Combined Use of Novel Endophytic and Rhizobacterial Strains Upregulates Antioxidant Enzyme Systems and Mineral Accumulation in Wheat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection and Inoculation of Wheat Seed
2.2. Pot Trial
2.3. Soil Analyses
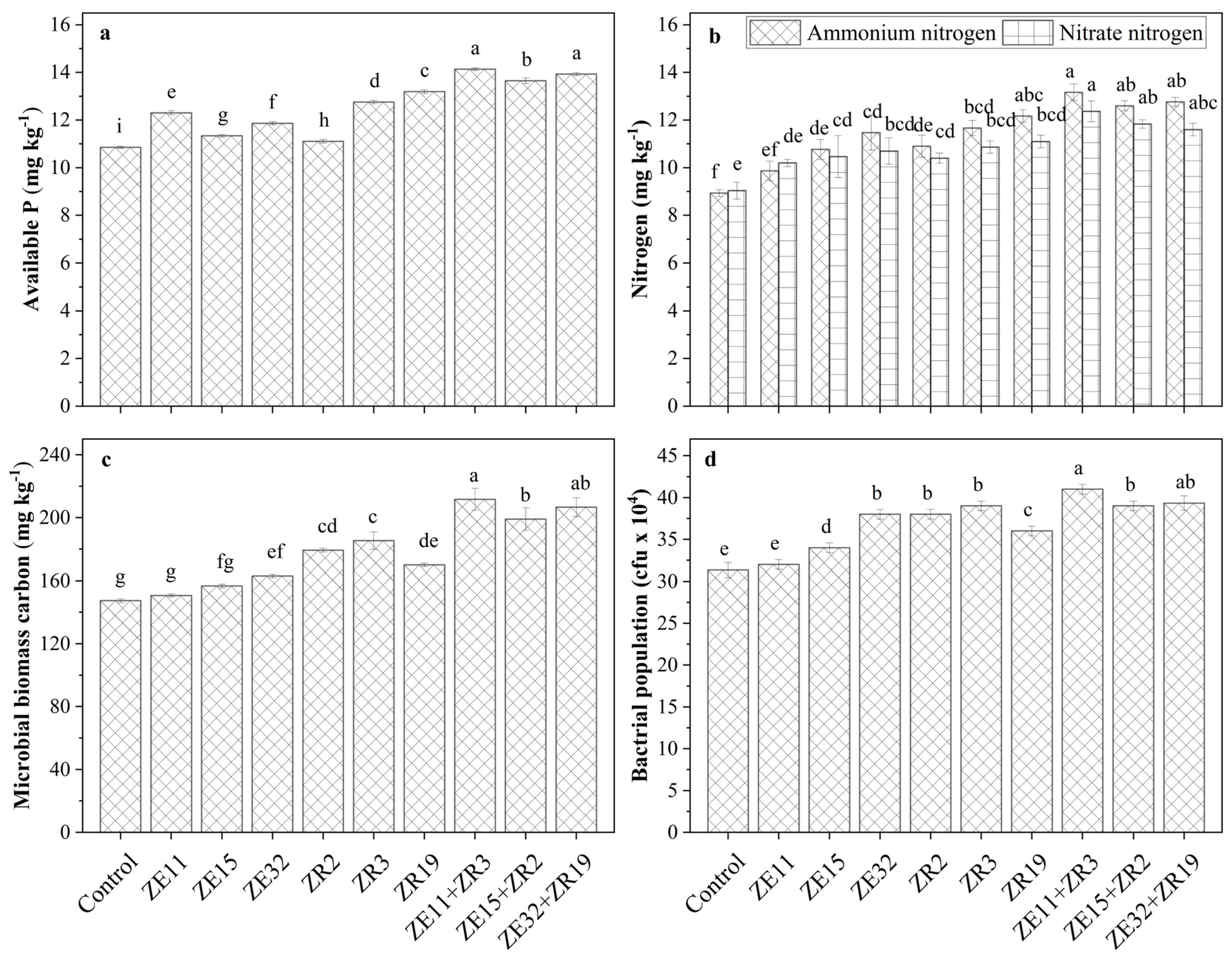
2.3.1. Determination of Microbial Population
2.3.2. Determination of Microbial Biomass Carbon
2.3.3. Determination of Nitrate and Ammonium Nitrogen
