1. Introduction
The Australian tomato industry has two distinct markets worth a combined AUD 645 million in the 2018–19 financial year [
1]. The processing market consists almost entirely of field-grown tomatoes, whereas a large proportion of the fresh-market produce is grown in protected-cropping facilities [
2]. Protected cropping presents unique opportunities to improve production efficiencies, although it can be accompanied by increased labour requirements [
3]. Tomato fruit production is heavily reliant on effective pollination services as the number of seeds within the fruit, produced through pollination, correlates with fruit size and weight [
4]. Some high-tech protected-cropping facilities (e.g., glasshouses and environmentally controlled polytunnels) in Australia can exclude natural pollination vectors such as wind or insects, and so artificial floral stimulation is needed to achieve higher production [
5]. It was estimated by that hand pollination costs the protected tomato growing industry approximately AUD 25,000/ha/year [
6]. These costs represent a significant cost to tomato production in Australia and are detailed in this review.
Environmental conditions have been shown to affect plant physiology and the ability of the flower to be pollinated [
7]. Extreme high temperatures (>34 °C) impact microsporogenesis, leading to less pollen with lower viability [
8]. Heat stress also limits sucrose translocation throughout the plant, reducing assimilate allocation to the fruit [
8]. Conversely, low temperatures <17 °C prevent floral anther fusion and change responses to mechanical vibration, as well as retarding pollen tube growth [
8]. A higher relative humidity affects pollen adhesion, both to itself and the stigma surface [
9]. While relative humidity and temperature can be controlled within most high-tech glasshouse facilities, these parameters can be difficult to control in low-tech protected-cropping facilities (e.g., polytunnels). The environmental influence on tomato flower pollination is a major factor affecting the success of pollination technology and ultimately the productivity of protected cropping facilities.
Tomatoes have unique pollen restriction adaptations that match the abundance and efficiency of natural pollinators in order to maximise the probability of successful reproduction [
10]. Their poricidal anthers require vibration to impart inertia on the pollen grains within the anther locule so that they can overcome adhesion forces and exit through the apical pore [
11]. Only specific stimulations can cause this reaction, which serves to distinguish between insects so that only the most efficient pollinators can extract pollen [
12]. This suggests that vibrational stimulation beyond that capable by insect morphologies may elicit greater pollen expulsion. However, the exact pollen expulsion mechanism is still poorly understood with a combination of centrifugal, kinetic and electrostatic forces hypothesised to be responsible [
13]. Tomato flower development and fruit quality production are discussed in this review in the context of the pollinator.
Tomato floral pollination by bumblebees (
Bombus terrestris/impatiens) resulted in a 74% increase in fruit weight compared with non-pollinated controls, with artificially pollinated tomatoes only achieving a 28% increase [
14]. Bumblebees offer the greatest benefit–cost ratio in terms of pollination services as a single bee can pollinate up to 500 plants per day [
15,
16]. Bees may also be able to make better use of optimal pollination windows as reared colonies are available to pollinate over an entire 24 h period, whereas human pollinators are restricted to certain working hours, and most commonly only operate three times per week [
17]. Australian biosecurity laws, however, prohibit the importation of commercially reared bumblebees for fear of contribution to the spread and pollination of significant weeds that already cost AUD 4.9 billion to the economy per annum [
5,
18]. As a consequence, there has been an increasing interest in the use of native solitary bees, such as
Amegilla zonata (the native blue-banded bee), as commercial pollinators [
19]. Their pollination capacity has been shown to be comparable to that of the bumblebee, although mass rearing and adaptation to protected-cropping environments have been noted as significant uptake challenges [
14]. Alternative insect pollination methods and their associated ecosystem benefits will be reviewed from a sustainability perspective relevant to the Australian tomato industry.
Insect pollination of the tomato flower involves physical and acoustic vibrations [
11]. Vibrations of the same frequency are not necessarily equal as they can vary in energy output with amplitudes of displacement, acceleration and velocity [
20]. This variation is related to the pollen extraction efficiency of different insect species through their capacity to generate vibrations. The role of acoustic vibrations during sonication is poorly understood, and acoustics could be a by-product of physical vibration [
21], even though acoustic stimulation alone can improve tomato pollination [
11]. These contrasting notions highlight the ambiguity around energy transmission from the pollinator to the flower. Vibrations are not equally transmitted through floral structures [
22], which means that the vibration must be applied around the natural frequency of the flower for a significant acceleration response to be experienced [
11]. Non-resonant frequencies are dampened by the floral structures causing the pollen to not be ejected [
23]. Bees have been shown to overcome this selectivity by extending the duration of the sonication event [
24]. The magnitude of energy transmission is dependent on the physical properties of the floral structure, which are highly correlated to turgor pressure [
11]. It is unknown whether the magnitude of the stimulus must change to suit the inherent variability in shape and structural properties of flowers from different cultivars or even individual plants. The mechanisms of buzz- and vibration-induced pollination detailed in this review enable a deeper understanding of how artificial technologies can be utilised within the tomato copping industry.
