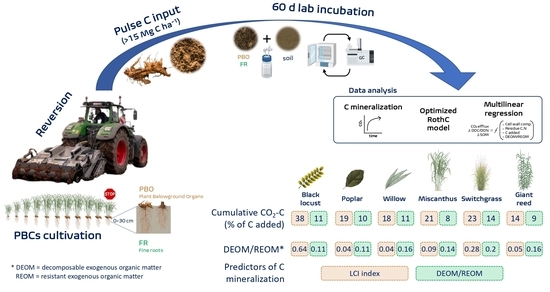
Short-Term Mineralization of Belowground Biomass of Perennial Biomass Crops after Reversion to Arable Land
Abstract
:1. Introduction
2. Materials and Methods
2.1. Source of BGB Residues for Incubation
2.2. Characterization of EOM Used in the Incubation
2.3. Incubation Experiment
2.3.1. Soil Used in the Incubation Experiment
2.3.2. Soil Incubation Experiment and CO2 Measurement
2.3.3. Soil C and N analysis
2.4. Estimation of RothC EOM Pool Parameters
- (1)
- fDEOM + fREOM = 1
- (2)
- kREOM > 0.02 yr−1
2.5. Statistical Analysis
3. Results
3.1. Stumps and Rhizomes C and N Mineralization
3.2. Fine Roots C and N Mineralization
3.3. Pool Size and Decomposition Rates of EOM Types
3.4. Modeling C and N Mineralization of EOM Types Using Multiple Linear Regression Models
4. Discussion
4.1. C and N Mineralization of BGB Residues during Reversion
4.2. Modeling C and N Mineralization of BGB Decomposition in the Soil
4.3. RothC Model Optimization of BGB Residues for Multiple PBCs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Glossary
| BGB | Belowground Biomass |
| DEOM | Decomposable Exogenous Organic Matter |
| DOC | Dissolved Organic Carbon |
| DON | Dissolved Organic Nitrogen |
| DPM | Decomposable Plant Material |
| EOM | Exogenous Organic Matter |
| f | Partitioning factor |
| FR | Fine Roots |
| HEOM | Humified Exogenous Organic Matter |
| HUM | Humified Carbon Pool |
| k | Decomposition rate constant |
| LFOM | Light Fraction Organic Matter |
| PBO | Plant Belowground Organs |
| PBCs | Perennial Biomass Crops |
| REOM | Resistant Exogenous Organic Matter |
| RPM | Resistant Plant Material |
| SOC | Soil Organic Carbon |
| SOM | Soil Organic Matter |
| SMB | Soil Microbial Biomass |
| TOC | Total Organic Carbon |
| WSC | Water Soluble Carbon |
| WSN | Water Soluble Nitrogen |
References
- Chimento, C.; Almagro, M.; Amaducci, S. Carbon Sequestration Potential in Perennial Bioenergy Crops: The Importance of Organic Matter Inputs and Its Physical Protection. GCB Bioenergy 2016, 8, 111–121. [Google Scholar] [CrossRef]
- Martani, E.; Ferrarini, A.; Serra, P.; Pilla, M.; Marcone, A.; Amaducci, S. Belowground Biomass C Outweighs Soil Organic C of Perennial Energy Crops: Insights from a Long-term Multispecies Trial. GCB Bioenergy 2020, 13, 1–14. [Google Scholar] [CrossRef]
- Ferrarini, A.; Serra, P.; Almagro, M.; Trevisan, M.; Amaducci, S. Multiple Ecosystem Services Provision and Biomass Logistics Management in Bioenergy Buffers: A State-of-the-Art Review. Renew. Sustain. Energy Rev. 2017, 73, 277–290. [Google Scholar] [CrossRef]
- Ferrarini, A.; Fornasier, F.; Serra, P.; Ferrari, F.; Trevisan, M.; Amaducci, S. Impacts of Willow and Miscanthus Bioenergy Buffers on Biogeochemical N Removal Processes along the Soil-Groundwater Continuum. GCB Bioenergy 2017, 9, 246–261. [Google Scholar] [CrossRef]
