a | |
| MS | 13.43 B ab | 7.90 B a | 37.70 A a | 2.25 A a | 7.12 A a | 3.62 A a | |
| RH | 16.18 A ab | 8.33 B a | 29.29 A ab | 2.68 A a | 7.62 A a | 3.00 A a | |
| WS | 14.25 A b | 7.29 B a | 29.99 A ab | 2.47 A a | 7.08 A a | 3.09 A a | |
| Stress | C | 15.35 A a | 9.23 A b | 27.99 A b | 2.77 A a | 8.37 A a | 2.89 A ab |
| PE | 17.51 A a | 9.82 A ab | 28.49 A b | 2.85 A a | 9.18 A a | 2.36 A b | |
| MS | 19.51 A a | 9.92 A ab | 41.24 A a | 2.31 A a | 8.89 A a | 3.96 A a | |
| RH | 16.82 A a | 11.11 A a | 30.62 A b | 3.06 A a | 9.51 A a | 3.82 A a | |
| WS | 15.66 A a | 10.76 A ab | 30.80 A b | 2.59 A a | 9.05 A a | 3.46 A ab | |
| Irrigation | Mulch | Cu (mg kg−1 DW) | Mn (mg kg−1 DW) | Zn (mg kg−1 DW) | B (mg kg−1 DW) | Cl (mg g−1 DW) | Na (mg g−1 DW) |
| Control | C | 1.31 A a | 26.26 A a | 8.75 B b | 25.82 A a | 29.68 A b | 12.46 A a |
| PE | 3.02 A a | 32.09 A a | 7.81 A b | 28.18 A a | 26.13 A b | 11.00 A a | |
| MS | 2.86 A a | 22.86 A a | 19.76 A a | 25.79 B a | 36.99 A a | 12.92 B a | |
| RH | 2.07 A a | 18.41 A a | 18.43 A ab | 26.33 A a | 26.73 B b | 10.56 A a | |
| WS | 1.84 A a | 25.77 A a | 12.37 B a | 26.04 A a | 30.31 A b | 11.88 A a | |
| Stress | C | 1.66 A a | 29.42 A a | 15.84 A b | 24.46 A a | 28.99 A a | 12.67 A a |
| PE | 1.30 A a | 27.68 A a | 12.02 A b | 27.05 A a | 28.25 A a | 13.45 A a | |
| MS | 2.68 A a | 30.23 A a | 24.36 A a | 30.08 A a | 40.63 A a | 15.49 A a | |
| RH | 1.59 A a | 22.08 A a | 17.55 A ab | 25.23 A a | 35.48 A a | 12.58 A a | |
| WS | 1.81 A a | 30.41 A a | 17.60 A ab | 25.85 A a | 32.20 A a | 13.89 A a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero-Muñoz, M.; Albacete, A.; Gálvez, A.; Piñero, M.C.; Amor, F.M.d.; López-Marín, J. The Use of Ecological Hydromulching Improves Growth in Escarole (Cichorium endivia L.) Plants Subjected to Drought Stress by Fine-Tuning Cytokinins and Abscisic Acid Balance. Agronomy 2022, 12, 459. https://doi.org/10.3390/agronomy12020459
Romero-Muñoz M, Albacete A, Gálvez A, Piñero MC, Amor FMd, López-Marín J. The Use of Ecological Hydromulching Improves Growth in Escarole (Cichorium endivia L.) Plants Subjected to Drought Stress by Fine-Tuning Cytokinins and Abscisic Acid Balance. Agronomy. 2022; 12(2):459. https://doi.org/10.3390/agronomy12020459
Chicago/Turabian StyleRomero-Muñoz, Miriam, Alfonso Albacete, Amparo Gálvez, María Carmen Piñero, Francisco M. del Amor, and Josefa López-Marín. 2022. "The Use of Ecological Hydromulching Improves Growth in Escarole (Cichorium endivia L.) Plants Subjected to Drought Stress by Fine-Tuning Cytokinins and Abscisic Acid Balance" Agronomy 12, no. 2: 459. https://doi.org/10.3390/agronomy12020459