Artificial mechanical strategies to control precision pollination are required to improve industry productivity. Vibrating probes are used to stimulate pollen expulsion and have been shown to increase glasshouse fruit yield by 75% [
20,
25]. Air jet pulses are also being investigated to improve pollination but in the past were found to be less effective than the vibrating wand [
26]. Contactless, acoustic excitation technology that simulate the vibration induced by a bee could provide an alternative. The cost of artificial pollination is a concern to the Australian tomato industry and advanced robotic technologies that automate pollination services have begun to emerge. While the lack of bumble bees for pollination is unique to Australia as the majority of foreign tomato-growing nations have endemic
Bombus spp., robotic non-contact techniques have superior potential to reduce overall production costs [
27]. This review investigates tomato floral pollination mechanics and artificial pollination methods suitable for maintaining tomato production. A deeper knowledge of the key pathways to simulate buzz pollination technology and optimise tomato floral pollination within a specific growth environment can direct future research towards developing precision pollination strategies.
2. Tomato Production in Australia
The two main markets for tomatoes in Australia, fresh tomatoes and processed tomato products, have unique management practices, growing conditions, and postharvest operations to ensure the product meets consumer requirements [
28]. Australia produced ~329,000 t of tomatoes from 4278 ha in 2018–19. About half (51%) of crop value is in the processing industry; the remainder of market value is in the production and postharvest management of fresh produce [
1]. The processing market is supplied almost entirely by field tomatoes that are predominantly grown in the Goulburn Valley region of Victoria and Murrumbidgee Irrigation Area of New South Wales [
29]. Figures from the 2018 Processing Industry report showed that the industry is supplied by eleven major growers and dominated by two major processors, Kagome and SPC Ardmona [
28]. Seasonality in production requires the importation of ~127,000 t (AUD 161 million) of tomato products, mainly from Italy, China, and the USA, to meet Australian consumer demand (
Figure 1). In 2017, Australia exported ~8000 t of tomato products, predominantly to New Zealand, Japan and Vietnam (
Figure 1). The most recent agricultural commodity reports from the Australian Bureau of Statistics, showed that ~1800 ha were planted in 2018–19, which produced ~169,000 t of tomatoes for processing (averaging 92.3 t/ha) with farmers in Victoria growing the majority (~163,000 t) on ~1600 ha of land [
1].
The fresh fruit market was supplied by ~2400 ha of outdoor and undercover production systems that produced ~159,000 t of tomatoes in 2018–19 [
1]. Most of the outdoor tomatoes were grown in Queensland around Bowen, Bundaberg, and the Darling Downs, with, 44,175 t of fresh tomatoes being grown on 1390 ha [
1]. Economies of scale are particularly important for undercover production systems [
31], leading to 61.5% of undercover tomatoes being produced by only three large suppliers—Costa, Flavorite and Perfection Fresh [
3]. Of these three suppliers, half of their infrastructure is devoted to tomatoes [
32]. Nationally, ~377 ha of protected-cropping infrastructure (that shelter crops and enable modification of growth conditions as well as protect from pests and adverse weather conditions) produced ~93,400 t of tomatoes in 2018–19. Protected cropping has been expanding at 60% p.a. since 2015, which exceeds the global expansion rate of 5% annually [
2].
Production increased by 205% between the 2008/9 and 2014/15 seasons due to the construction of larger, more technical facilities by corporate growers such as Costa [