- Ferrarini, A.; Martani, E.; Fornasier, F.; Amaducci, S. High C Input by Perennial Energy Crops Boosts Belowground Functioning and Increases Soil Organic P Content. Agric. Ecosyst. Environ. 2020, 308, 107247. [Google Scholar] [CrossRef]
- Lewandowski, I. The Role of Perennial Biom Ass Crops in a Growing Bioeconomy. In Perennial Biomass Crops for a Resource-Constrained World; Springer: Cham, Switzerland, 2016; pp. 1–313. [Google Scholar] [CrossRef]
- Dufossé, K.; Drewer, J.; Gabrielle, B.; Drouet, J.L. Effects of a 20-Year Old Miscanthus × Giganteus Stand and Its Removal on Soil Characteristics and Greenhouse Gas Emissions. Biomass Bioenergy 2014, 69, 198–210. [Google Scholar] [CrossRef]
- Ledo, A.; Smith, P.; Zerihun, A.; Whitaker, J.; Vicente-Vicente, J.L.; Qin, Z.; McNamara, N.P.; Zinn, Y.L.; Llorente, M.; Liebig, M.; et al. Changes in Soil Organic Carbon under Perennial Crops. Glob. Chang. Biol. 2020, 26, 4158–4168. [Google Scholar] [CrossRef]
- Agostini, F.; Gregory, A.S.; Richter, G.M. Carbon Sequestration by Perennial Energy Crops: Is the Jury Still Out? Bioenergy Res. 2015, 8, 1057–1080. [Google Scholar] [CrossRef] [Green Version]
- Toenshoff, C.; Georg, R.; Stuelpnagel, R.; Wachendorf, C. Dynamics of Soil Organic Carbon Fractions One Year after the Re-Conversion of Poplar and Willow Plantations to Arable Use and Perennial Grassland. Agric. Ecosyst. Environ. 2013, 174, 21–27. [Google Scholar] [CrossRef]
- McCalmont, J.P.; Rowe, R.; Elias, D.; Whitaker, J.; McNamara, N.P.; Donnison, I.S. Soil Nitrous Oxide Flux Following Land-Use Reversion from Miscanthus and SRC Willow to Perennial Ryegrass. GCB Bioenergy 2018, 10, 914–929. [Google Scholar] [CrossRef]
- Rowe, R.L.; Keith, A.M.; Elias, D.M.O.; Mcnamara, N.P. Soil Carbon Stock Impacts Following Reversion of Miscanthus × Giganteus and Short Rotation Coppice Willow Commercial Plantations into Arable Cropping. GCB Bioenergy 2020, 12, 680–693. [Google Scholar] [CrossRef]
- Mangold, A.; Lewandowski, I.; Kiesel, A. How Can Miscanthus Fields Be Reintegrated into a Crop Rotation? GCB Bioenergy 2019, 11, 1348–1360. [Google Scholar] [CrossRef]
- Moore, C.E.; Berardi, D.M.; Blanc-Betes, E.; Dracup, E.C.; Egenriether, S.; Gomez-Casanovas, N.; Hartman, M.D.; Hudiburg, T.; Kantola, I.; Masters, M.D.; et al. The Carbon and Nitrogen Cycle Impacts of Reverting Perennial Bioenergy Switchgrass to an Annual Maize Crop Rotation. GCB Bioenergy 2020, 12, 941–954. [Google Scholar] [CrossRef]
- Toenshoff, C.; Stuelpnagel, R.; Joergensen, R.G.; Wachendorf, C. Carbon in Plant Biomass and Soils of Poplar and Willow Plantations—Implications for SOC Distribution in Different Soil Fractions after Re-Conversion to Arable Land. Plant Soil 2013, 367, 407–417. [Google Scholar] [CrossRef]
- Beuschel, R.; Piepho, H.P.; Joergensen, R.G.; Wachendorf, C. Effects of Converting a Temperate Short-Rotation Coppice to a Silvo-Arable Alley Cropping Agroforestry System on Soil Quality Indicators. Agrofor. Syst. 2020, 94, 389–400. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Blagodatskaya, E. Microbial Hotspots and Hot Moments in Soil: Concept & Review. Soil Biol. Biochem. 2015, 83, 184–199. [Google Scholar] [CrossRef]