2.4. Plant Analyses
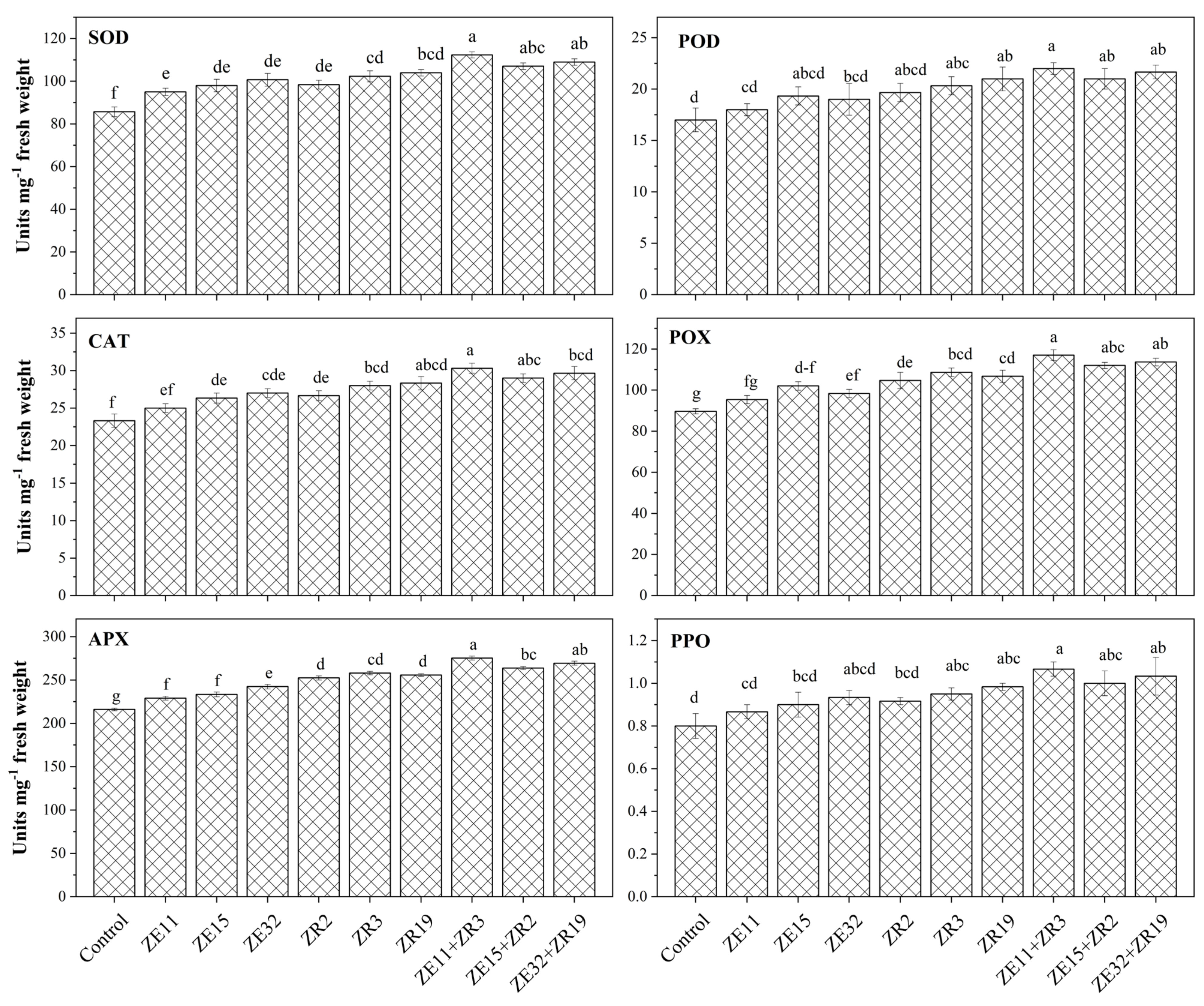
Determination of Antioxidant Enzymes
2.5. Statistical Analyses
3. Results
3.1. Effect of Phosphate-Solubilizing Bacillus and Paenibacilus Strains on Soil Biological Properties
3.2. Plant Antioxidative Status Affected by Inoculation of Phosphate-Solubilizing Endophytic and Rhizobacteria
3.3. Effect of Endophytic Bacteria and Rhizobacteria Strains on Growth, Physiology and Yield of Wheat