3]. Geographical location of the facilities prioritises transport linkages to market destinations and distributors, proximity to resources and labour, with climate as a secondary consideration. Availability and the cost of labour is seen as the primary constraint to future expansion. An estimated 11,293 h/ha of labour is required in the greenhouse for tomato production (pruning, pollinating, and picking), which equates to ~7 workers/ha. High employee retention is difficult because of the seasonality of foreign workers and the use of unskilled labour. The greenhouse production efficiency associated with precise control over the environmental conditions and nutrient treatment are unquestionable. The productivity of greenhouse production has been shown to be significantly greater than field production revealing a favourable future for this industry (
Table 1) [
33].
3. Tomato Flower Development and Fruit Production
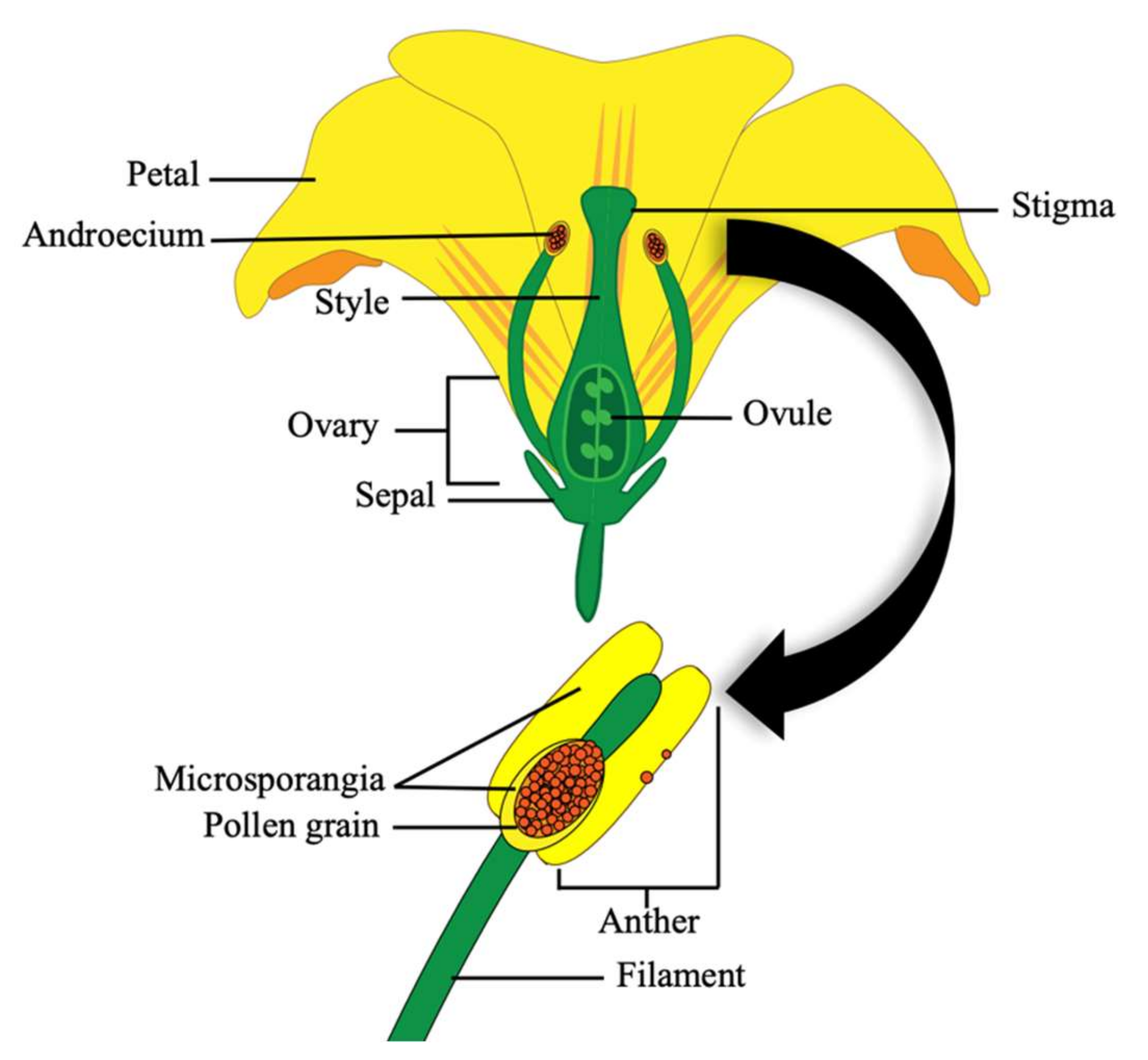
Tomato fruit production is heavily reliant on effective pollination of tomato flowers. The tomato flower is comprised of four concentric whorls, with the outermost being the calyx (sepals), followed by the corolla (petals), then the androecium (stamens) and the innermost gynoecium (pistil and carpels) (
Figure 2) [
34]. The inflorescence meristem has a cymose growth pattern, with laterally alternating floral meristems, that produce a zig zag shape along the truss [
35]. Floral morphology may impact pollen deposition pathways. Herkogamy is the separation in space of the anther and stigma of the flower, which may encourage cross pollination [
36]. If the stigma extends beyond the anthers (approach herkogamy) cross pollination may be favoured as the pollinator (e.g., a bee) contacts the stigma before collecting pollen from that flower on its body [
37,
38]. Herkogamy may become prominent when the availability of pollinators is scarce [
36]. Herkogamy of the tomato flower favours cross-pollination, or outcrossing [
39], whereas a smaller separation between the anthers and the stigma would increase the chance of self-fertilisation and therefore the risk of inbreeding depression and increase the prevalence of deleterious mutations in breeding populations [
40]. Commercial tomato plants are grown from new seed each year, which means self- or cross-pollination is not important to the producer, but rather an area of interest to tomato breeders when developing new cultivars [
41].
Pollination does not usually occur until the floral petals emerge fully developed (
Figure 3A). Pollination is part of the flowering process whereby pollen grains are released from the microsporangia within the dehisced poricidal anther and fall onto the receptive stigma surface at the end of the style (
Figure 2) [
42].
Fertilization occurs 24–48 h after pollination when the flower is mature (
Figure 3A; stages 8–9) [
42]. Dehiscence is the mechanical rupture of the anther’s epidermal cells after the anthers reach maturity. This occurs 24–48 h after the opening of the corolla (petals). Vibrating the flower causes movement of the epidermal cells of the anther and this gives the pollen grains enough inertia to overcome their adhesion forces, causing them to be shed [
11]. Tomatoes are receptive to their own pollen, making them self-fertile, but the stigma is often receptive for several days prior to anther dehiscence from the same flower, which allows the opportunity for cross-pollination from other flowers on the same and/or neighbouring tomato plants [
43]. Once the pollen sticks to the stigma, a pollen tube grows down the style into the ovary (
Figure 2), where ovules are fertilised to become seeds and the ovary transitions into a fruit (
Figure 3B) [
42]. The flesh of the fruit is derived from, and genetically identical to the female structures, whilst the seeds are a hybrid of the parental genomes [
44].
Extrinsic factors, such as plant density, floral morphology, and pollinator abundance, as well as behaviour, create sufficient variation that can dilute outcrossing ability in natural populations [
36]. In controlled environments, herkogamy could significantly influence the success of pollination. Long styles (e.g., due to low solar irradiance and high fertility) increase pollen transfer between separate individual flowers, whereas short styles (high solar irradiance) increase the chances of self-fertilisation [
45].