- Janzen, H.H. The Soil Carbon Dilemma: Shall We Hoard It or Use It? Soil Biol. Biochem. 2006, 38, 419–424. [Google Scholar] [CrossRef]
- Zhu, X.; Jackson, R.D.; DeLucia, E.H.; Tiedje, J.M.; Liang, C. The Soil Microbial Carbon Pump: From Conceptual Insights to Empirical Assessments. Glob. Chang. Biol. 2020, 26, 6032–6039. [Google Scholar] [CrossRef]
- Kramer, S.; Marhan, S.; Ruess, L.; Armbruster, W.; Butenschoen, O.; Haslwimmer, H.; Kuzyakov, Y.; Pausch, J.; Scheunemann, N.; Schoene, J.; et al. Carbon Flow into Microbial and Fungal Biomass as a Basis for the Belowground Food Web of Agroecosystems. Pedobiologia 2012, 55, 111–119. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Soong, J.L.; Horton, A.J.; Campbell, E.E.; Haddix, M.L.; Wall, D.H.; Parton, W.J. Formation of Soil Organic Matter via Biochemical and Physical Pathways of Litter Mass Loss. Nat. Geosci. 2015, 8, 776–779. [Google Scholar] [CrossRef]
- Kallenbach, C.M.; Frey, S.D.; Grandy, A.S. Direct Evidence for Microbial-Derived Soil Organic Matter Formation and Its Ecophysiological Controls. Nat. Commun. 2016, 7, 13630. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Kästner, M.; Joergensen, R.G. Microbial Necromass on the Rise: The Growing Focus on Its Role in Soil Organic Matter Development. Soil Biol. Biochem. 2020, 150, 2018–2021. [Google Scholar] [CrossRef]
- Abiven, S.; Recous, S.; Reyes, V.; Oliver, R. Mineralisation of C and N from Root, Stem and Leaf Residues in Soil and Role of Their Biochemical Quality. Biol. Fertil. Soils 2005, 42, 119–128. [Google Scholar] [CrossRef]
- Toenshoff, C.; Joergensen, R.G.; Stuelpnagel, R.; Wachendorf, C. Initial Decomposition of Post-Harvest Crown and Root Residues of Poplars as Affected by N Availability and Particle Size. Biol. Fertil. Soils 2014, 50, 675–683. [Google Scholar] [CrossRef]
- Mondini, C.; Cayuela, M.L.; Sinicco, T.; Fornasier, F.; Galvez, A.; Sánchez-monedero, M.A. Modification of the RothC Model to Simulate Soil C Mineralization of Exogenous Organic Matter. Biogeosciences 2017, 14, 3253–3274. [Google Scholar] [CrossRef] [Green Version]
- Amaducci, S.; Facciotto, G.; Bergante, S.; Perego, A.; Serra, P.; Ferrarini, A.; Chimento, C. Biomass Production and Energy Balance of Herbaceous and Woody Crops on Marginal Soils in the Po Valley. GCB Bioenergy 2016, 9, 31–45. [Google Scholar] [CrossRef] [Green Version]
- Amaducci, S.; Zatta, A.; Raffanini, M.; Venturi, G. Characterisation of Hemp (Cannabis Sativa L.) Roots under Different Growing Conditions. Plant Soil 2008, 313, 227–235. [Google Scholar] [CrossRef]
- Monti, A.; Zatta, A. Root Distribution and Soil Moisture Retrieval in Perennial and Annual Energy Crops in Northern Italy. Agric. Ecosyst. Environ. 2009, 132, 252–259. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Mondini, C.; Sinicco, T.; Cayuela, M.L.; Sanchez-Monedero, M.A. A Simple Automated System for Measuring Soil Respiration by Gas Chromatography. Talanta 2010, 81, 849–855. [Google Scholar] [CrossRef]
