Hydrogen Sulfide Interacts with 5-Aminolevulinic Acid to Enhance the Antioxidant Capacity of Pepper (Capsicum annuum L.) Seedlings under Chilling Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Treatments and Experimental Design
2.3. Biomass, Morphology, and Physiological Indexes
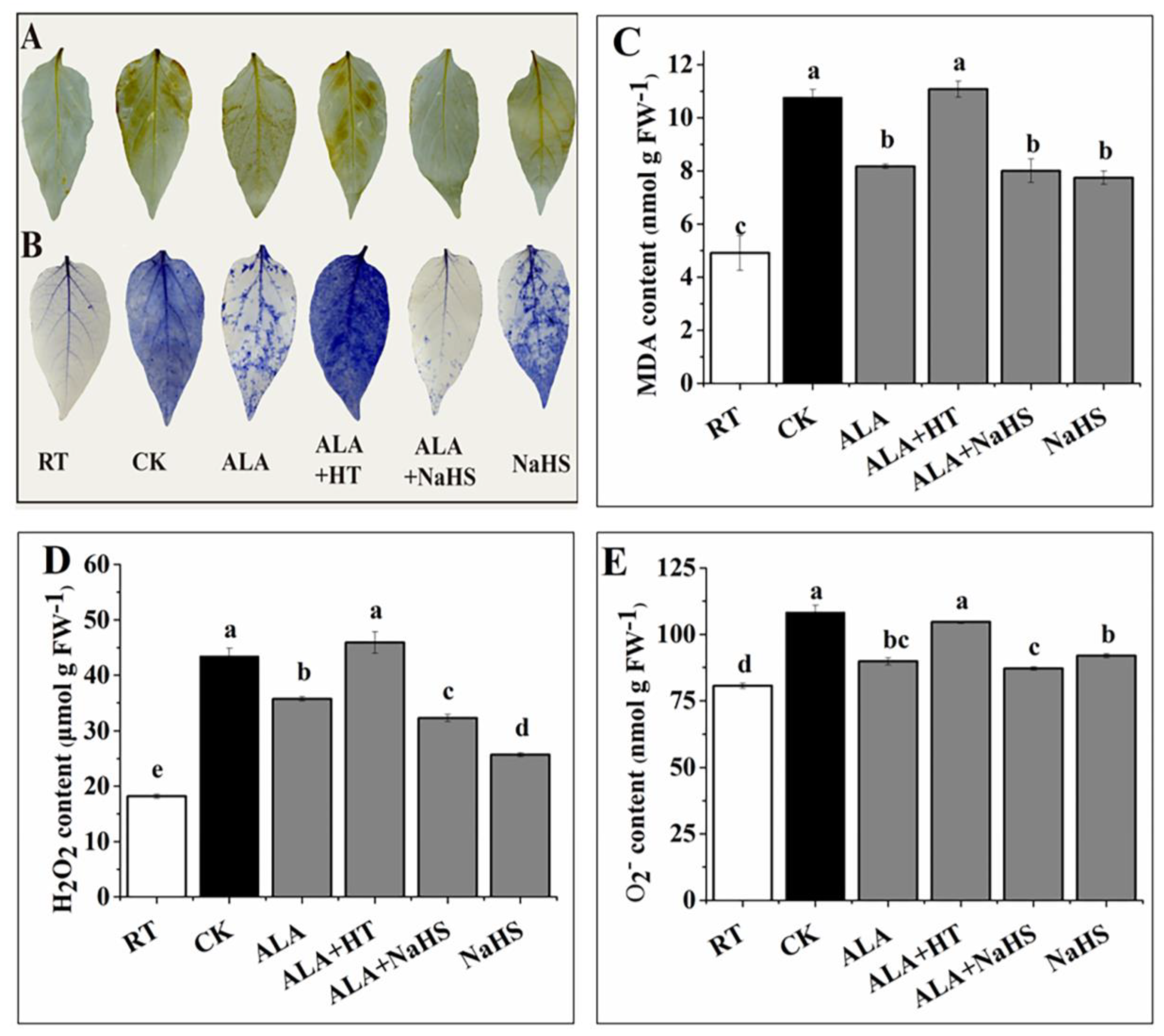
2.4. Histochemical Staining and the Contents of H2O2, O2•−, and MDA
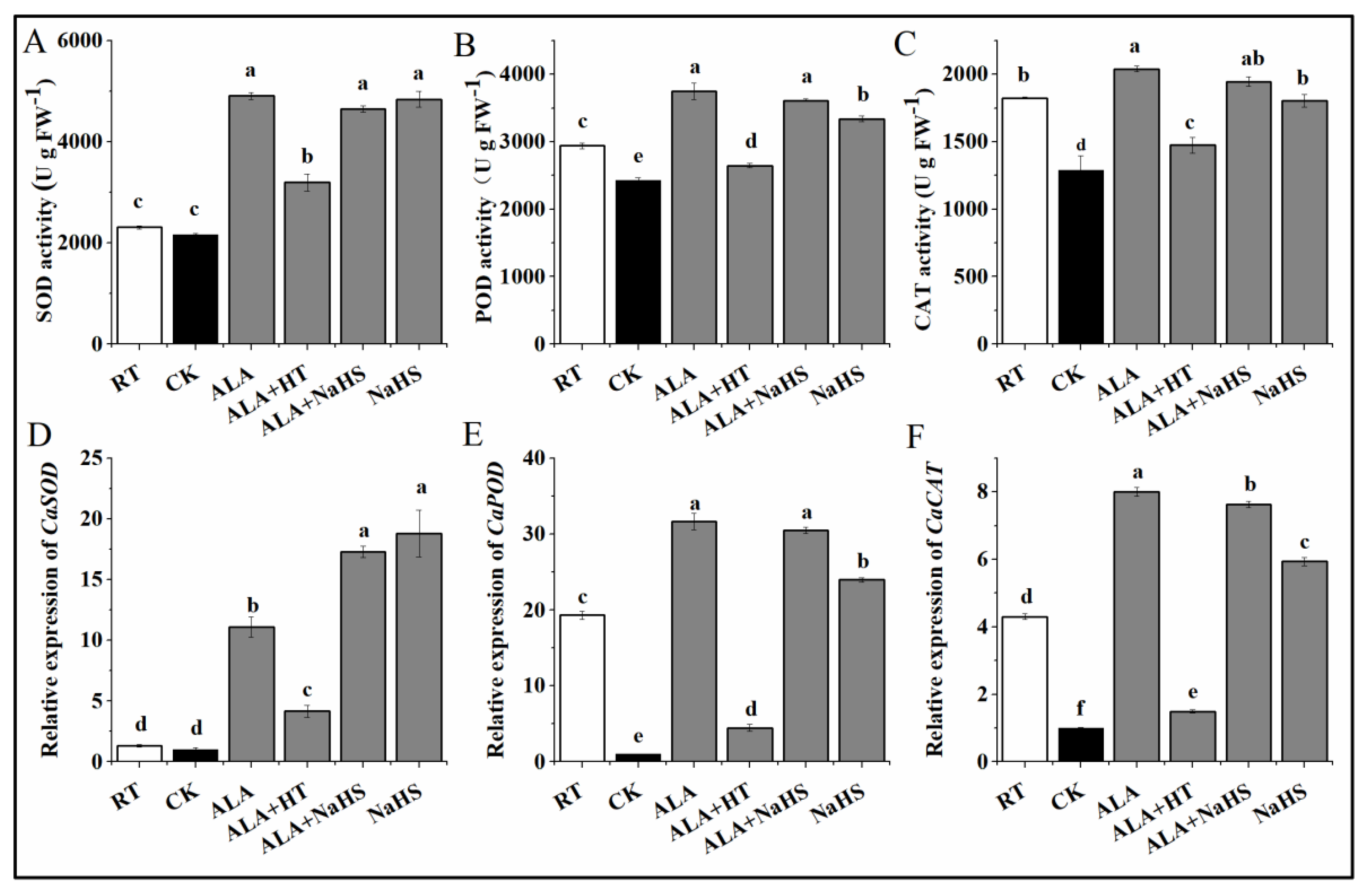
2.5. Antioxidant Enzyme Activities
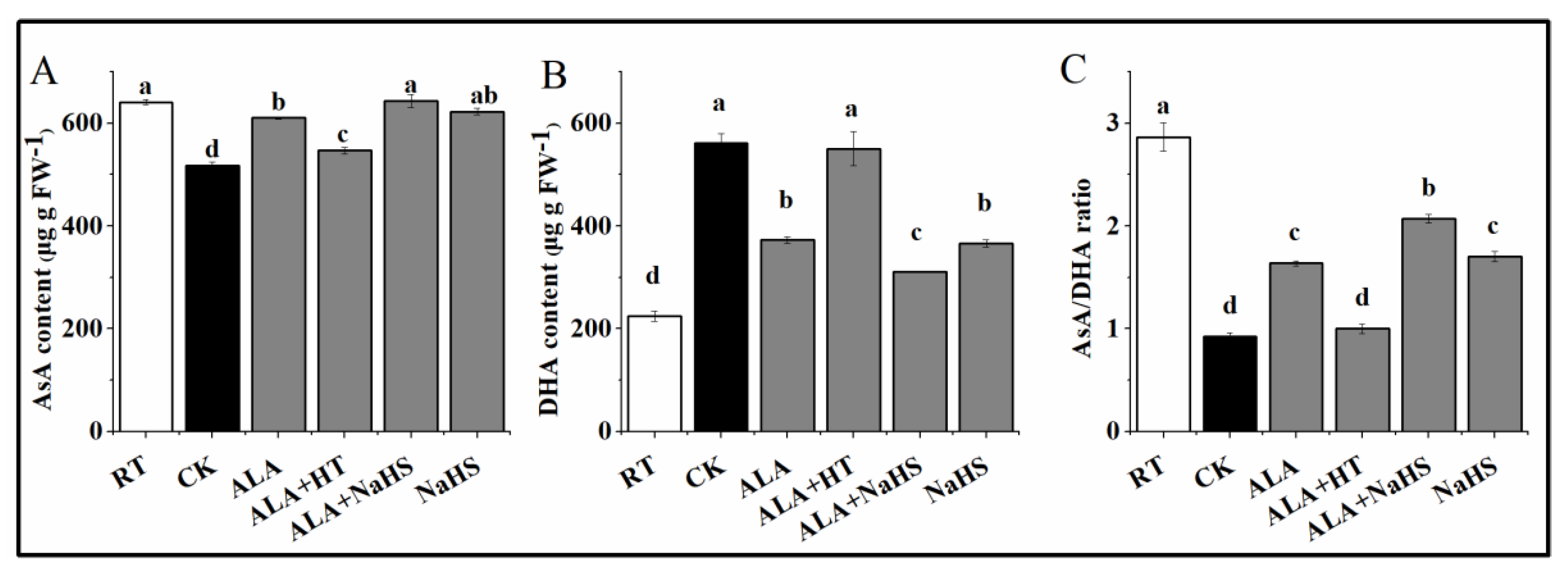
2.6. The Contents of GSH, GSSG, AsA, and DHA
2.7. RNA Extraction and qRT-PCR
2.8. Statistical Analysis
3. Results
3.1. Effects of ALA and/or H2S on the Growth of Pepper Seedlings under Chilling Stress
3.2. Effects of ALA and/or H2S on H2O2, O2•−, and MDA under Chilling Stress
3.3. Effects of ALA and/or H2S on the Activities of SOD, POD, and CAT under Chilling Stress
3.4. Effects of ALA and/or H2S on the Contents of GSH and GSSG under Chilling Stress
3.5. Effects of ALA and/or H2S on the Contents of AsA and DHA under Chilling Stress
3.6. Effects of ALA and/or H2S on APX and GR Activity under Chilling Stress
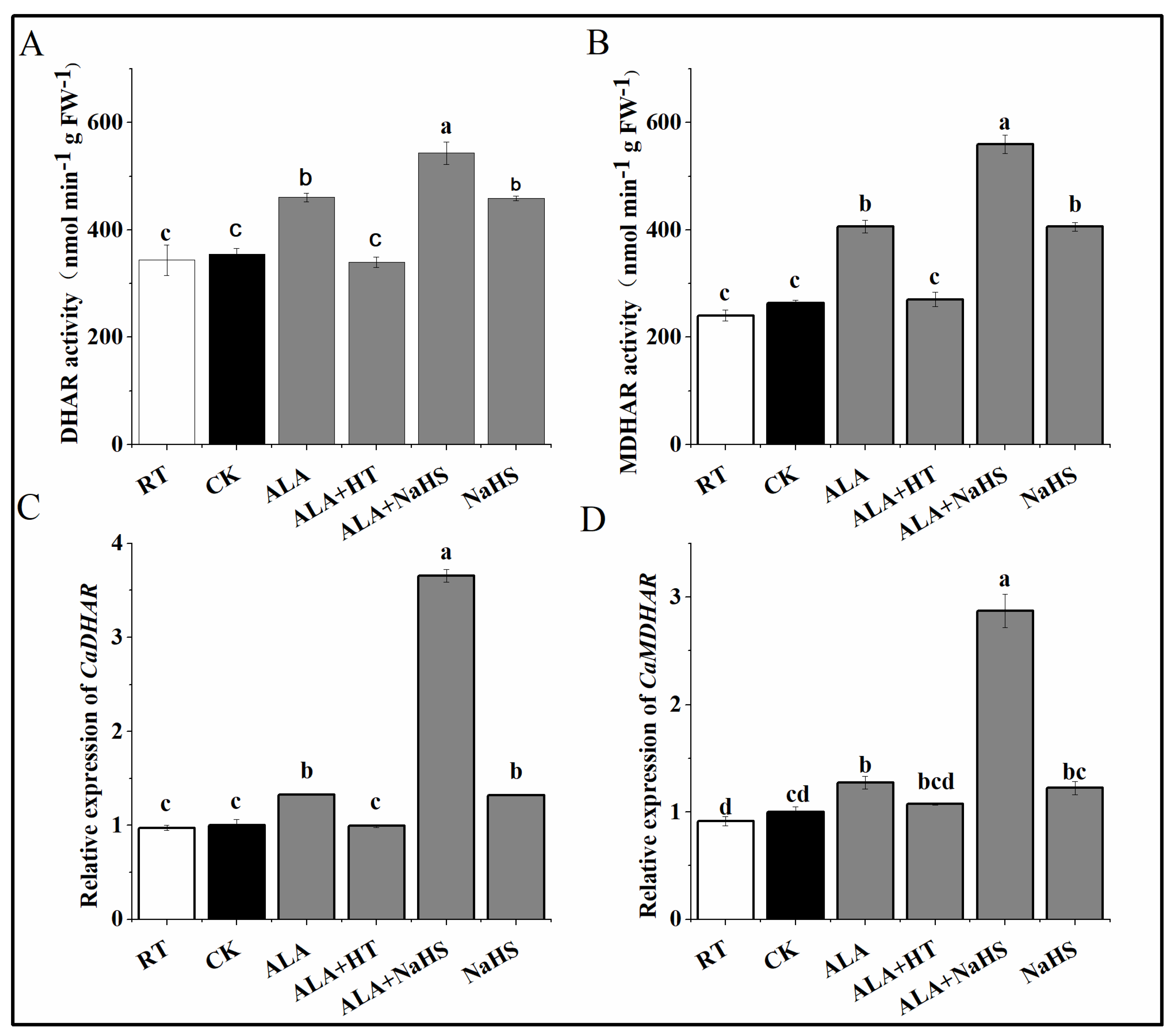
3.7. Effects of ALA and/or H2S on DHAR and MDHAR Activity under Chilling Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tang, C.; Xie, J.; Lv, J.; Li, J.; Zhang, J.; Wang, C.; Liang, G. Alleviating damage of photosystem and oxidative stress from chilling stress with exogenous zeaxanthin in pepper (Capsicum annuum L.) seedlings. Plant Physiol. Biochem. 2021, 162, 395–409. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.L.; Chen, R.G.; Gong, Z.H.; Yin, Y.X.; Li, D.W.; Pandey, G.K. Suppression Subtractive Hybridization Analysis of Genes Regulated by Application of Exogenous Abscisic Acid in Pepper Plant (Capsicum annuum L.) Leaves under Chilling Stress. PLoS ONE 2013, 8, e66667. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.K. Cold stress tolerance mechanisms in plants. A review. Agron. Sustain. Dev. 2010, 30, 515–527. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Hu, Y.; Snyder, R.L.; Kent, E.R. Tea leaf’s microstructure and ultrastructure response to low temperature in indicating critical damage temperature. Inf. Processing Agric. 2019, 6, 247–254. [Google Scholar] [CrossRef]
