Improving Rabbiteye Blueberry Performance in a Calcareous Soil by Growing Plants in Pits Filled with Low-CaCO3 Growth Media
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of the Experiments
2.2. Characterization and Analysis of the Growth Media
2.3. Plant Measurements and Analyses
2.3.1. Plant Size and Chlorophyll Concentrations
2.3.2. Mineral Concentrations
2.4. pH of the Growth Media
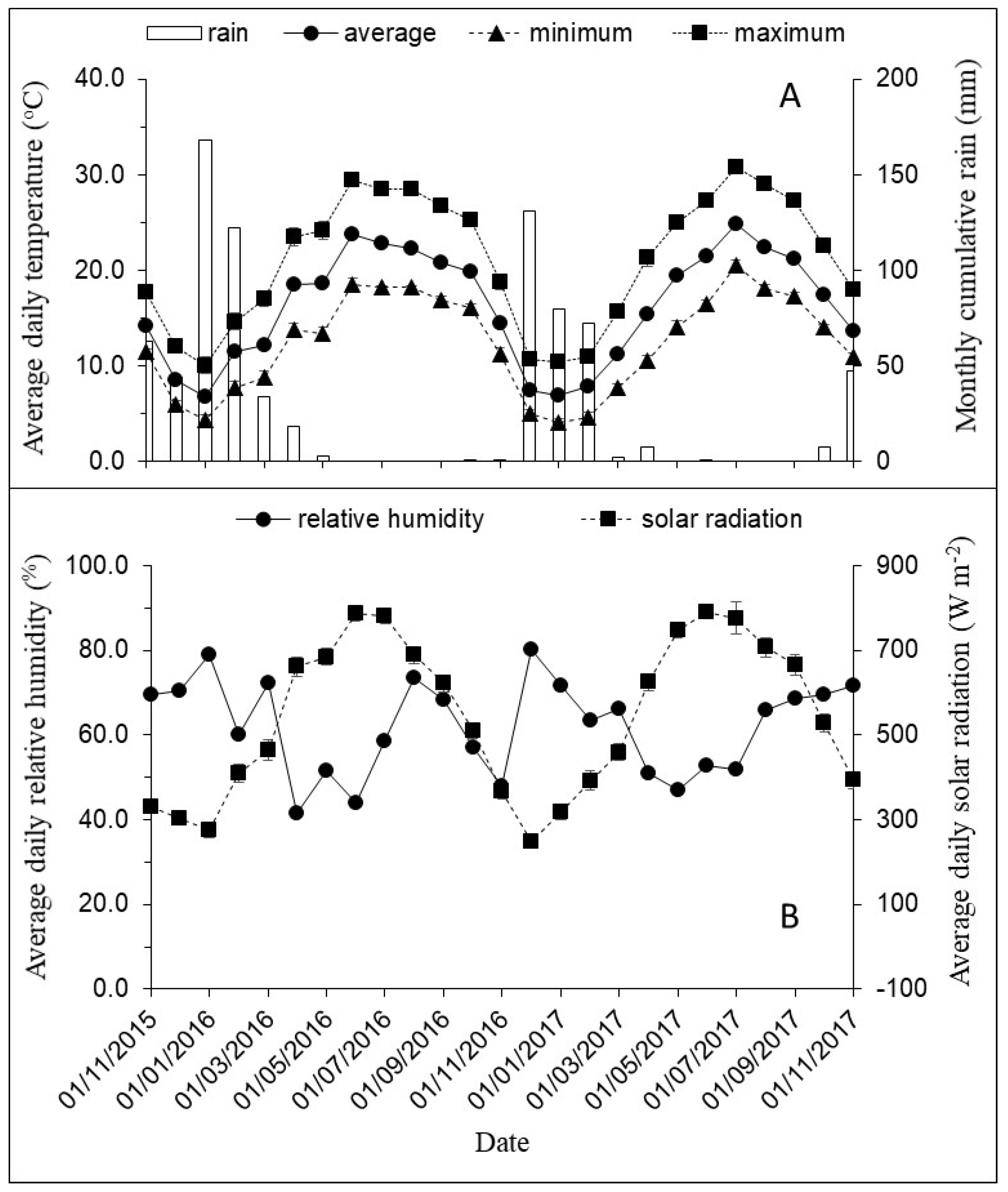
2.5. Environmental Conditions
2.6. Statistical Analysis
3. Results
3.1. Effects of RNH4+ on the pH of the Different Growth Media
3.2. Combined Effects of Growth Medium and RNH4+ on Plant Performance
3.3. Combined Effect of Growth Medium and RNH4+ on Mineral Concentrations in the Leaves
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Blueberry production, crops/regions/world list. In UN and Food organization, Corporate Statistical Database; (FAOSTAT), Food and Agriculture Organization of the United Nations: Rome, Italy, 2019; Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 3 March 2021).