- Walsh, S.; Diamond, D. Non-Linear Curve Fitting Using Microsoft Excel Solver. Talanta 1995, 42, 561–572. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Groempings, U.; Lehrkamp, M. Package ‘Relaimpo’. In Relative Importance of Regressors in Linear Models; R Fundation for Statstical Computing: Vienna, Austria, 2021. [Google Scholar]
- Kong, A.Y.Y.; Six, J.; Bryant, D.C.; Denison, R.F.; van Kessel, C. The Relationship between Carbon Input, Aggregation, and Soil Organic Carbon Stabilization in Sustainable Cropping Systems. Soil Sci. Soc. Am. J. 2005, 69, 1078–1085. [Google Scholar] [CrossRef]
- Martani, E.; Ferrarini, A.; Amaducci, S. Reversion of Perennial Biomass Crops to Save C and N: A Meta-Analysis. Agronomy 2021, 12, 232. [Google Scholar] [CrossRef]
- Wang, H.; Boutton, T.W.; Xu, W.; Hu, G.; Jiang, P.; Bai, E. Quality of Fresh Organic Matter Affects Priming of Soil Organic Matter and Substrate Utilization Patterns of Microbes. Sci. Rep. 2015, 5, 10102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahbaz, M.; Kuzyakov, Y.; Heitkamp, F. Decrease of Soil Organic Matter Stabilization with Increasing Inputs: Mechanisms and Controls. Geoderma 2017, 304, 76–82. [Google Scholar] [CrossRef]
- Wickings, K.; Grandy, A.S.; Reed, S.C.; Cleveland, C.C. The Origin of Litter Chemical Complexity during Decomposition. Ecol. Lett. 2012, 15, 1180–1188. [Google Scholar] [CrossRef]
- Johnson, J.M.-F.; Barbour, N.W.; Weyers, S.L. Chemical Composition of Crop Biomass Impacts Its Decomposition. Soil Sci. Soc. Am. J. 2007, 71, 155–162. [Google Scholar] [CrossRef] [Green Version]
- Chimento, C.; Amaducci, S. Characterization of Fine Root System and Potential Contribution to Soil Organic Carbon of Six Perennial Bioenergy Crops. Biomass Bioenergy 2015, 83, 116–122. [Google Scholar] [CrossRef]
- Walela, C.; Daniel, H.; Wilson, B.; Lockwood, P.; Cowie, A.; Harden, S. The Initial Lignin: Nitrogen Ratio of Litter from above and below Ground Sources Strongly and Negatively Influenced Decay Rates of Slowly Decomposing Litter Carbon Pools. Soil Biol. Biochem. 2014, 77, 268–275. [Google Scholar] [CrossRef]
- Rubino, M.; Dungait, J.A.J.; Evershed, R.P.; Bertolini, T.; de Angelis, P.; D’Onofrio, A.; Lagomarsino, A.; Lubritto, C.; Merola, A.; Terrasi, F. Carbon Input Belowground Is the Major C Flux Contributing to Leaf Litter Mass Loss: Evidences from a 13C Labelled-Leaf Litter Experiment. Soil Biol. Biochem. 2010, 42, 1009–1016. [Google Scholar] [CrossRef]
- Castellano, M.J.; Mueller, K.E.; Olk, D.C.; Sawyer, J.E.; Six, J. Integrating Plant Litter Quality, Soil Organic Matter Stabilization, and the Carbon Saturation Concept. Glob. Chang. Biol. 2015, 21, 3200–3209. [Google Scholar] [CrossRef]