4. Environmental Influences on Pollination
Plant responses to environmental factors are intrinsically linked, which means multiple physiological pathways controlling pollination may be affected simultaneously (
Figure 4) [
7]. The myriad of processes adversely affected by extreme temperatures include: meiotic division in the androecium (anthers and filaments) and gynoecium (style and ovaries), stylar exertion, morphological development in the anthers, pollen germination and tube growth, fertilization, as well as pollen and ovule viability [
46]. Increasing temperatures above 27 °C are associated with fruit yield losses and a rise in parthenocarpy, with greater consequences seen for pollen development than the ovules [
46]. Heat stress is less deleterious to post-fertilization processes [
46]. There can be adverse effects on pollen production, dehiscence and ovule viability when night temperatures exceed the range of 17–24 °C, although there is a degree of cultivar variability that remains under explored [
47].
Extremely high temperatures (>34 °C) affect microsporogenesis, which reduces pollen number and decreases viability [
8,
47]. The androecium is most sensitive to heat stress 7–15 d before anthesis as this coincides with tissue differentiation in the anther, and meiotic division [
48]. Abnormalities in morphological development of the anther tissues affect pollen rupture characteristics, which often reduces the amount of pollen that is released, and subsequent fruit set [
8,
48]. Extremely high temperatures may also impair gametogenesis, which tends to be less sensitive than microsporogenesis [
8].
Moderately high temperature (>27 °C) does not necessarily reduce meiotic divisions, however pollen grains are less viable from a reduction in carbohydrate supply during microsporogenesis [
8]. Under more optimal conditions, dehiscence is initiated by the rupture of endothelium cells within the anther, during the rapid expansion of the maturing pollen grain. Moderately high temperatures that produce irregularly shaped pollen, attenuated by low carbohydrate availability, will result in dehiscence failure. Additionally, under these conditions, stylar carbohydrate provision is insufficient to sustain adequate pollen tube growth [
8].
Lower temperatures (<17 °C) were found to increase the number of floral organs with multiplication and fissure of larger primordia [
8]. Anther fusion was also prevented at lower temperatures, which attenuated the structure of the staminal cone, limiting dehiscence and pollen release. An increase in heterostyly (a polymorphism in style length between flowers of the same species), and retarded growth of pollen tubes, was associated with reduced pollination outcomes. Generally, female organ viability was not impaired by extreme low temperatures to the same extent as the male organs [
8].
Low light is associated with low auxin production, which retards floral structure development and creates high rates of flower abscission [
49]. Light intensity and temperature determine the availability of assimilates, which impact pollen viability and sex organ development [
8,
50,
51]. Additionally, low light and moderately high temperatures are associated with heterostyly, which impacts the rate of self-fertilization [
7,
51].
Pollen production increases with soil moisture in water-scarce environments, as the number of pollen mother cells is pre-determined during primordia development [
52]. Relative humidity (RH) is pertinent to pollen release and stigma receptivity [
8]. Acutely high RH (>70%) was shown to increase pollen adhesion within the anthers, which reduced pollen release. When the RH >70% was sustained for >24 h flower abscission was enhanced, while higher night humidity only limited flower opening [
8]. When the RH was <70% coupled with temperatures outside the optimal range of 17–24 °C pollen adhesion to the stigmatic surface was reduced. Moreover, low RH and high temperatures cause stigmatic drying, which impairs pollen receptivity [
8,
9]. Extreme temperature events negatively impact the formation of gametes and tissue development of the sexual organs, which is detrimental to the process of pollination. Similarly, fluctuating light and moisture outside of the optimum range was shown to adversely affect floral morphology and fertilisation events. Improved pollination (from bees or artificially) and CO
2 enrichment are methods that can help ameliorate unfavourable climatic conditions [
17].
5. Natural Pollination Methods and Ecosystem Benefits
Ecosystem services are the benefits human beings derive from ecological interactions—such as in bee-mediated pollination [
53]. Tomato pollination influences crop yield, and so is a critical process ecosystem benefit that requires further understanding [
20]. Animal pollination (zoophily) is responsible for 35% of total global production of pollinated crops, whilst 60% of global production comes from wind pollination (anemophily) (5% is unknown) [
53]. Although zoophilous crops cover less cultivated area and produce less total volume than anemophilous crops, they produce greater revenue per unit area [
54]. Pollination benefits to yield are also impacted by agronomic and environmental conditions that modulate plant growth [
55]. Tamburini and colleagues showed that assisted pollination can help perennial and indeterminate plants (some tomato varieties are indeterminate) maintain yields under abiotic stress. They were able to demonstrate that perennial and indeterminate plants could adequately re-allocate substrates to maintain reproduction when they were stressed, which impacted management decisions on pollination services [
56].
Tomatoes have hermaphroditic flowers in which pollen acquisition acts as an attractant for insect pollinators [
14]. Self-fertilisation of tomatoes is improved by facultative pollination from wind or insect visitation that stimulates pollen release from their poricidal anthers [
14,
56]. That is, anemophily can increase the success of pollination events in the absence of, or with the variability of, biotic vectors such as bees [
57]. It is passive and strongly affected by external conditions such as moisture and wind speed and is typical of open and dry environments. Grasses have the typical morphology of anemophilous plants with their reduced perianth and dry pollen. Field tomatoes can be anemophilous under suitable conditions [
57].
Biotic pollinators vastly increase the success of pollination outcomes for tomatoes, although a decline in land suitability, and subsequent insect biodiversity, from agricultural intensification has caused a decline in pollinator availability [
53]. Economic valuation of interspecific pollinator interactions shows that conservation of wild bees buffers the detrimental effects of commercial pollinator scarcity (e.g., the honeybee) [
58]. Strategies that conserve nesting habitats in close proximity to tomato field crops are important as a gradient from maximum pollination to minimum pollination can occur over less than a 1 km range [
58].