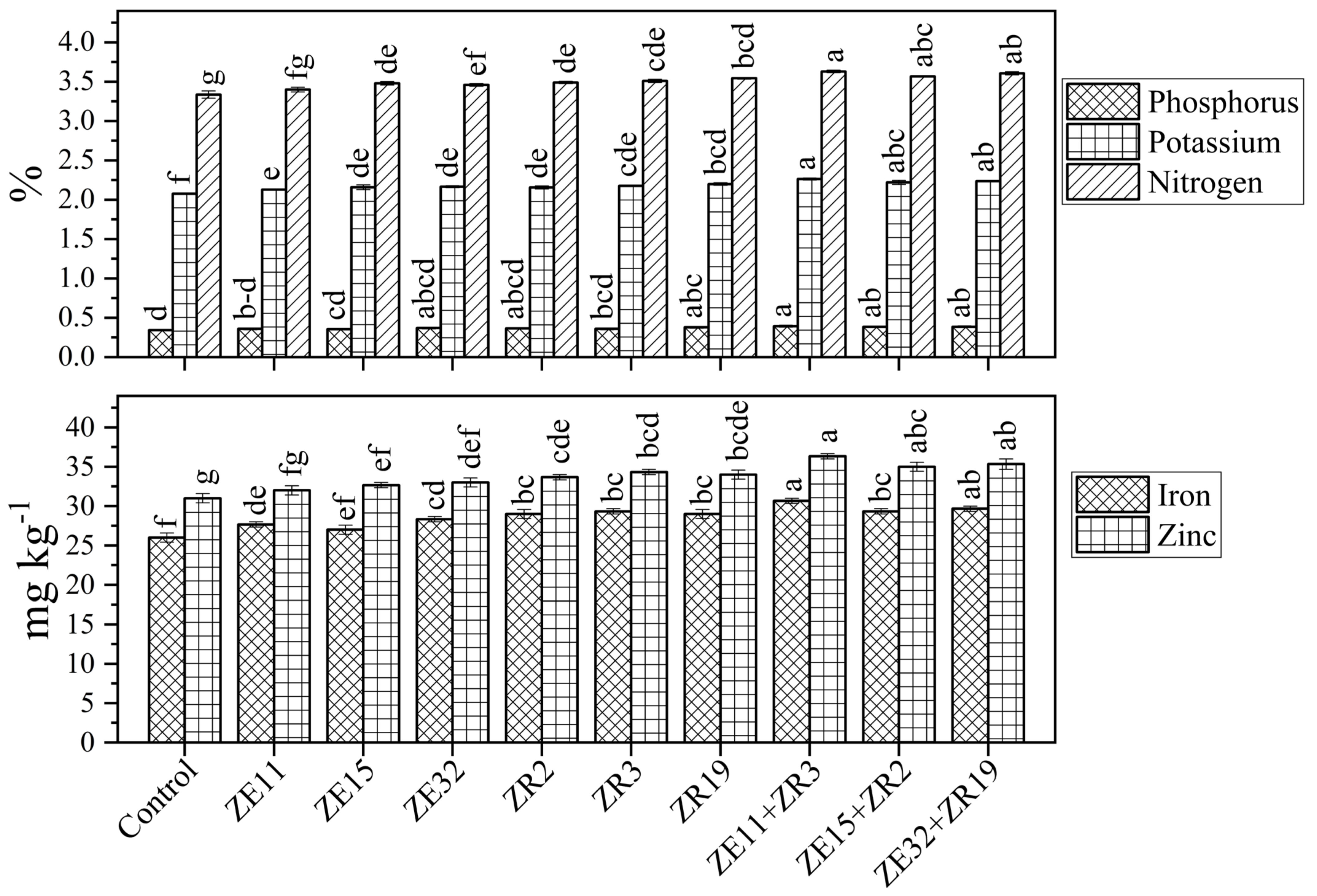
3.4. Effect of Endophytic Bacteria and Rhizobacteria Strains on Mineral Concentration in Wheat Grain
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raza, A.; Williams, D. Grain and Feed Update-Pakistan; Gain Report Number, PK1704; Global Agricultural information Network, USDA Foreign Agriculture Service, USDA Staff: Washington, DC, USA, 2017.