- Anwar, A.; Yan, Y.; Liu, Y.; Li, Y.; Yu, X. 5-Aminolevulinic Acid Improves Nutrient Uptake and Endogenous Hormone Accumulation, Enhancing Low-Temperature Stress Tolerance in Cucumbers. Int. J. Mol. Sci. 2018, 19, 3379. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Chen, C.; Yang, M.; Dong, X.; Lv, W.; Meng, Q. Cold-regulated protein (SlCOR413IM1) confers chilling stress tolerance in tomato plants. Plant Physiol. Biochem. 2018, 124, 29–39. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Kapoor, D.; Kumar, S.; Singh, S.; Dhanjal, D.S.; Datta, S.; Samuel, J.; Dey, P.; Wang, S.; et al. Revealing on hydrogen sulfide and nitric oxide signals co-ordination for plant growth under stress conditions. Physiol. Plant. 2020, 168, 301–317. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Ullah, F.; Zhou, D.X.; Yi, M.; Zhao, Y. Mechanisms of ROS Regulation of Plant Development and Stress Responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Schippers, J.; Nguyen, H.M.; Lu, D.; Schmidt, R.; Mueller-Roeber, B. ROS homeostasis during development: An evolutionary conserved strategy. Cell. Mol. Life Sci. 2012, 69, 3245. [Google Scholar] [CrossRef] [PubMed]
- Seth, C.S.; Remans, T.; Keunen, E.; Jozefczak, M.; Gielen, H.; Opdenakker, K.; Weyens, N.; Vangronsveld, J.; Cuypers, A. Phytoextraction of toxic metals: A central role for glutathione. Plant Cell Environ. 2012, 35, 334–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaya, C.; Ashraf, M.; Akram, N.A. Hydrogen sulfide regulates the levels of key metabolites and antioxidant defense system to counteract oxidative stress in pepper ( Capsicum annuum L.) plants exposed to high zinc regime. Environ. Sci. Pollut. Res. 2018, 25, 12612–12618. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, F.; Hancock, J.T. Hydrogen sulfide in horticulture: Emerging roles in the era of climate change. Plant Physiol. Biochem. 2020, 155, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Hu, X.; Zhang, J.; Zhang, J.; Du, Q.; Li, J. H2O2 mediates ALA-induced glutathione and ascorbate accumulation in the perception and resistance to oxidative stress in Solanum lycopersicum at low temperatures. BMC Plant Biol. 2018, 18, 34. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Liao, W.; Dawuda, M.M.; Hu, L.; Yu, J. 5-Aminolevulinic acid (ALA) biosynthetic and metabolic pathways and its role in higher plants: A review. Plant Growth Regul. 2018, 87, 357–374. [Google Scholar] [CrossRef] [Green Version]