Effective pollen foraging by insects reduces pollen availability for fertilization [
12]. Thus the pollen restriction mechanisms of tomato enable the plant to match the abundance and pollen transfer efficiency of the endemic pollinators [
10]. Floral mechanisms that allow restriction can be grouped as (a) packaging—that periodises pollen production so that only a proportion of its total reserve is available to pollinators at any one time; and (b) dispensing—that controls the proportion of pollen a pollinator is able to remove [
12].
Many bee species are voracious foragers, which diminishes the benefits to plant reproduction upon each subsequent visit [
12]. Bees can determine pollen availability from each flower, allowing them to optimise their grooming habits to maximise acquisition. Poricidal anthers (those that open by a terminal pore, as in tomato) do not expel pollen from incidental contact, which would be wasteful, but require the ‘buzz’ of correct sonication for pollen release [
12]. In essence, this morphology filters the taxa able to extract pollen through vibrations, as a means of ensuring its pollinators are effective at transmitting its gametes to the appropriate destinations [
59]. When visits decrease, the pollen released per extraction is increased, as a compensatory mechanism [
12]. Pollen reward is largest upon the first visit, but multiple or extended visits are necessary for complete extraction. Because of this, bees spend more time on newly opened flowers [
59].
The amount of pollen released can increase with higher vibration velocities when artificially simulated [
59]. In terms of the poricidal anther dispensing mechanisms, the vibration frequencies used by bees are likely to expel less pollen compared to higher optimal mechanical frequencies that are unattainable by a bee. Field tomatoes are generally more sensitive to wind stimulation, which allows for adequate pollination outcomes [
14]. Some studies have shown improved fruit set and quality (e.g., size, weight and seed set) from artificially pollinated tomatoes compared with field controls, which may account for enhanced production/m
2 in controlled-environment horticulture [
56,
60]. Understanding the natural pollination mechanisms of the tomato plant can allow management decisions to support these processes and inspire novel concepts pertaining to these mechanisms that may enhance control and automation in protected settings.
6. Sustainability, Diversity and Utilization of Native Bee Pollinators
Field tomatoes are predominantly wind pollinated so it is extremely challenging to estimate the value of bee pollination to tomato production [
61]. Multiple studies have shown bee pollinators to be of value to field crops, such as [
61], whereas [
62] found they were of little benefit. More recent studies reported that field tomatoes pollinated by the native blue-banded bee (were 21% larger than similar wind-pollinated tomatoes [
56]. This study emphasises the importance of maintaining abundant and diverse bee communities to offer their pollination services to agriculture.
Several farming practices are harmful to local apifauna [
63]. Global bee populations have been in decline as a result of reckless pesticide use, habitat loss and fragmentation, and the spread of Varroa mite (
Varroa destructor) with reared pollinators [
64]. Climate modelling studies have shown that, due to harmful farming practices, the bee habitats of Brazil and the main Brazilian tomato production areas may no longer overlap in the future, reducing production [
65]. Bee diversity is a better predictor than bee abundance for crop production, as pollination efficiency is improved by species interaction [
63]. Wild bee populations may vary dramatically from year to year, so the design of management plans must focus on maintaining diversity [
61].
The lack of pollen vectors indoors necessitates intervention to improve pollination outcomes [
5]. Methods that are currently employed are trellis tapping, wand pollination, air gun pollination and the use of reared pollinators [
5]. Pollination by bumblebees (
Bombus terrestris or
B. impatiens) can increase fruit weight by 74% compared with non-pollinated greenhouse tomatoes [
14], which is why 95% of commercially reared bumblebees are used in the greenhouse tomato industry [
66]. Bumblebees offer the greatest cost benefit ratio when compared with other pollination methods [
5,
67]. A single bee is capable of pollinating up to 500 tomato plants (or 250 m
2) per day [
16]. This means that in a greenhouse, about 10–15 colonies/ha would be needed, depending on flower density [
16,
67]. Colony distribution is important because pollen collection by bees is greatest within 50 m of the colony and over-visitation can cause malformed fruit [
16,
67].
Studies have shown that bee-pollinated tomatoes perform better across a variety of consumer market traits and are produced at a lower cost compared to mechanically pollinated fruits [
67,
68]. Despite this, the indoor tomato industry in Australia predominantly uses hand pollination as bumblebees are prohibited [
14]. Importation of commercially reared bumblebees is not permitted by the Australian Government (Environment Protection and Biodiversity Conservation Act 1999) due to a range of potential detrimental effects of their pollination activities on natural and agricultural ecosystems [
5].
Native solitary bees are being investigated for commercial rearing and have been shown to sonicate comparably to bumblebees [
5]. Identified native alternatives,
Amegilla chlorocyanea and
A. zonata, have been shown to increase tomato production ~20% above that achieved by artificial pollination [
19,
56]. A stocking rate of about 280 nesting females/ha would be required, which is lower than for bumblebees. However, the stocking rate varies with the tomato cultivar [
19]. Currently, utilization of native bees is impractical due to the difficulties in rearing selected species at commercial scales, and the incompatibility of the insects’ life histories with greenhouse design [
14].
Artificial buzz pollination has been shown to increase fruit weight by 28% compared with non-pollinated controls [
14]. However, this costs the Australian tomato industry ~AUD 25,000/ha/year in manual labour [
6]. Mechanical pollination is performed routinely (typically 3 times a week) whereas several different bees may visit each flower, so there are potentially multiple visitations each day [
17]. The disparity in frequency helps to explain the significantly heavier fruit produced by bee pollination. Pollinating by hand may miss optimal pollination conditions or under-stimulate some flowers highlighting the need for further research and innovation.