- Haynes, R.J.; Swift, R.S. Effects of liming on the extractability of Fe, Mn, Zn and Cu from a peat medium and the growth and micronutrient uptake of highbush blueberry plants. Plant Soil 1985, 84, 213–223. [Google Scholar] [CrossRef]
- Tamir, G.; Zilkah, S.; Dai, N.; Shawahna, R.; Cohen, S.; Bar-Tal, A. Combined Effects of CaCO3 and the proportion of N-NH4+ among the total applied inorganic N on the growth and mineral uptake of rabbiteye blueberry. J. Soil Sci. Plant Nutr. 2021, 21, 35–48. [Google Scholar] [CrossRef]
- Darnell, R.L.; Hiss, S.A. Uptake and assimilation of nitrate and iron in two Vaccinium species as affected by external nitrate concentration. J. Amer. Soc. Hort. Sci. 2006, 131, 5–10. [Google Scholar] [CrossRef] [Green Version]
- Ochmian, I.; Malinowski, R.; Kubus, M.; Malinowska, K.; Sotek, Z.; Racek, M. The feasibility of growing highbush blueberry (V. corymbosum L.) on loamy calcic soil with the use of organic substrates. Sci. Hortic. 2019, 257, 108690. [Google Scholar] [CrossRef]
- Kafkafi, U.; Tarchizky, J. Fertigation—A Tool for Efficient Fertilizer and Water Management; International Fertilizer Industry Association (IFA), International Potash Institute (IPI): Paris, France, 2011. [Google Scholar]
- Silber, A.; Bar-Tal, A. Nutrition of substrates-grown plants. Chapter 6. In Soilless Culture: Theory and Practice, 2nd ed.; Raviv, M., Lieth, J.H., Bar-Tal, A., Eds.; Academic Press, Elsevier: London, UK, 2019; pp. 197–257. [Google Scholar] [CrossRef]
- Bryla, D.R.; Strik, B.C. Effects of cultivar and plant spacing on the seasonal water requirements of highbush blueberry. J. Amer. Soc. Hort. Sci. 2007, 132, 270–277. [Google Scholar] [CrossRef] [Green Version]
- Conyers, M.M.; Uren, N.C.; Helyar, K.R. Causes of changes in pH in acidic mineral soils. Soil Biol. Biochem. 1995, 27, 1383–1392. [Google Scholar] [CrossRef]
- Liu, M.; Kissel, D.E.; Sonon, L.S.; Cabrera, M.L.; Vendrell, P.F. Effects of biological nitrogen reactions on soil lime requirement determined by incubation. Soil Sci. Soc. Am. J. 2008, 72, 720–726. [Google Scholar] [CrossRef]
- Molina, J.A.E. Components of rates of ammonium oxidation in soil. Soil Sci. Soc. Am. J. 1985, 49, 603–609. [Google Scholar] [CrossRef]