- Incerti, G.; Bonanomi, G.; Giannino, F.; Angela, F.; Piermatteo, D.; Castaldi, S.; De Marco, A.; Fierro, A.; Fioretto, A.; Maggi, O.; et al. Litter Decomposition in Mediterranean Ecosystems: Modelling the Controlling Role of Climatic Conditions and Litter Quality. Appl. Soil Ecol. 2011, 49, 148–157. [Google Scholar] [CrossRef]
- Sall, S.; Bertrand, I.; Chotte, J.L.; Recous, S. Separate Effects of the Biochemical Quality and N Content of Crop Residues on C and N Dynamics in Soil. Biol. Fertil. Soils 2007, 43, 797–804. [Google Scholar] [CrossRef]
- Wachendorf, C.; Stuelpnagel, R.; Wachendorf, M. Influence of Land Use and Tillage Depth on Dynamics of Soil Microbial Properties, Soil Carbon Fractions and Crop Yield after Conversion of Short-Rotation Coppices. Soil Use Manag. 2017, 33, 379–388. [Google Scholar] [CrossRef]
- Marzi, M.; Shahbazi, K.; Kharazi, N.; Rezaei, M. The Influence of Organic Amendment Source on Carbon and Nitrogen Mineralization in Different Soils. J. Soil Sci. Plant Nutr. 2020, 20, 177–191. [Google Scholar] [CrossRef]
- Amougou, N.; Bertrand, I.; Machet, J.-M.; Recous, S. Quality and Decomposition in Soil of Rhizome, Root and Senescent Leaf from Miscanthus x Giganteus, as Affected by Harvest Date and N Fertilization. Plant Soil 2010, 338, 83–97. [Google Scholar] [CrossRef]
- Jesmin, T.; Mitchell, D.T.; Mulvaney, R.L. Short-Term Effect of Nitrogen Fertilization on Carbon Mineralization during Corn Residue Decomposition in Soil. Nitrogen 2021, 2, 444–460. [Google Scholar] [CrossRef]
- Henriksen, T.M.; Breland, T.A. Nitrogen Availability Effects on Carbon Mineralization, Fungal and Bacterial Growth, and Enzyme Activities during Decomposition of Wheat Straw in Soil. Soil Biol. Biochem. 1999, 31, 1121–1134. [Google Scholar] [CrossRef]
- De Graaff, M.-A.; Jastrow, J.D.; Gillette, S.; Johns, A.; Wullschleger, S.D. Differential Priming of Soil Carbon Driven by Soil Depth and Root Impacts on Carbon Availability. Soil Biol. Biochem. 2014, 69, 147–156. [Google Scholar] [CrossRef]
- Rivas, F.A.; Tabatabai, M.A.; Olk, D.C.; Thompson, M.L. Kinetics of Short-Term Carbon Mineralization in Roots of Biofuel Crops in Soils. Biol. Fertil. Soils 2014, 50, 527–535. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Reed, S.C.; Keller, A.B.; Nemergut, D.R.; O’Neill, S.P.; Ostertag, R.; Vitousek, P.M. Litter Quality versus Soil Microbial Community Controls over Decomposition: A Quantitative Analysis. Oecologia 2014, 174, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The Microbial Efficiency-Matrix Stabilization (MEMS) Framework Integrates Plant Litter Decomposition with Soil Organic Matter Stabilization: Do Labile Plant Inputs Form Stable Soil Organic Matter? Glob. Chang. Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poeplau, C.; Don, A.A. Soil Carbon Changes under Miscanthus Driven by C4 Accumulation and C3 Decompostion—Toward a Default Sequestration Function. GCB Bioenergy 2013, 6, 327–338. [Google Scholar] [CrossRef]