- Balestrasse, K.B.; Tomaro, M.L.; Batlle, A.; Noriega, G.O. The role of 5-aminolevulinic acid in the response to cold stress in soybean plants. Phytochemistry 2010, 71, 2038–2045. [Google Scholar] [CrossRef]
- Akram, N.A.; Ashraf, M. Regulation in Plant Stress Tolerance by a Potential Plant Growth Regulator, 5-Aminolevulinic Acid. J. Plant Growth Regul. 2013, 32, 663–679. [Google Scholar] [CrossRef]
- Rhaman, M.S.; Imran, S.; Karim, M.M.; Chakrobortty, J.; Mahamud, M.A.; Sarker, P.; Tahjib-Ul-Arif, M.; Robin, A.H.K.; Ye, W.; Murata, Y.; et al. 5-aminolevulinic acid-mediated plant adaptive responses to abiotic stress. Plant Cell Rep. 2021, 40, 1451–1469. [Google Scholar] [CrossRef]
- Liu, T.; Xu, J.; Zhang, J.; Li, J.; Hu, X. Exogenous 5-aminolevulinic acid pretreatment ameliorates oxidative stress triggered by low-temperature stress of Solanum lycopersicum. Acta Physiol. Plant. 2018, 40, 1–11. [Google Scholar] [CrossRef]
- Zhao, J.J.; Zhou, N.; Feng, N.J.; Zheng, D.F. Effects of 5-aminolevulinic acid on osmotic adjustment and antioxidant system in mung bean under chilling stress. Biol. Plant. 2020, 64, 736–743. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Gu, W.; Qian, Z.; Tian, L.; Guo, S.; Wei, S. Exogenous application of 5-aminolevulinic acid improves low-temperature stress tolerance of maize seedlings. Crop Pasture Sci. 2018, 69, 587–593. [Google Scholar] [CrossRef]
- Sheteiwy, M.; Shen, H.; Xu, J.; Guan, Y.; Song, W.; Hu, J. Seed polyamines metabolism induced by seed priming with spermidine and 5-aminolevulinic acid for chilling tolerance improvement in rice (Oryza sativa L.) seedlings. Environ. Exp. Bot. 2017, 137, 58–72. [Google Scholar] [CrossRef]
- Wang, R. Gasotransmitters: Growing pains and joys. Trends Biochem. Sci. 2014, 39, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Gotor, C.; García, I.; Aroca, N.; Laureano-Marín, A.; Romero, L.C. Signaling by hydrogen sulfide and cyanide through posttranslational modification. J. Exp. Bot. 2019, 70, 4251–4265. [Google Scholar] [CrossRef]
- Du, X.; Jin, Z.; Liu, D.; Yang, G.; Pei, Y. Hydrogen sulfide alleviates the cold stress through MPK4 in Arabidopsis thaliana. Plant Physiol. Biochem. 2017, 120, 112–119. [Google Scholar] [CrossRef]