- Hawkesford, M.; Horst, W.; Kichey, T.; Lambers, H.; Schjoerring, J.; Møller, I.S.; White, P. Functions of macronutrients. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Academic Press, Elsevier: Amsterdam, The Netherlands, 2012; pp. 135–189. [Google Scholar]
- Tamir, G.; Bar-Tal, A.; Zilkah, S.; Rotbaum, A.; Oren, A.; Freund, G.; Dai, N. The effects of pH level and calcium carbonate on biomass and mineral uptake of blueberry grown in tissue-culture medium. Acta Hort. 2018, 1265, 203–210. [Google Scholar] [CrossRef]
- Black, B.L.; Zimmerman, R.H. Mixtures of coal ash and compost as substrates for highbush blueberry. J. Amer. Soc. Hort. 2002, 127, 869–877. [Google Scholar] [CrossRef] [Green Version]
- Ben-Noah, I.; Friedman, S.P. Review and evaluation of root respiration and of natural and agricultural processes of soil aeration. Vadose Zone J. 2018, 17, 170119. [Google Scholar] [CrossRef] [Green Version]
- Raviv, M.; Lieth, J.H.; Bar-Tal, A. Growing plants in soilless culture: Operational conclusions. Chapter 14. In Soilless Culture: Theory and Practice, 2nd ed.; Raviv, M., Lieth, J.H., Bar-Tal, A., Eds.; Academic Press, Elsevier: London, UK, 2019; pp. 637–669. [Google Scholar] [CrossRef]
- Tamir, G.; Afik, G.; Zilkah, S.; Dai, N.; Bar-Tal, A. The use of increasing proportions of N-NH4+ among the total applied inorganic N to improve acidification and the nutritional status and performance of blueberry plants in soilless culture. Sci. Hortic. 2021, 276, 109754. [Google Scholar] [CrossRef]
- Whidden, A. Commercial blueberry production methods in Hillsborough County. Proc. Fla. State Hort. Soc. 2008, 121, 36–37. [Google Scholar]
- Martin, C.A.; Ingram, D.L. Container dimension affects rooting medium temperature patterns. HortScience 1993, 28, 18–19. [Google Scholar] [CrossRef] [Green Version]
- Xu, G.; Wolf, S.; Kafkafi, U. Interactive effect of nutrient concentration and container volume on flowering, fruiting, and nutrient uptake of sweet pepper. J. Plant Nutr. 2001, 24, 479–501. [Google Scholar] [CrossRef]