- Zatta, A.; Clifton-Brown, J.; Robson, P.; Hastings, A.; Monti, A. Land Use Change from C3 Grassland to C4 Miscanthus: Effects on Soil Carbon Content and Estimated Mitigation Benefit after Six Years. GCB Bioenergy 2014, 6, 360–370. [Google Scholar] [CrossRef] [Green Version]
- Dondini, M.; Hastings, A.; Saiz, G.; Jones, M.B.; Smith, P. The Potential of Miscanthus to Sequester Carbon in Soils: Comparing Field Measurements in Carlow, Ireland to Model Predictions. GCB Bioenergy 2009, 1, 413–425. [Google Scholar] [CrossRef]
- Hastings, A.; Clifton-Brown, J.; Wattenbach, M.; Mitchell, C.P.; Smith, P. The Development of MISCANFOR, a New Miscanthus Crop Growth Model: Towards More Robust Yield Predictions under Different Climatic and Soil Conditions. GCB Bioenergy 2009, 1, 154–170. [Google Scholar] [CrossRef]
- Clifton-Brown, J.; Schwarz, K.U.; Hastings, A. History of the Development of Miscanthus as a Bioenergy Crop: From Small Beginnings to Potential Realisation. Biol. Environ. 2015, 115B, 1–13. [Google Scholar] [CrossRef]
- Gabrielle, B.; Da-Silveira, J.; Houot, S.; Michelin, J. Field-Scale Modelling of Carbon and Nitrogen Dynamics in Soils Amended with Urban Waste Composts. Agric. Ecosyst. Environ. 2005, 110, 289–299. [Google Scholar] [CrossRef]
- Kaboré, W.T.; Pansu, M.; Hien, E.; Houot, S.; Zombré, N.P.; Masse, D. Usefulness of TAO Model to Predict and Manage the Transformation in Soil of Carbon and Nitrogen Forms from West-Africa Urban Solid Wastes. Waste Manag. 2011, 31, 154–167. [Google Scholar] [CrossRef]
- Noirot-Cosson, P.E.; Vaudour, E.; Gilliot, J.M.; Gabrielle, B.; Houot, S. Modelling the Long-Term Effect of Urban Waste Compost Applications on Carbon and Nitrogen Dynamics in Temperate Cropland. Soil Biol. Biochem. 2016, 94, 138–153. [Google Scholar] [CrossRef]
- Vidal-Beaudet, L.; Grosbellet, C.; Forget-Caubel, V.; Charpentier, S. Modelling Long-Term Carbon Dynamics in Soils Reconstituted with Large Quantities of Organic Matter. Eur. J. Soil Sci. 2012, 63, 787–797. [Google Scholar] [CrossRef]
- Robertson, A.; Smith, P.; Davies, C.; Bottoms, E.; Mcnamara, N. Longevity of Contributions to SOC Stocks from Roots and Aboveground Plant Litter below a Miscanthus Plantation. In Proceedings of the EGU General Assembly 2013, Vienna, Austria, 7–12 April 2013; Volume 15, pp. 1920–2013. [Google Scholar]
- Robertson, A.D.; Davies, C.A.; Smith, P.; Dondini, M.; Mcnamara, N.P. Modelling the Carbon Cycle of Miscanthus Plantations: Existing Models and the Potential for Their Improvement. GCB Bioenergy 2015, 7, 405–421. [Google Scholar] [CrossRef] [Green Version]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The Importance of Anabolism in Microbial Control over Soil Carbon Storage. Nat. Microbiol. 2017, 2, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Singh, B.P.; Cowie, A.; Wang, W.; Arachchi, M.H.; Wang, H.; Tavakkoli, E. Balancing Nutrient Stoichiometry Facilitates the Fate of Wheat Residue-carbon in Physically Defined Soil Organic Matter Fractions. Geoderma 2019, 354, 113883. [Google Scholar] [CrossRef]