7. Buzz Pollination Mechanics
Pollination involving vibrations is called buzz pollination or sonication. It is a method of collecting and incidentally spreading pollen, employed by approximately half of all bee species to a diverse range of angiosperms [
13,
22]. Functional specialisation in the male physiology of some species necessitates sonication for pollen release (
Figure 5) [
13]. These structures, which include poricidal anthers (
Figure 5), serve as dispensing mechanisms that divide their gamete resources amongst pollinator visits and thereby maximise the chance of dispersal by a variety of pollinators [
24].
The precise mechanism of buzz-mediated pollen ejection is not fully understood, but current knowledge suggests centrifugal forces from anther excitation by a pollinator imparts kinetic energy onto pollen grains that results in elastic collisions and subsequent pollen ejection from the apical pore [
20]. Furthermore, it has been theorised that triboelectric charging (the electrification of a pollen grain when it is separated from another material with which it made contact) between the pollen grains and the anther walls may also play a significant role in pollen expulsion, although no direct evidence has been found to confirm this mechanism [
69]. The combination of centrifugal, kinetic and electrostatic forces is the most likely outcome of sonication that facilitates pollen release [
13].
Buzz pollination was initially thought to involve both substrate-borne and acoustic components [
11], although more recent studies have revealed that auditory feedback from sonication is a by-product of thoracic vibrations rather than a direct contribution to pollen release and that energy transmission to the flower is by physical contact [
21]. Acoustic and substrate-borne vibrations do not share the same properties in energy transfer to the anther [
13]. Vibrations of the same frequency do not have equivalent energy outputs as they may have varied amplitudes of displacement (the magnitude of a wave property), acceleration, or velocity (
Figure 6). This fact is pertinent to the force a bee can create through vibration, which has implications for the amount of energy transmitted to the flower and the resulting pollen release [
13,
20]. The physiological range of frequencies created by bees (e.g., 240–400 Hz) has a relatively modest effect on pollen release, whereas artificially produced frequencies (e.g., 450–1000 Hz) have been shown to extract twice the amount of pollen compared to that produced by a bee [
12].
Vibrations are not equally transmitted through different floral structures or spatial axes, although the dominant frequency of a vibration is conserved when it is transmitted through the different structures. Variation in transmittance between structures may indicate that the application strategy can influence the magnitude of the vibration and the resultant pollen release [
22]. The amplitude experienced by the anthers can be 400% greater than the input amplitude experienced at the receptacle of the flower, which suggests that the anthers amplify the amplitude of input vibrations, whilst the frequency remains unchanged [
22,
70]. The apical pores therefore experience more intense forces than the distal components of the flower and pollen ejection can be influenced by peak amplitude, whilst frequency had no directional influence over pollen release. Finally, the buzz duration was found to be positively correlated with pollen release, which could be a method to overcome morphological limitations that limit amplitude [
22,
70].
The effects of each vibrational component on pollen release can be distinguished. The effect of frequency on pollination increased when amplitude, as velocity, was a covariate, but the effect decreased when amplitude, as acceleration, was the covariate [
13]. This implied pollen release was influenced by a combination of vibrational characteristics, so studying the sound component variables in isolation cannot fully describe the forces transmitted to the pollen grains by a pollinator. In contrast, the acceleration amplitude experienced by the floral structure can be influenced more by the inter-species variance in floral mechanical properties of the plant opposed to variation of the applied vibration associated with morphological traits (e.g., as body size) of the pollinator [
71].
Greater pollen removal per visit has diminishing returns for the probability of pollen dispersal, thus plants restrict the amount of pollen release per visit to spread the risk of dispersal to several pollinators [
70]. Conversely, bumblebees aim to maximise pollen extraction per visit through single continuous vibrations of a few seconds or multiple vibration pulses of ten-hundreds of milliseconds each [
13,
70]. Bumblebees will adjust their sonicating behaviour to circumvent the frequency restrictions imposed by poricidal anthers [
70]. Asynchronous flight muscles, usually responsible for wing movement, form part of a resonant system that varies vibrational frequency depending on the mass it is driving. Preventing wing movement reduces the mass imposed on the system so the vibrational frequency can be increased [
24]. It was suggested that larger bumblebees are able to produce greater peak amplitudes, which allows better pollen extraction [
70], although it was also found that body size was poorly correlated to the acoustic components of floral vibration [
71]. Size-dependent specialization in foraging communities has been observed by morphologically similar individuals selecting flowers that allowed efficient and maximal pollen extraction [
70]. Bees have also been observed manipulating their visitation habits, through frequency and duration of vibration, when they become familiar with the characteristics of a certain plant species [
13].
Different bee species produce different vibrational displacements (
Figure 6) even though frequency may be the same [
71]. Vibration frequencies show a large degree of intra- and inter-species variation [
13]. A study examining the sonicating behaviours of the Australian blue-banded bee and North American bumblebee (
Bombus impatiens) found that the blue-banded bee sonicated at a higher frequency (e.g., ~350 Hz) when compared with the bumblebee of (e.g., ~240 Hz) [
24]. The bumblebee tended to spend more time at each visitation, although the duration of individual sonications was similar. The two sonicate by slightly different mechanisms, with the blue-banded bee tapping the anther cone with its head at high frequencies generated by its resonant system, whereas the bumblebee grasps the staminal cone with its mandibles and vibrates it with its thorax. The shorter duration of each visit and higher average frequency of sonication may increase its pollination efficiency within the greenhouse [
24]. As such, the blue-banded bee might be a viable alternative to bumblebees for greenhouse tomato pollination in Australia.