- Ochmian, I.; Grajkowski, J.; Mikiciuk, G.; Ostrowska, K.; Chelpinski, P. Mineral composition of high blueberry leaves and fruits depending on substrate type used for cultivation. J. Elementol. 2009, 14, 509–516. [Google Scholar] [CrossRef]
- Ochmian, I.; Grajkowski, J.; Skupien, K. Effect of substrate type on the field performance and chemical composition of highbush blueberry cv. Patriot. Agr. Food Sci. 2010, 19, 69–80. [Google Scholar] [CrossRef] [Green Version]
- Gee, G.W.; Or, D. Particle-size analysis. In Methods of Soil Analysis. Part 4. Physical Methods; Sparks, D., Ed.; Book Series 5; Soil Science Society of America: Madison, WI, USA, 2002; pp. 255–293. [Google Scholar]
- Rhoades, J.D. Soluble salts. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties, 2nd ed.; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy and Soil Science Society of America: Madison, WI, USA, 1982; pp. 167–178. [Google Scholar]
- Antil, R.S.; Bar-Tal, A.; Fine, P.; Hadas, A. Predicting nitrogen and carbon mineralization of composted manure and sewage sludge in soil. Comp. Sci. Util. 2011, 19, 33–43. [Google Scholar] [CrossRef]
- Nelson, R.E. Carbonate and gypsum. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties, 2nd ed.; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy and Soil Science Society of America: Madison, WI, USA, 1982; pp. 181–196. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis: Chemical and Microbiological Properties. Part 2, 2nd ed.; Page, A.L., Ed.; American Society of Agronomy and Soil Science Society of America: Madison, WI, USA, 1983; pp. 539–579. [Google Scholar] [CrossRef]
- Barnes, J.D.; Balaguer, L.; Manrique, E.; Elvira, S.; Davison, A.W. A reappraisal of the use of DMSO for the extraction and determination of chlorophylls a, and b, in lichens and higher plants. Environ. Exp. Bot. 1992, 32, 85–100. [Google Scholar] [CrossRef]
- Snell, F.D.; Snell, C.T. Colorimetric Methods of Analysis Including Some Turbidimeteric and Nephelometeric Methods; D. Van Nostrand Company, Inc.: Toronto, ON, Canada; New York, NY, USA; London, UK, 1953; Volume 3. [Google Scholar]
- Shahak, Y.; Gussakovsky, E.E.; Gal, E.; Ganelevin, R. ColorNets: Crop protection and light-quality manipulation in one technology. Acta Hort. 2004, 659, 143–161. [Google Scholar] [CrossRef]