- Chen, T.; Tian, M.; Han, Y. Hydrogen sulfide: A multi-tasking signal molecule in the regulation of oxidative stress responses. J. Exp. Bot. 2020, 71, 2862–2869. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, Y.; He, X.; Yong, B.; Peng, Y.; Zhang, X.; Xiao, M.; Yan, Y.; Huang, L.; Nie, G. The hydrogen sulfide, a downstream signaling molecule of hydrogen peroxide and nitric oxide, involves spermidine-regulated transcription factors and antioxidant defense in white clover in response to dehydration. Environ. Exp. Bot. 2018, 161, 255–264. [Google Scholar] [CrossRef]
- Fu, P.; Wang, W.; Hou, L.; Liu, X. Hydrogen sulfide is involved in the chilling stress response in Vitis vinifera L. Acta Soc. Bot. Pol. 2013, 82, 295–302. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.W.; Liu, F.J.; Zhai, J.; Li, F.D.; Bi, H.G.; Ai, X.Z. Auxin acts as a downstream signaling molecule involved in hydrogen sulfide-induced chilling tolerance in cucumber. Planta 2020, 251, 69. [Google Scholar] [CrossRef]
- Fu, J.J.; Chu, X.T.; Sun, Y.F.; Xu, Y.F.; Hu, T.M. Involvement of nitric oxide in 5-aminolevulinic acid-induced antioxidant defense in roots of Elymus nutans exposed to cold stress. Biol. Plant. 2016, 60, 1–10. [Google Scholar] [CrossRef]
- Liu, T.; Xu, J.; Li, J.; Hu, X. NO is involved in JA- and H2O2-mediated ALA-induced oxidative stress tolerance at low temperatures in tomato. Environ. Exp. Bot. 2019, 161, 334–343. [Google Scholar] [CrossRef]
- Pan, D.Y.; Fu, X.; Zhang, X.W.; Liu, F.J.; Bi, H.G.; Ai, X.Z. Hydrogen sulfide is required for salicylic acid-induced chilling tolerance of cucumber seedlings. Protoplasma 2020, 257, 1543–1557. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, L.; Cui, W.; Xu, S.; Shen, W.; Wang, R. Hydrogen sulfide enhances alfalfa (Medicago sativa) tolerance against salinity during seed germination by nitric oxide pathway. Plant Soil 2012, 351, 107–119. [Google Scholar] [CrossRef]
- Li, Z.G.; Yang, S.Z.; Long, W.B.; Yang, G.X.; Shen, Z.Z. Hydrogen sulfide may be a novel downstream signal molecule in nitric oxide-induced heat tolerance of maize (Zea mays L.) seedlings. Plant Cell Environ. Plant Cell Environ. 2013, 36, 1564–1572. [Google Scholar] [CrossRef]