- PARC-Pakistan Agriculture Research Council. Wheat in Pakistan: A status paper. In National Coordinator Wheat, Plant Sciences Division; Pakistan Agriculture Research Council: Islamabad, Pakistan, 2017. [Google Scholar]
- Mustafa, A.; Naveed, M.; Abbas, T.; Saeed, Q.; Hussain, A.; Ashraf, M.N.; Minggang, X. Growth response of wheat and associated weeds to plant antagonistic rhizobacteria and fungi. Ital. J. Agron. 2019, 14, 191–198. [Google Scholar] [CrossRef]
- Abd El-Latief, E.A. Use of Azospirillum and Azobacter bacteria as biofertilizers in cereal crops: A review. Int. J. Res. Eng. Appl. Sci. 2016, 6, 36–44. [Google Scholar]
- Rasche, F.; Blagodatskaya, E.; Emmerling, C.; Belz, R.; Musyoki, M.K.; Zimmermann, J.; Martin, K. A preview of perennial grain agriculture: Knowledge gain from biotic interactions in natural and agricultural ecosystems. Ecosphere 2017, 8, e02048. [Google Scholar] [CrossRef] [Green Version]
- Umar, W.; Ayub, M.A.; Ur Rehman, M.Z.; Ahmad, H.R.; Farooqi, Z.U.R.; Shahzad, A.; Rehman, U.; Mustafa, A.; Nadeem, M. Nitrogen and phosphorus use efficiency in agroecosystems. In Resources Use Efficiency in Agriculture; Springer: Singapore, 2020; pp. 213–257. [Google Scholar]
- Hardoim, P.; Overbeek, L.S.; Berg, G.; Pirttilea, A.M.; Compant, S.; Campisano, A.; Doring, M.; Sessitsch, A. The hidden world within plants: Ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saeed, Q.; Xiukang, W.; Haider, F.U.; Kučerik, J.; Mumtaz, M.Z.; Holatko, J.; Naseem, M.; Kintl, A.; Ejaz, M.; Naveed, M.; et al. Rhizosphere Bacteria in Plant Growth Promotion, Biocontrol, and Bioremediation of Contaminated Sites: A Comprehensive Review of Effects and Mechanisms. Int. J. Mol. Sci. 2021, 22, 10529. [Google Scholar] [CrossRef]
- Ahmad, M.; Ahmad, I.; Hilger, T.H.; Nadeem, S.M.; Akhtar, M.F.Z.; Jamil, M.; Hussain, A.; Zahir, Z.A. Preliminary study on phosphate solubilizing Bacillus subtilis strain Q3 and Paenibacillus sp. strain Q6 for improving cotton growth under alkaline conditions. Peer J. 2018, 6, e5122. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.A.; Naveed, M.; Mustafa, A.; Abbas, A. The Good, the Bad, and the Ugly of Rhizosphere Microbiome: Probiotics and Plant Health; Springer: Singapore, 2017; pp. 253–290. [Google Scholar]
- Ahmad, M.; Zahir, Z.A.; Jamil, M.; Nazli, F.; Iqbal, Z. Field application of ACC-deaminase biotechnology for improving chickpea productivity in Bahawalpur. Soil Environ. 2017, 36, 93–102. [Google Scholar]
- Chittora, D.; Meena, M.; Barupal, T.; Swapnil, P.; Sharma, K. Cyanobacteria as a source of biofertilizers for sustainable agriculture. Biochem. Biophys. 2020, 22, 100737. [Google Scholar] [CrossRef]
- Mumtaz, M.Z.; Ahmad, M.; Jamil, M.; Asad, S.A.; Hafeez, F. Bacillus strains as potential alternate for zinc biofortification of maize grains. Int. J. Agric. Biol. 2018, 20, 1779–1786. [Google Scholar]
- Dalve, P.D.; Mane, S.V.; Nimbalkar, R.R. Effect of biofertilizers on growth, flowering and yield of gladiolus. Asian J. Hortic. 2009, 4, 227–229. [Google Scholar]