- SAS Institute. SAS User’s Guide: Statistics; SAS Institute: Cary, NC, USA, 2005. [Google Scholar]
- Bloom, P.R.; Skyllberg, U.L.; Sumner, M.E. Soil acidity. In Chemical Processes in Soils; Tabatabai, M.A., Sparks, D.L., Eds.; Book Series 8; Soil Science Society of America: Madison, WI, USA, 2005; pp. 411–460. [Google Scholar]
- Kafkafi, U.; Ganmore Neumann, R. Correction of iron chlorosis in peanut (Arachis hypogea Shulamit) by ammonium sulfate and nitrification inhibitor. J. Plant Nut. 1985, 8, 303–309. [Google Scholar] [CrossRef]
- Zhang, H. Organic matter incorporation affects mechanical properties of soil aggregates. Soil Tillage Res. 1994, 31, 263–275. [Google Scholar] [CrossRef]
- Retamales, J.B.; Hancock, J.F. Blueberries; CAB International: Wallingford, UK, 2012. [Google Scholar]
- Scagel, C.F. Growth and nutrient use of ericaceous plants grown in media amended with sphagnum moss peat or coir dust. HortScience 2003, 31, 46–54. [Google Scholar] [CrossRef]
- Turner, A.J.; Arzola, C.I.; Nunez, G.H. High pH stress affects root morphology and nutritional status of hydroponically grown rhododendron (Rhododendron spp.). Plants 2020, 9, 1019. [Google Scholar] [CrossRef] [PubMed]

| Soil Texture | WC 1 | AC 2 | BD 3 | Porosity | OM 4 | CaCO3 | pH | CEC 5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sand | Silt | Clay | Air dry | Field capacity | Saturation | Field capacity | |||||||
| Growth medium | g kg−1 | % w/w | % v/v | g cm−3 | % | g kg−1 | mmolc kg−1 | ||||||
| Tuff/peat mixture (TP) | 2.89 | 33.4 | 58.9 | 47.3 | 0.74 | 72 | 50 * | 0.0 | 6.8 | 161 | |||
| Sandy soil (S) | 889 | 40 | 71 | 0.67 | 8.9 | 21.2 | 33.4 | 1.42 | 46 | 6.0 | 8.0 | 7.3 | 40 |
| Calcareous clayey soil (CC) | 269 | 320 | 411 | 6.66 | 34.8 | 66.2 | 17.0 | 1.15 | 57 | 64 | 149 | 7.8 | 247 |
| Treatment | pH |
|---|---|
| Variable | Significance: F < 0.05 |
| RNH4+ | <0.0001 |
| Growth medium | <0.0001 |
| Block | 0.0008 |
| Date | <0.0001 |
| RNH4+ × Growth medium | 0.0003 |
| RNH4+ × Date | 0.003 |
| Growth medium × Date | <0.0001 |
| RNH4+ × Growth medium × Date | 0.0007 |
| Separate analysis for each growth medium | |