- Fang, H.; Jing, T.; Liu, Z.; Zhang, L.; Jin, Z.; Pei, Y. Hydrogen sulfide interacts with calcium signaling to enhance the chromium tolerance in Setaria italica. Cell Calcium Int. Interdiscip. Forum Res. Calcium 2014, 56, 472–481. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Z.; Luo, S.; Li, J.; Zhang, J.; Li, L.; Xie, J. 5-Aminolevulinic acid and hydrogen sulphide alleviate chilling stress in pepper (Capsicum annuum L.) seedlings by enhancing chlorophyll synthesis pathway. Plant Physiol. Biochem. 2021, 167, 567–576. [Google Scholar] [CrossRef]
- Korkmaz, A.; Korkmaz, Y.; Demirkıran, A.R. Enhancing chilling stress tolerance of pepper seedlings by exogenous application of 5-aminolevulinic acid. Environ. Exp. Bot. 2010, 67, 495–501. [Google Scholar] [CrossRef]
- Wu, G.X.; Cai, B.B.; Zhou, C.F.; Li, D.D.; Bi, H.G.; Ai, X.Z. Hydrogen sulfide-induced chilling tolerance of cucumber and involvement of nitric oxide. J. Plant Biol. Res. 2016, 5, 58–69. [Google Scholar]
- Duan, B.; Ma, Y.; Jiang, M.; Yang, F.; Ni, L.; Lu, W. Improvement of photosynthesis in rice (Oryza sativa L.) as a result of an increase in stomatal aperture and density by exogenous hydrogen sulfide treatment. Plant Growth Regul. 2014, 75, 33–44. [Google Scholar] [CrossRef]
- Suzuki, N.; Koussevitzky, S.; Mittler, R.; Miller, G. ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ. 2012, 35, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, A.; Dietz, K.-J. Reactive Oxygen Species and the Redox-Regulatory Network in Cold Stress Acclimation. Antioxidants 2018, 7, 169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, Z.B.; Li, J.T.; Zhang, M.M.; Bi, Z.Z.; Li, Z.L. He–Ne laser pretreatment protects wheat seedlings against cadmium-induced oxidative stress. Ecotoxicol. Environ. Saf. 2013, 88, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhang, M.; Shen, S. Effect of salt on malondialdehyde and antioxidant enzymes in seedling roots of Jerusalem artichoke (Helianthus tuberosus L.). Acta Physiol. Plant. 2010, 33, 273–278. [Google Scholar] [CrossRef]
- Wu, Y.; Hu, L.; Liao, W.; Mujitaba Dawuda, M.; Lyu, J.; Xie, J.; Feng, Z.; Calderón-Urrea, A.; Yu, J. Foliar application of 5-aminolevulinic acid (ALA) alleviates NaCl stress in cucumber (Cucumis sativus L.) seedlings through the enhancement of ascorbate-glutathione cycle. Sci. Hortic. 2019, 257, 108761. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, Y.; Jia, C.; Wang, X. TransgenicArabidopsis overexpressing Mn-SOD enhanced salt-tolerance. Plant Sci. 2004, 167, 671–677. [Google Scholar] [CrossRef]