- Ahmad, M.; Hussain, A.; Akhtar, M.F.Z.; Hye, M.Z.; Iqbal, Z.; Naz, T.; Iqbal, M.M. Effectiveness of multi-strain biofertilizer in combination with organic sources for improving the productivity of chickpea in drought ecology. Asian J. Agric. Biol. 2017, 5, 228–237. [Google Scholar]
- Singh, D.; Prasanna, R. Potential of microbes in the biofortification of Zn and Fe in dietary food grains. A review. Agron. Sustain. Dev. 2020, 40, 15. [Google Scholar] [CrossRef]
- Kumar, K.; Dasgupta, C.N.; Das, C. Cell growth kinetics of chlorella sorokiniana and nutritional values of its biomass. Bioresour. Technol. 2014, 167, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Ali, S.; Shahid, M.A.; Mustafa, A.; Sayyed, R.Z.; Curá, J.A. Insights into the Interactions among Roots, Rhizosphere, and Rhizobacteria for Improving Plant Growth and Tolerance to Abiotic Stresses: A Review. Cells 2021, 10, 1551. [Google Scholar] [CrossRef]
- Mumtaz, M.Z.; Barry, K.M.; Baker, A.L.; Nichols, D.S.; Ahmad, M.; Zahir, Z.A.; Britz, M.L. Production of lactic and acetic acids by Bacillus sp. ZM20 and Bacillus cereus following exposure to zinc oxide: A possible mechanism for Zn solubilization. Rhizosphere 2019, 12, 100170. [Google Scholar] [CrossRef]
- Hussain, A.; Ahmad, M.; Mumtaz, M.Z.; Ali, S.; Sarfraz, R.; Naveed, M.; Jamil, M.; Damalas, C.A. Integrated application of organic amendments with alcaligenes sp. AZ9 improves nutrient uptake and yield of maize (Zea mays). J. Plant Growth Regul. 2020, 29, 1277–1292. [Google Scholar] [CrossRef]
- Ahmad, M.; Naseer, I.; Hussain, A.; Mumtaz, M.Z.; Mustafa, A.; Hilger, T.H.; Zahir, Z.A.; Xu, M. Appraising endophyte–plant symbiosis for improved growth, nodulation, nitrogen fixation and abiotic stress tolerance: An experimental investigation with chickpea. (Cicer arietinum L.). Agronomy 2019, 9, 621. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, Z.; Ahmad, M.; Jamil, M.; Akhtar, M.F.Z. Appraising the potential of integrated use of bacillus strains for improving wheat growth. Int. J. Agric. Biol. 2020, 24, 1439–1448. [Google Scholar]
- Bertani, G. Studies on lysogenesis I: The mode of phage liberation by lysogenic Escherichia coli. J. Bacteriol. 1951, 62, 293–300. [Google Scholar] [CrossRef] [Green Version]
- Alexander, J.; Parsons, B.V. Functional Family Therapy; Brooks/Cole Publishing Company: Three Lakes, WI, USA, 1982. [Google Scholar]
- Anderson, J.M.; Ingram, J.S.I. Tropical Soil Biology and Fertility: A Handbook of Methods; CAB International: Wallingford, UK, 1993; p. 12. [Google Scholar]
- Okalebo, J.R.; Gathua, K.W.; Woomer, P.L. Laboratory Methods of Soil and Plant Analysis: A Working Manual; Tropical Soil Biology and Fertility Programme: Nairobi, Kenya, 1993. [Google Scholar]
- Brewer, P.G.; Riley, J.P. The automatic determination of nitrate in sea water. Deep. Sea Res. Oceanogr. Abstr. 1965, 12, 765–772. [Google Scholar] [CrossRef]
- Anderson, C.R.; Condron, L.M.; Clough, T.J.; Fiers, M.; Stewart, A.; Hill, R.A.; Sherlock, R.R. Biochar induced soil microbial community change: Implications for biogeochemical cycling of carbon, nitrogen and phosphorus. Pedobiologia 2011, 54, 309–320. [Google Scholar] [CrossRef]