8. Mechanical and Other Artificial Pollination Strategies
Contemporary methods used by commercial growers to artificially pollinate tomatoes include trellis-tapping, vibration probes (tuning fork/vibration wand) and air guns [
5]. Vibrating probes can be used to oscillate the staminal cone to elicit pollen expulsion (
Figure 7) [
20]. Artificial vibrations of individual inflorescences have been reported to increase fruit weights by 10–17% in open field systems [
72] and overall fruit yield by 75% in greenhouse production [
25], when compared with non-treated controls. Electric toothbrushes (
Figure 7) have also been reported to achieve comparable results to tuning fork treatments, noting that the effect of frequency on pollination outcomes was negligible but was more dependent on the buzzing duration and plant species [
73].
Some studies concluded that artificial pollination was inferior to bee pollination, across all pollination outcomes [
67,
74]. In contrast, a comparative study of mechanical-stimulated pollination, using a vibrating wand, and bee pollination, found that artificial and bee pollination treatments were not significantly different when pollen production was not limited by environmental constraints [
17]. It was found that in cases of severe weather conditions bee pollination was superior, likely due to the greater number of visits per flower compared with the three treatments per week of mechanical vibrations. When mechanical pollination was repeated, the pollination outcomes, such as seed number and fruit weight, were not statistically distinguishable between treatments. Similarly, when pollen production was not limited, bee and artificial pollination had comparable outcomes [
75,
76].
Trellis tapping (
Figure 7) is a similar mechanism of mechanical stimulation that has also been shown to increase the number of seeds/fruit in five hybrid tomato cultivars when conducted for 5 s and twice-daily during anthesis [
77]. Increased fruit production (t/ha) by 34% was also observed when compared with untreated controls, but this was 15% less than bumblebee-pollinated tomatoes [
78]. Other studies investigating tomatoes and eggplants (also buzz-pollinated) also found that tapping of the trellis wires improved fruit set and seed number compared with untreated plants, however the effects were significantly inferior compared with bee pollinated trials [
79,
80]. Conversely, studies using other Solanaceae crops (eggplants and sweet peppers) found no significant effect of trellis tapping on seed set and fruit weight, across multiple cultivars and treatment groups (e.g., untreated plants, and indoor and outdoor grown crops) [
72,
81]. The efficacy of the technique seems to vary across cultivars and growth environments, with different costs to the industry (
Table 2).
Air jet pulses were shown to increase the effectiveness of pollination in tomato flowers as air velocity increases [
26]. Flowers being tested had a natural frequency (22 Hz) in their test range of 5–60 Hz, however this was specific to variety and the prevailing conditions. It was concluded that the vibrating wand was more effective than the air treatment, but both had a more significant effect than the untreated control, and that pulse frequency of air jets was a critical parameter in determining fruit size. It was suggested that the air treatment was feasible and that three pulses per inflorescence was sufficient to induce satisfactory pollination [
26]. In most studies, labour cost of mechanical stimulation appears to be the major concern, with a] estimated labour requirement to be in the vicinity of 12 person h/ha [
75]. The development of new precision technologies (e.g., drones and robots) that dispel air can enable flower movement and might lower manual labour costs (
Figure 7). With the sensory attributes of a tomato being positively related to seed number, it was found that a panel of untrained test subjects preferred the taste of bee-pollinated tomatoes over mechanically vibrated tomatoes [
68]. Clearly, more research is needed to understand the postharvest effects from artificial pollination technologies.
Acoustic turbulence and mechanical vibration were proposed to be the components of sonication that initiate pollen movement in the anther [
11]. To investigate the effect of sound the anther apparatus as a non-uniform cantilever beam with associated structural coefficients and found that the optimum stimulatory frequency was that which was equal to the natural frequency of the structure. Experimental results showed that application of 4.5 kHz, which was closest to the natural frequency, outperformed the other test frequencies (15, 12 and 9.9 kHz) by producing larger fruit [
11]. The interplay of frequency and acceleration is attributed to the mechanism that determines the metering rate of poricidal anthers [
23]. In a sinusoidal vibration, the vibration force acting on a particle (in this case a pollen grain), is proportional to the acceleration (as force = mass × acceleration), whilst frequency determines the time that a force may act on the particle, and hence low frequency vibrations with comparative accelerations to higher frequencies, will produce larger displacements [
23]. Thus, the magnitude of the acceleration is as critical to the mechanism as frequency. When the structure has a low damping property, vibrating the structure at its natural frequency drastically increases the displacement [
23]. This phenomenon was observed and indicated that the elasticity and shear modulus were both highly dependent on cell turgor pressure and were variable properties that contributed to vibration transmission through the anther [
11]. This reveals that there is a wide variation in the degree of damping amongst anther cones [
23]. In the study by DeTar et al. (1968), it was found acoustic vibrations at 4.5 kHz and 155 dB produced 75% fruit set, which was less than the fruit set of hand pollinated controls. They concluded that the acoustics most likely scattered loosely packed pollen from the anther locule, instead of initiating anther rupture and pollen ejection. Future research will be necessary to determine the utility of speakers to emit acoustic waves as a precision pollination technology within the tomato cropping industry.