- Wang, X. Effects of exogenous SOD on physiological characteristics of Petunia hybrida uder low temperature stress. Seed 2021, 40, 108–112. [Google Scholar]
- Juszczak, I.; Cvetkovic, J.; Zuther, E.; Hincha, D.K.; Baier, M. Natural Variation of Cold Deacclimation Correlates with Variation of Cold-Acclimation of the Plastid Antioxidant System in Arabidopsis thaliana Accessions. Front. Plant Sci. 2016, 7, 305. [Google Scholar] [CrossRef] [Green Version]
- Ali, B.; Huang, C.R.; Qi, Z.Y.; Ali, S.; Daud, M.K.; Geng, X.X.; Liu, H.B.; Zhou, W.J. 5-Aminolevulinic acid ameliorates cadmium-induced morphological, biochemical, and ultrastructural changes in seedlings of oilseed rape. Environ. Sci. Pollut. Res. 2013, 20, 7256–7267. [Google Scholar] [CrossRef]
- Luo, S.; Tang, Z.; Yu, J.; Liao, W.; Xie, J.; Lv, J.; Feng, Z.; Dawuda, M.M. Hydrogen sulfide negatively regulates cd-induced cell death in cucumber (Cucumis sativus L) root tip cells. BMC Plant Biol. 2020, 20, 480. [Google Scholar] [CrossRef]
- Li, Z.G. Synergistic effect of antioxidant system and osmolyte in hydrogen sulfide and salicylic acid crosstalk-induced heat tolerance in maize (Zea mays L.) seedlings. Plant Signal. Behav. 2015, 10, e1051278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aghdam, M.S.; Mahmoudi, R.; Razavi, F.; Rabiei, V.; Soleimani, A. Hydrogen sulfide treatment confers chilling tolerance in hawthorn fruit during cold storage by triggering endogenous H 2 S accumulation, enhancing antioxidant enzymes activity and promoting phenols accumulation. Sci. Hortic. 2018, 238, 264–271. [Google Scholar] [CrossRef]
- Avashthi, H.; Pathak, R.K.; Pandey, N.; Arora, S.; Mishra, A.K.; Gupta, V.K.; Ramteke, P.W.; Kumar, A. Transcriptome-wide identification of genes involved in Ascorbate–Glutathione cycle (Halliwell–Asada pathway) and related pathway for elucidating its role in antioxidative potential in finger millet (Eleusine coracana (L.)). 3 Biotech 2018, 8, 499. [Google Scholar] [CrossRef]
- Rahantaniaina, M.S.; Tuzet, A.; Mhamdi, A.; Noctor, G. Missing links in understanding redox signaling via thiol/disulfide modulation: How is glutathione oxidized in plants? Front. Plant Sci. 2013, 4, 477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fotopoulos, V.; Ziogas, V.; Tanou, G.; Molassiotis, A. Involvement of AsA/DHA and GSH/GSSG Ratios in Gene and Protein Expression and in the Activation of Defence Mechanisms Under Abiotic Stress Conditions. In Ascorbate-Glutathione Pathway and Stress Tolerance in Plants; Springer: Dordrecht, The Netherlands, 2010; pp. 265–302. [Google Scholar]