- Kirkby, C.A.; Richardson, A.E.; Wade, L.J.; Conyers, M.; Kirkegaard, J.A. Inorganic Nutrients Increase Humification Efficiency and C-Sequestration in an Annually Cropped Soil. PLoS ONE 2016, 11, e0153698. [Google Scholar] [CrossRef] [Green Version]
- Kirkby, C.A.; Richardson, A.E.; Wade, L.J.; Batten, G.D.; Blanchard, C.; Kirkegaard, J.A. Carbon-Nutrient Stoichiometry to Increase Soil Carbon Sequestration. Soil Biol. Biochem. 2013, 60, 77–86. [Google Scholar] [CrossRef]
- Zhang, Y.; Lavallee, J.M.; Robertson, A.D.; Even, R.; Ogle, S.M.; Paustian, K.; Cotrufo, M.F. Simulating Measurable Ecosystem Carbon and Nitrogen Dynamics with the Mechanistically Defined MEMS 2.0 Model. Biogeosciences 2021, 18, 3147–3171. [Google Scholar] [CrossRef]
- Kyker-Snowman, E.; Wieder, W.; Frey, S.; Grandy, A.S. Stoichiometrically Coupled Carbon and Nitrogen Cycling in the MIcrobial-MIneral Carbon Stabilization Model (MIMICS-CN). Geosci. Model Dev. Discuss. 2019, 13, 1–32. [Google Scholar] [CrossRef]




| Site | Land Use | Sand | Silt | Clay | pH | CaCO3 | SOC | STN | C/N |
|---|---|---|---|---|---|---|---|---|---|
| (%) | (%) | (%) | (%) | (g kg−1) | (g kg−1) | ||||
| Gariga | Arable | 11 | 68 | 21 | 6.9 | Negligible | 7.5 | 0.98 | 7.6 |
| EOM Group | Crop | EOM Type | pH | OM | TOC | NTOT | TOC/NTOT | WSC | WSN | NH4+ | NO3− |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (%) | (%) | (%) | (g kg−1) | (g kg−1) | (mg kg−1) | (mg kg−1) | |||||
| Woody PBCs | Black locust | Stump | 5.37 | 86.0 | 44 | 1.9 | 23.1 | 42.8 | 5.4 | 20.20 | 45.82 |
| Poplar | 5.29 | 89.7 | 44 | 0.7 | 62.8 | 29.2 | 1.6 | 6.79 | 45.90 | ||
| Willow | 4.92 | 83.2 | 44 | 0.4 | 110.0 | 31.5 | 0.7 | 9.35 | 20.45 | ||
| Herbaceous PBCs | Miscanthus | Rhizome | 4.94 | 74.8 | 44 | 0.9 | 48.9 | 32.9 | 1.8 | 7.78 | 35.10 |
| Switchgrass | 5.08 | 78.4 | 40 | 0.5 | 80.0 | 27.4 | 0.9 | 6.24 | 38.38 | ||
| Giant reed | 4.17 | 80.3 | 44 | 0.8 | 55.0 | 54.8 | 1.3 | 8.54 | 10.23 | ||
| Woody PBCs | Black locust | Fine roots | 5.00 | 69.9 | 29 | 2.1 | 13.8 | 12.4 | 2.6 | 123.67 | 78.88 |
| Poplar | 4.94 | 83.2 | 35 | 1.2 | 16.7 | 13.1 | 1.1 | 85.77 | 202.34 | ||
| Willow | 4.19 | 69.9 | 31 | 1.0 | 31.0 | 13.2 | 0.7 | 60.88 | 184.63 | ||
| Herbaceous PBCs | Miscanthus | Fine roots | 5.40 | 68.2 | 32 | 0.9 | 35.6 | 9.0 | 0.7 | 28.14 | 141.06 |
| Switchgrass | 4.86 | 68.4 | 36 | 0.7 | 51.4 | 26.1 | 0.6 | 11.97 | 38.43 | ||
| Giant reed | 5.47 | 70.3 | 30 | 0.7 | 42.9 | 9.5 | 0.8 | 26.33 | 166.35 |
| EOM Group | Crop | EOM Type | NDF | ADF | ADL | Hemicellulose | Cellulose | Lignin | Ash | Soluble | LCI | Lignin/N |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (%) | (%) | (%) | (%) | (%) | ||||||||
| Woody PBCs | Black locust | Stump | 62.87 | 46.29 | 14.30 | 16.58 | 31.99 | 11.71 | 2.60 | 37.13 | 0.27 | 6.2 |
| Poplar | 74.41 | 67.71 | 28.49 | 6.70 | 39.21 | 25.43 | 3.07 | 25.59 | 0.39 | 36.3 | ||
| Willow | 67.76 | 66.03 | 29.43 | 1.73 | 36.60 | 26.22 | 3.22 | 32.24 | 0.42 | 65.5 | ||