| Tuff/Peat mixture (TP) | |
| RNH4+ (%) | pH |
| 33 | 6.52 a |
| 66 | 6.24 b |
| 100 | 5.97 b |
| Variable | Significance: F < 0.05 |
| RNH4+ | <0.0001 |
| Block | n.s. |
| Date | <0.0001 |
| RNH4+ × Date | 0.008 |
| Sandy soil (S) | |
| RNH4+ (%) | pH |
| 33 | 6.04 a |
| 66 | 5.62 b |
| 100 | 4.21 c |
| Variable | Significance: F < 0.05 |
| RNH4+ | <0.0001 |
| Block | 0.002 |
| Date | <0.0001 |
| RNH4+ × Date | 0.0004 |
| Calcareous clayey soil (CC) | |
| RNH4+ (%) | pH |
| 33 | 7.15 a |
| 66 | 6.95 a |
| 100 | 7.10 a |
| Variable | Significance: F < 0.05 |
| RNH4+ | n.s. |
| Block | n.s. |
| Date | 0.02 |
| RNH4+ × Date | n.s. |
| RNH4+ | Medium | a | b | c | r2 | Time of Minimal pH | Minimum pH Value |
|---|---|---|---|---|---|---|---|
| % | pH day−2 | pH day−1 | pH | day | |||
| 33 | S | 1.42 × 10−5 (6.98 × 10−6) | −0.0118 (0.0049) | 7.75 (0.69) | 0.50 | 416 | 5.29 |
| 33 | TP | 2.93 × 10−6 (2.51 × 10−6) | −0.0034 (0.0018) | 7.24 (0.25) | 0.59 | 574 | 6.27 |
| 33 | CC | 5.40 × 10−6 (1.29 × 10−6) | −0.0041 (0.0009) | 7.72 (0.13) | 0.75 | 379 | 6.94 |
| 66 | S | 9.99 × 10−6 (4.62 × 10−6) | −0.0101 (0.0033) | 7.40 (0.46) | 0.74 | 506 | 4.84 |
| 66 | TP | 4.13 × 10−6 (9.51 × 10−7) | −0.0045 (0.0007) | 7.12 (0.09) | 0.94 | 543 | 5.90 |
| 66 | CC | 6.22 × 10−6 (1.83 × 10−6) | −0.0049 (0.0013) | 7.76 (0.18) | 0.69 | 394 | 6.79 |
| 100 | S | 1.78 × 10−5 (3.85 × 10−6) | −0.0157 (0.0027) | 7.70 (0.38) | 0.87 | 441 | 4.25 |
| 100 | TP | 9.72 × 10−6 (3.64 × 10−6) | −0.0088 (0.0026) | 7.37 (0.36) | 0.72 | 454 | 5.39 |
| 100 | CC | 9.96 × 10−6 (4.34 × 10−6) | −0.0076 (0.0031) | 7.96 (0.43) | 0.48 | 384 | 6.49 |
| Treatment | Chlorophyll Concentration | Canes Diameter | Height |
| Variable | Significance: F < 0.05 | ||
| RNH4+ | <0.0001 | n.s. | 0.04 |
| Growth medium | <0.0001 | <0.0001 | <0.0001 |
| Date | <0.0001 | <0.0001 | <0.0001 |
| Block | n.s. | <0.0001 | <0.0001 |
| RNH4+ × Growth medium | <0.0001 | 0.008 | <0.0001 |
| RNH4+ × Date | n.s. | n.s. | n.s. |
| Growth medium × Date | <0.0001 | <0.0001 | <0.0001 |
| RNH4+ × Growth medium × Date | n.s. | n.s. | n.s. |
| Separate Analysis for each growth medium | |||
| Tuff/peat mixture (TP) | |||