- Szalai, G.; Kells, T.; Galiba, G.; Kocsy, G. Glutathione as an Antioxidant and Regulatory Molecule in Plants under Abiotic Stress Conditions. J. Plant Growth Regul. 2009, 28, 66–80. [Google Scholar] [CrossRef]
- Liu, D.; Pei, Z.F.; Naeem, M.S.; Ming, D.F.; Liu, H.B.; Khan, F.; Zhou, W.J. 5-Aminolevulinic Acid Activates Antioxidative Defence System and Seedling Growth in Brassica napus L. under Water Deficit Stress. J. Agron. Crop Sci. 2011, 197, 284–295. [Google Scholar] [CrossRef]
- Singh, V.P.; Singh, S.; Kumar, J.; Prasad, S.M. Hydrogen sulfide alleviates toxic effects of arsenate in pea seedlings through up-regulation of the ascorbate-glutathione cycle: Possible involvement of nitric oxide. J. Plant Physiol. 2015, 181, 20–29. [Google Scholar] [CrossRef]
- Yabuta, Y.; Motoki, T.; Yoshimura, K.; Takeda, T.; Ishikawa, T.; Shigeoka, S. Thylakoid membrane-bound ascorbate peroxidase is a limiting factor of antioxidative systems under photo-oxidative stress. Plant J. 2002, 32, 915–925. [Google Scholar] [CrossRef]
- Broadbent, P.; Creissen, G.P.; Kular, B.; Wellburn, A.R.; Mullineaux, P.M. Oxidative stress responses in transgenic tobacco containing altered levels of glutathione reductase activity. Plant J. 1995, 8, 247–255. [Google Scholar] [CrossRef]
- Li, Q.; Li, Y.; Li, C.; Yu, X. Enhanced Ascorbic Acid Accumulation through Overexpression of Dehydroascorbate Reductase Confers Tolerance to Methyl Viologen and Salt Stresses in Tomato. Czech J. Genet. Plant Breed. 2012, 48, 74–86. [Google Scholar] [CrossRef] [Green Version]
- Sultana, S.; Khew, C.Y.; Morshed, M.M.; Namasivayam, P.; Napis, S.; Ho, C.L. Overexpression of monodehydroascorbate reductase from a mangrove plant (AeMDHAR) confers salt tolerance on rice. J. Plant Physiol. 2012, 169, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Eltayeb, A.E.; Kawano, N.; Badawi, G.H.; Kaminaka, H.; Sanekata, T.; Shibahara, T.; Inanaga, S.; Tanaka, K. Overexpression of monodehydroascorbate reductase in transgenic tobacco confers enhanced tolerance to ozone, salt and polyethylene glycol stresses. Planta 2007, 225, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Zhen, A.; Bie, Z.L.; Huang, Y.; Liu, Z.X.; Fan, M.L. Effects of 5-aminolevulinic acid on the H2O2-content and antioxidative enzyme gene expression in NaCl-treated cucumber seedlings. Biol. Plant. 2012, 56, 566–570. [Google Scholar] [CrossRef]