Despite the promise of these results, worker health and safety must be considered for the successful application of the technology. It is generally cited by numerous health organisations, including the United States Occupational Safety and Health Association and the World Health Organisation, that the permissible exposure level to sound should not exceed 85 dB for 8 h per day (in the range of 4–6 kHz), above which noise induced hearing loss begins [
83]. Damage to the cochlear hair cells depends on sound pressure level (measured in dB) and duration of exposure, with the effect being cumulative [
84]. Decibels are a logarithmic measurement, which means a 15 min exposure to 100 dB is approximately equivalent to 8 h exposure to 85 dB [
84]. The frequency of the sound wave modulates the sound pressure level and exposure time that is sufficient to cause damage [
85]. Generally, the permissible sound pressure level exposure will decrease as frequency decreases, with headaches and nausea being reported from extended exposures to 16 kHz at 80 dB [
85]. These safety concerns need to be addressed before wide-spread uptake of artificial acoustic or air jet technologies can be adopted within a commercial workplace. Additionally, consideration must be given to any associated socioeconomic impacts caused by the redundancy of hand-pollination jobs due to their automation. With many of these technologies still at the point of inception, it is difficult to gauge the relative impact of each.
9. Precision Robotic Pollination Technology
Handheld mechanical pollination devices are a suitable alternative to bee pollination in tomatoes, however the associated labour costs are prohibitive to large-scale industry applications (
Table 2). Assisted pollination in the tomato industry consists of inspection and precision practices, which are currently carried out by humans, which may lead to missed opportunity or pollination latency. As a subset of artificial intelligence, robotic machine learning is currently being trialled in multiple industries, such as the oil palm industry, for utility in autonomous pollination systems, as it provides efficient and cost-effective services (
Figure 7) [
86]. Machine learning can solve large non-linear problems autonomously, in real-time, from multiple datasets and sources [
86]. Wireless sensor networks consisting of multiple nodes collecting environmental data, such as humidity and temperature, can be mounted to autonomous vehicles that communicate and process data independently [
87] (
Figure 7). This idea of integrating RGB (red, green and blue light) sensor information with meteorological data has been demonstrated in the wine grape industry to quantify the number of flowers on a vine and correlate it to final yield. A mobile sensing platform at night used to capture RGB images of the grapevine canopy were then computed by an algorithm developed through the Convolutional Neural Network (CNN) using SegNEt architecture [
88]. A correlation coefficient (R
2) of 0.91 between detected number of flowers and the actual number was achieved, and a coefficient of 0.70 was achieved for estimated number of flowers and final yield. The technology required for robotic autonomy exists, and algorithms that make it applicable for agricultural use have been developed and are constantly improving. Novel methods currently under trial for robotic pollination in the tomato industry include the air jet originally proposed by [
26] (
Figure 7). However, there are concerns for the air jet technology because it may facilitate the spread of wind-borne fungal pathogens that are economically detrimental to production (
Table 3) [
89]. Modern technology and advanced research methods that can potentially automate precision pollination devices would be particularly valuable for the Australian protected-cropping industry, as the importation of commercial bumblebees is still prohibited in Australia. Further research is needed to understand the mechanics of poricidal pollen ejection to create viable technologies that cause effective pollen release under different environmental conditions (
Table 3). Such research requires interdisciplinary collaboration among plant scientists, engineers, and industry cooperation to levy funding that can advance pollination efficacy for the Australian tomato industry.
10. Conclusions and Future Prospects
Glasshouse tomato production in Australia is predominantly aimed at the fresh fruit market. Protected cultivation enables production output and efficiencies unattainable by field production, which serve as contributing factors to its rapid expansion. However, indoor production reduces the opportunity to utilise natural insect or wind pollinators. Alternative methods are necessary to increase flower pollination and fruit productivity as well as quality. Artificial manual pollination has been estimated to cost the Australian indoor tomato growing industry AUD 25,000/ha/year and the utilisation of robotic precision pollination technologies has the potential to revolutionise the industry (
Table 3).
Tomato flowers have poricidal anthers that require specific frequencies achievable by mechanical or acoustic stimulation that can accelerate pollen expulsion. The success of tomato pollination is correlated with larger fruit size, as fertilised ovules that become seeds, produce growth regulators that increase the fruit’s demand for assimilates. Tomatoes are adapted to acquire the necessary vibration stimulation from insects, particularly bees capable of sonication, wind movement, and mechanical vibration. Pollination can be affected by environmental conditions that limit plant growth and development. Temperature extremes reduce the assimilates available for reproduction, which can result in impotent gametes or malformed sexual structures that are not conducive to typical pollination pathways. Similarly, nutritional, and hydrological limitations to the plant impose stress effects that abate sexual performance, and ultimately affect the amount and quality of fruit.
Assisted pollination, via mechanical contact or contactless acoustic treatment can improve crop production compared with untreated controls and is somewhat comparable to that achieved by natural bee-induced pollination. Various methods have been trialled in the industry, although many have practical and financial constraints. Buzz-probe and trellis-tapping treatments have similar efficacies but are manual-labour intensive. Air jet pulses are an improved mechanical treatment that have precision automation potential when coupled with environmental sensors. Acoustic treatments might emulate the frequency of bee sonication to elicit pollen expulsion from the anther. It is envisioned that acoustic pollination technologies could facilitate precision robotic automation of self-pollination in crops such as tomato and strawberry, thereby advancing the productivity of the Australian protected-cropping industry.