- Sahrawat, K.L.; Ponnamperuma, F.N. Measurement of exchangeable NH+4 in tropical rice soils. Soil Sci. Soc. Am. J. 1978, 42, 282–283. [Google Scholar] [CrossRef] [Green Version]
- Ryan, J.; Estefan, G.; Rashid, A. Soil and Plant Analysis Laboratory Manual, 2nd Ed; International Center for Agriculture in Dry Areas (ICARDA): Aleppo, Syria, 2001; 172p. [Google Scholar]
- Bach, D.R.; Friston, K.J.; Dolan, R.J. An improved algorithm for model-based analysis of evoked skin conductance responses. Biol. Psychol. 2013, 94, 490–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steel, R.G.D.; Torrie, J.H.; Dicky, D.A. Principles and Procedures of Statistics: A Biometrical Approach, 3rd ed.; McGraw-Hill Book International Co.: Singapore, 1997. [Google Scholar]
- Hussain, A.; Zahir, Z.A.; Ditta, A.; Tahir, M.U.; Ahmad, M.; Mumtaz, M.Z.; Hayat, K.; Hussain, S. Production and implication of bio-activated organic fertilizer enriched with zinc-solubilizing bacteria to boost up maize (Zea mays L.) production and biofortification under two cropping seasons. Agronomy 2020, 10, 39. [Google Scholar] [CrossRef] [Green Version]
- Anli, M.; Baslam, M.; Tahiri, A.; Raklami, A.; Symanczik, S.; Boutasknit, A.; Ait-El-Mokhtar, M.; Ben-Laouane, R.; Toubali, S.; Ait Rahou, Y. Biofertilizers as strategies to improve photosynthetic apparatus, growth, and drought stress tolerance in the date palm. Front. Plant Sci. 2020, 11, 516818. [Google Scholar] [CrossRef]
- Sun, B.; Bai, Z.; Bao, L.; Xue, L.; Zhang, S.; Wei, Y.; Zhang, Z.; Zhuang, G.; Zhuang, X. Bacillus subtilis biofertilizer mitigating agricultural ammonia emission and shifting soil nitrogen cycling microbiomes. Environ. Int. 2020, 144, 105989. [Google Scholar] [CrossRef]
- Kumar, S.M.; Reddy, C.G.; Phogat, M.; Korav, S. Role of bio-fertilizers towards sustainable agricultural development: A review. J. Pharmacogn. Phytochem. 2018, 7, 1915–1921. [Google Scholar]
- Usmani, Z.; Kumar, V.; Rani, R.; Gupta, P.; Chandra, A. Changes in physico-chemical, microbiological and biochemical parameters during composting and vermicomposting of coal fy ash: A comparative study. Int. J. Environ. Sci. Technol. 2018, 16, 4647–4664. [Google Scholar] [CrossRef]
- Lukashe, N.S.; Mupambwa, H.A.; Green, E.; Mnkeni, P.N.S. Inoculation of fy ash amended vermicompost with phosphate solubilizing bacteria (Pseudomonas fuorescens) and its infuence on vermi-degradation, nutrient release and biological activity. Waste Manag. 2019, 84, 14–22. [Google Scholar] [CrossRef]
- Mustafa, A.; Naveed, M.; Saeed, Q.; Ashrah, M.N.; Hussain, A.; Abbas, T.; Kamran, M.; Minggang, X. Application potentials of plant growth promoting rhizobacteria and fungi as an alternative to conventional weed control methods. Sustain. Crop Prod. 2019. [Google Scholar] [CrossRef] [Green Version]
- Shaharoona, B.; Arshad, M.; Zahir, Z.A. Effect of plant growth promoting rhizobacteria containing ACC-deaminase on maize (Zea mays L.) growth under axenic conditions and on nodulation in mung bean (Vigna radiata L.). Lett. Appl. Microbiol. 2006, 42, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, S.M.; Hamed, M.; Asghar, M.J.; Abbas, G.; Saeed, N.A. Screening of mungbean (Vigna radiate L. Wilczek) genotypes against sucking insect pests under natural field conditions. Pak. J. Zool. 2014, 46, 863–866. [Google Scholar]