| Genes | Accessions | Forward Primer | TM | Reverse Primer | TM |
|---|---|---|---|---|---|
| CaSOD | AF036936.2 | GCTTCATCACCAGAAACATCATCAGAC | 57.79 | ATGACCTCCGCCATTGAACTTGATAG | 58.83 |
| CaPOD | FJ596178.1 | GCCATTACTGCTAGGGACTCTGTTG | 59.71 | GAAGTAGGAGGAGGAATGCTGCTATTG | 58.98 |
| CaCAT | AB007190.1 | TTAACGCTCCCAAGTGTGCTCATC | 59.68 | GGGCAAATAATCCACCTCCTCATCG | 60.08 |
| CaAPX | DQ002888.1 | GAGCAGTTTCCCACACTCTCCTATG | 59.47 | CATCAGGTCCTCCAGTAACTTCAACAG | 59.01 |
| CaGR | AY547351.1 | GTTAATTCAACTGGATGGCACCAAGATG | 58.11 | ATTCCTGGACGATGAGCCCTACTAC | 60.19 |
| CaDHAR2 | JW079767.1 | CCGTCACTAGAATCCTTGCTCTTCAG | 59.17 | TACCCAAATCCCTCTCTCGTTACTCC | 59.69 |
| CaMDHAR | AY652702.1 | GAGCAAGACCACTCACGACTCTATTC | 59.07 | AACATCACCTACAGCGTACACATCAG | 58.60 |
| CaActin | XM_016722297.1 | GTCCTTCCATCGTCCACAGG | 58.38 | GAAGGGCAAAGGTTCACAACA | 55.84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Liu, Z.; Li, J.; Luo, S.; Zhang, J.; Xie, J. Hydrogen Sulfide Interacts with 5-Aminolevulinic Acid to Enhance the Antioxidant Capacity of Pepper (Capsicum annuum L.) Seedlings under Chilling Stress. Agronomy 2022, 12, 572. https://doi.org/10.3390/agronomy12030572
Wang H, Liu Z, Li J, Luo S, Zhang J, Xie J. Hydrogen Sulfide Interacts with 5-Aminolevulinic Acid to Enhance the Antioxidant Capacity of Pepper (Capsicum annuum L.) Seedlings under Chilling Stress. Agronomy. 2022; 12(3):572. https://doi.org/10.3390/agronomy12030572
Chicago/Turabian StyleWang, Huiping, Zeci Liu, Jing Li, Shilei Luo, Jing Zhang, and Jianming Xie. 2022. "Hydrogen Sulfide Interacts with 5-Aminolevulinic Acid to Enhance the Antioxidant Capacity of Pepper (Capsicum annuum L.) Seedlings under Chilling Stress" Agronomy 12, no. 3: 572. https://doi.org/10.3390/agronomy12030572
APA StyleWang, H., Liu, Z., Li, J., Luo, S., Zhang, J., & Xie, J. (2022). Hydrogen Sulfide Interacts with 5-Aminolevulinic Acid to Enhance the Antioxidant Capacity of Pepper (Capsicum annuum L.) Seedlings under Chilling Stress. Agronomy, 12(3), 572. https://doi.org/10.3390/agronomy12030572