| Herbaceous PBCs | Miscanthus | Rhizome | 68.02 | 47.38 | 21.06 | 20.64 | 26.32 | 13.99 | 7.07 | 31.98 | 0.35 | 15.5 |
| Switchgrass | 77.36 | 53.26 | 21.17 | 24.10 | 32.09 | 18.96 | 2.20 | 22.64 | 0.41 | 40.1 | ||
| Giant reed | 69.97 | 52.08 | 23.03 | 17.90 | 29.04 | 20.03 | 3.00 | 30.03 | 0.37 | 23.7 | ||
| Woody PBCs | Black locust | Fine roots | 70.85 | 42.03 | 28.08 | 7.73 | 16.77 | 20.94 | 16.77 | 37.78 | 0.56 | 8.4 |
| Poplar | 62.22 | 54.49 | 37.71 | 28.82 | 13.95 | 17.63 | 10.45 | 29.15 | 0.56 | 17.5 | ||
| Willow | 57.03 | 56.32 | 31.42 | 0.72 | 24.90 | 19.60 | 11.82 | 42.97 | 0.44 | 19.6 | ||
| Herbaceous PECb | Miscanthus | Fine roots | 67.99 | 53.80 | 30.05 | 14.19 | 23.75 | 13.99 | 16.05 | 32.01 | 0.37 | 15.5 |
| Switchgrass | 64.79 | 45.46 | 20.14 | 19.33 | 25.32 | 12.77 | 7.37 | 35.21 | 0.34 | 18.2 | ||
| Giant reed | 69.23 | 49.38 | 26.18 | 19.85 | 23.20 | 15.54 | 10.64 | 30.77 | 0.40 | 22.2 |
| EOM Type | C and N Mineralization Variables | Residual SE | Adjusted R2 | F-Statistic | p-Value | Significant Predictors (% Relative Importance) * |
|---|---|---|---|---|---|---|
| Plant belowgroundorgans | C mineralization (cumulative C-CO2) | 44.3 | 0.98 | 72.81 | 0.013 | LCI index (51%) a, C added (34%) a, Lignin (15%) b |
| C mineralization (% of added C-CO2) | 0.37 | 0.99 | 825.8 | 0.001 | LCI index (49%) a, DEOM/REOM (46%) a, Hemicellulose (6%) b | |
| ΔDOC (μg C g−1) | 7.5 | 0.65 | 10.08 | 0.033 | C added (100%) a | |
| ΔDON (μg N g−1) | 9.4 | 0.55 | 6.78 | 0.045 | N content (48%) a, DEOM/REOM (37%) a, Cellulose (15%) b | |
| ΔSOM (μg SOM g−1) | 0.09 | 0.92 | 29.78 | 0.011 | C added (83%) a, Ash (16%) b | |
| Fine roots | C mineralization (cumulative C-CO2) | 9.44 | 0.97 | 53.4 | 0.018 | DEOM/REOM (42%) a, DEOM (40%) a, C/N ratio (17%) b |
| C mineralization (% of added C) | 2.01 | 0.87 | 12.6 | 0.05 | DEOM/REOM (44%) a, DEOM (42%) a, C/N ratio (15%) b | |
| ΔDOC (μg C g−1) | 1.07 | 0.94 | 25.6 | 0.038 | Lignin/N ratio (53%) a, Soluble (32%) a, Lignin (17%) b | |
| ΔDON (μg N g−1) | 0.07 | 1 | 9139 | <0.001 | C/N ratio (52%) a, Cellulose (26%) b, Ash (22%) b | |
| ΔSOM (μg SOM g−1) | 0.01 | 0.94 | 37.2 | 0.008 | C/N ratio (87%) a, Cellulose (13%) b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrarini, A.; Martani, E.; Mondini, C.; Fornasier, F.; Amaducci, S. Short-Term Mineralization of Belowground Biomass of Perennial Biomass Crops after Reversion to Arable Land. Agronomy 2022, 12, 485. https://doi.org/10.3390/agronomy12020485
Ferrarini A, Martani E, Mondini C, Fornasier F, Amaducci S. Short-Term Mineralization of Belowground Biomass of Perennial Biomass Crops after Reversion to Arable Land. Agronomy. 2022; 12(2):485. https://doi.org/10.3390/agronomy12020485
Chicago/Turabian StyleFerrarini, Andrea, Enrico Martani, Claudio Mondini, Flavio Fornasier, and Stefano Amaducci. 2022. "Short-Term Mineralization of Belowground Biomass of Perennial Biomass Crops after Reversion to Arable Land" Agronomy 12, no. 2: 485. https://doi.org/10.3390/agronomy12020485