- Ahmad, M.; Zahir, Z.A.; Nadeem, S.M.; Nazli, F.; Jamil, M.; Jamshaid, M.U. Physiological response of mung bean to Rhizobium and Pseudomonas based biofertilizers under salinity stress. Pak. J. Agric. Sci. 2014, 51, 555–562. [Google Scholar]
- El-Husseini, M.M.; Helmut, B.; Helmut, J. The biofertilising effect of seed dressing with PGPR Bacillus amyloliquefaciens FZB 42 combined with two levels of mineral fertilizing in African cotton production. Arch. Phytopathol. 2012, 45, 2261–2271. [Google Scholar] [CrossRef]
- Ahmad, M.; Zahir, Z.A.; Asghar, H.N.; Asghar, M. The combined application of rhizobial strains and plant growth promoting rhizobacteria improves growth and productivity of mung bean (Vigna radiata L.) under salt-stressed conditions. Ann. Microbiol. 2012, 62, 1321–1330. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Khalid, M.; Shahzad, S.M.; Ahmad, M.; Soleman, N.; Akhtar, N. Integrated use of Rhizobium leguminosarum, plant growth promoting rhizobacteria and enriched compost for improving growth, nodulation and yield of lentil (Lens culinaris Medik.). Chil. J. Agric. Res. 2013, 72, 104–110. [Google Scholar] [CrossRef] [Green Version]
- Aziz, M.Z.; Yaseen, M.; Naveed, M.; Wang, X.; Fatima, K.; Saeed, Q.; Mustafa, A. Polymer-Paraburkholderia phytofirmans PsJN coated diammonium phosphate enhanced microbial survival, phosphorous use efficiency, and production of wheat. Agronomy 2020, 10, 1344. [Google Scholar] [CrossRef]
- Ahmad, M.; Zahir, Z.A.; Asghar, H.N.; Asghar, M. Inducing salt tolerance in mung bean through co-inoculation with rhizobia and plant growth promoting rhizobacteria (PGPR) containing 1-aminocyclopropane-1-carboxylate deaminase. Can. J. Microbiol. 2011, 57, 578–589. [Google Scholar] [CrossRef]
- Williamson, J.D.; Hirsch-Wyncott, M.E.; Larkins, B.A.; Gelvin, S. Differential accumulation of a transcript driven by the CaMV 35S promoter in transgenic tobacco. Plant Physiol. 1989, 90, 1570–1576. [Google Scholar] [CrossRef]
- Foyer, C.; Lelandais, M.; Galap, C.; Kunert, K.J. Effects of elevated cytosolic glutathione reductase activity on the cellular glutathione pool and photosynthesis in leaves under normal and stress conditions. Plant Physiol. 1991, 97, 863–872. [Google Scholar] [CrossRef] [Green Version]
- Mumtaz, M.; Ahmad, M.; Jamil, M.; Hussain, T. Zinc solubilizing Bacillus spp. potential candidates for biofortification in maize. Microbiol. Res. 2017, 202, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Rabiei, Z.; Hosseini, S.J.; Pirdashti, H.; Hazrati, S. Physiological and biochemical traits in coriander affected by plant growth-promoting rhizobacteria under salt stress. Heliyon 2020, 6, e05321. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Akhtar, M.F.U.Z.; Jamil, M.; Latif, M.; Ahmad, I. Pesticide tolerant plant growth promoting rhizobacteria isolated from rhizosphere of okra. Soil Environ. 2015, 34, 111–118. [Google Scholar]
- Ahmad, M.; Pataczek, L.; Hilger, T.H.; Zahir, Z.A.; Hussain, A.; Rasche, F.; Schafleitner, R.; Solberg, S.O. Perspectives of Microbial Inoculation for Sustainable Development and Environmental Management. Front. Microbiol. 2018, 9, 2992. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, A.; Mumtaz, M.Z.; Wang, X.; Ahmad, M.; Saqib, M.; Maqbool, H.; Zaheer, A.; Wang, W.; Mustafa, A. Insights Into Manganese Solubilizing Bacillus spp. for Improving Plant Growth and Manganese Uptake in Maize. Front. Plant Sci. 2021, 12, 719504. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Arshad, M.; Zahir, Z.A.; Asghar, M. Prospects of zinc solubilizing bacteria for enhancing growth of maize. Pak. J. Agric. Sci. 2015, 52, 915–992. [Google Scholar]
| Treatments | Spade Value | Relative Water Contents | Dry Shoot Biomass | Dry Root Biomass | Grain Yield | Straw Yield | Harvest Index |
|---|---|---|---|---|---|---|---|
| % | g plant−1 | g plant−1 | g pot−1 | g pot−1 | % | ||
| Control | 33.1 c | 64.0 d | 8.6 c | 4.40 e | 10.6 e | 13.8 e | 43.4 a |
| ZE11 | 34.9 bc | 65.3 bcd | 9.2 bc | 4.60 de | 11.7 d | 14.5 de | 44.7 a |
| ZE15 | 35.5 abc | 64.7 cd | 9.1 bc | 4.70 cd | 11.8 d | 15.3 cd | 43.5 a |
| ZE32 | 37.0 ab | 65.3 bcd | 9.6 abc | 4.73 cd | 12.3 cd | 15.8 bc | 43.8 a |
| ZR2 | 36.6 ab | 66.0 bcd | 9.7 abc | 4.77 bcd | 12.6 bcd | 15.5 cd | 44.9 a |
| ZR3 | 36.0 ab | 66.0 bcd | 10.3 ab | 4.87 bc | 12.8 abc | 15.8 bc | 44.8 a |
| ZR19 | 36.7 ab | 65.7 bcd | 9.8 ab | 4.80 bcd | 13.2 abc | 15.8 bc | 45.5 a |
| ZE11+ZR3 | 38.4 a | 68.7 a | 10.7 a | 5.13 a | 13.8 a | 17.2 a | 44.6 a |
| ZE15+ZR2 | 37.2 ab | 66.7 a–c | 10.3 ab | 4.90 abc | 13.4 ab | 16.5 ab | 44.9 a |
| ZE32+ZR19 | 37.5 ab | 67.3 ab | 10.4 a | 5.00 ab | 13.4 ab | 16.7 ab | 44.5 a |
| LSD (p ≤ 0.05) | 2.8894 | 2.3061 | 1.2027 | 0.2429 | 1.0133 | 0.9671 | 2.7290 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, Z.; Bushra; Hussain, A.; Dar, A.; Ahmad, M.; Wang, X.; Brtnicky, M.; Mustafa, A. Combined Use of Novel Endophytic and Rhizobacterial Strains Upregulates Antioxidant Enzyme Systems and Mineral Accumulation in Wheat. Agronomy 2022, 12, 551. https://doi.org/10.3390/agronomy12030551
Iqbal Z, Bushra, Hussain A, Dar A, Ahmad M, Wang X, Brtnicky M, Mustafa A. Combined Use of Novel Endophytic and Rhizobacterial Strains Upregulates Antioxidant Enzyme Systems and Mineral Accumulation in Wheat. Agronomy. 2022; 12(3):551. https://doi.org/10.3390/agronomy12030551
Chicago/Turabian StyleIqbal, Zafar, Bushra, Azhar Hussain, Abubakar Dar, Maqshoof Ahmad, Xiukang Wang, Martin Brtnicky, and Adnan Mustafa. 2022. "Combined Use of Novel Endophytic and Rhizobacterial Strains Upregulates Antioxidant Enzyme Systems and Mineral Accumulation in Wheat" Agronomy 12, no. 3: 551. https://doi.org/10.3390/agronomy12030551
APA StyleIqbal, Z., Bushra, Hussain, A., Dar, A., Ahmad, M., Wang, X., Brtnicky, M., & Mustafa, A. (2022). Combined Use of Novel Endophytic and Rhizobacterial Strains Upregulates Antioxidant Enzyme Systems and Mineral Accumulation in Wheat. Agronomy, 12(3), 551. https://doi.org/10.3390/agronomy12030551