| RNH4+ | Chlorophyll Concentration | Canes Diameter | Height |
| (%) | mg m−2 | mm | cm |
| 33 | 3.90a | 11.7 a | 121.2 a |
| 66 | 3.88a | 10.9 a | 118.1 ab |
| 100 | 4.22a | 11.3 a | 109.8 b |
| Variable | Significance: F < 0.05 | ||
| RNH4+ | n.s. | n.s. | 0.04 |
| Block | n.s. | <0.0001 | <0.0001 |
| Date | <0.0001 | <0.0001 | <0.0001 |
| RNH4+ × Date | n.s. | n.s. | n.s. |
| Sandy soil (S) | |||
| RNH4+ | Chlorophyll Concentration | Canes Diameter | Height |
| (%) | mg m−2 | mm | cm |
| 33 | 4.02 b | 8.55 a | 92.4 b |
| 66 | 4.35 a | 9.01 a | 99.1 b |
| 100 | 4.36 a | 9.36 a | 109.2 a |
| Variable | Significance: F < 0.05 | ||
| RNH4+ | 0.01 | 0.05 | 0.0006 |
| Block | n.s. | <0.0001 | <0.0001 |
| Date | <0.0001 | <0.0001 | <0.0001 |
| RNH4+ × Date | n.s. | n.s. | n.s. |
| Calcareous clayey soil (CC) | |||
| RNH4+ | Chlorophyll Concentration | Canes Diameter | Height |
| (%) | mg m−2 | mm | cm |
| 33 | 2.07 b | 7.3 b | 74.6 b |
| 66 | 3.64 a | 8.0 a | 87.8 a |
| 100 | 3.10 a | 8.1 a | 79.5 b |
| Variable | Significance: F < 0.05 | ||
| RNH4+ | <0.0001 | 0.039 | 0.0004 |
| Block | 0.04 | 0.018 | <0.0001 |
| Date | <.0001 | <0.0001 | n.s. |
| RNH4+ × Date | 0.018 | n.s. | n.s. |
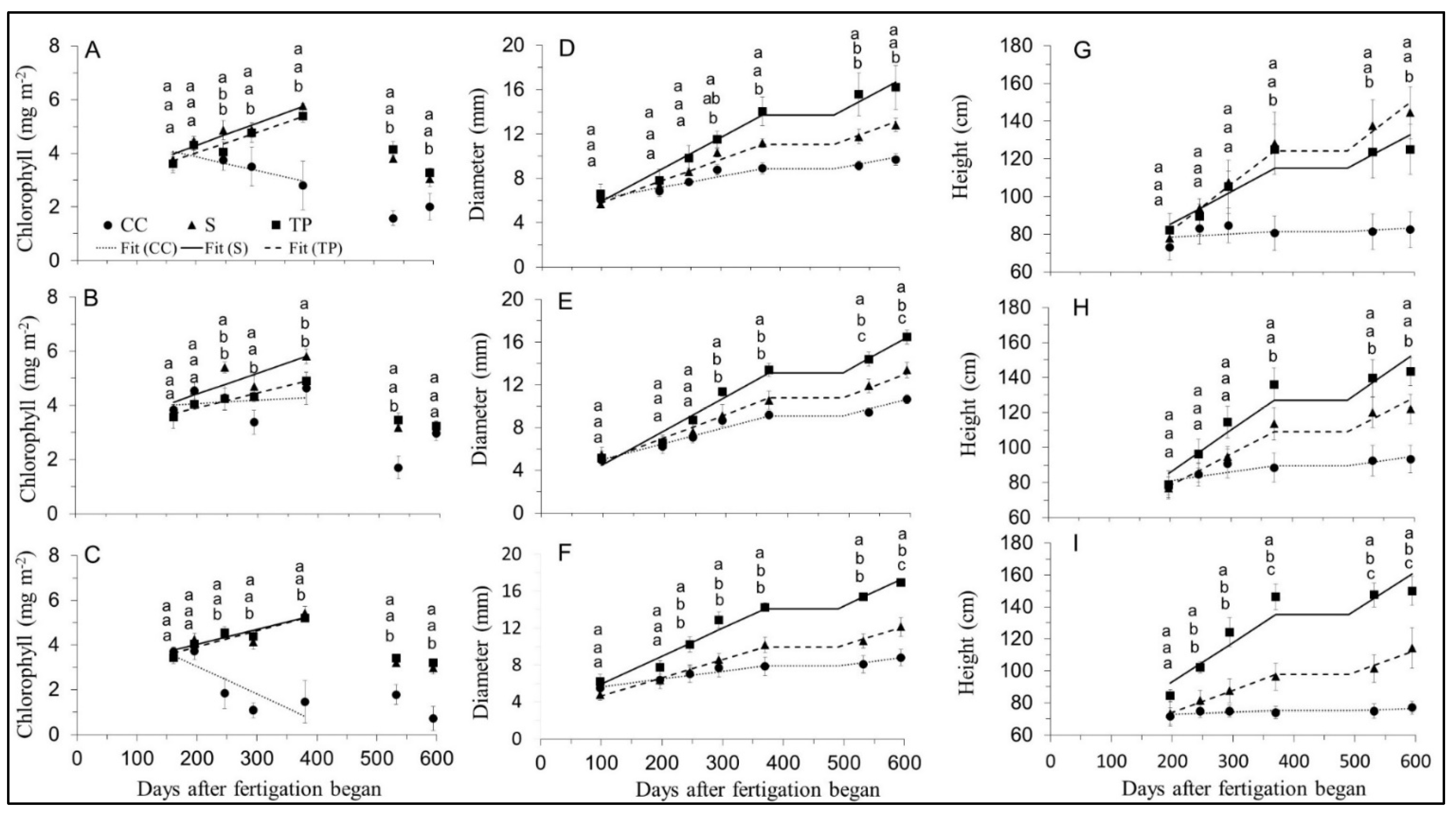
| Chlorophyll—Figure 3A–C | ||||||
| Prob. of F < 0.05 | ||||||
| RNH4+ | Medium | a | b | r2 | a | b |
| % | mg m−2 day−1 | mg m−2 | ||||
| 33 | S | 0.0066 | 2.72 | 0.76 | 0.05 | 0.017 |
| 33 | TP | 0.0071 | 2.49 | 0.88 | 0.02 | 0.009 |
| 33 | CC | −0.0123 | 5.51 | 0.71 | n.s. | 0.02 |
| 66 | S | 0.0077 | 2.88 | 0.70 | n.s. | 0.03 |
| 66 | TP | 0.0055 | 2.81 | 0.94 | 0.006 | 0.0009 |
| 66 | CC | 0.0012 | 3.82 | 0.04 | n.s. | 0.03 |
| 100 | S | 0.0081 | 2.66 | 0.93 | 0.008 | 0.005 |
| 100 | TP | 0.0075 | 2.51 | 0.89 | 0.017 | 0.009 |
| 100 | CC | −0.0050 | 4.86 | 0.64 | n.s. | 0.003 |
| Cane Diameter—Figure 3D–F | ||||||
| Prob. of F < 0.05 | ||||||
| RNH4+ | Medium | a | b | r2 | a | b |
| % | mm day−1 | mm | ||||
| 33 | S | 0.0198 | 2.643 | 0.68 | <0.0001 | 0.0004 |
| 33 | TP | 0.0303 | 2.886 | 0.89 | <0.0001 | 0.0003 |
| 33 | CC | 0.0084 | 4.798 | 0.18 | 0.005 | <0.0001 |
| 66 | S | 0.0220 | 2.635 | 0.70 | <0.0001 | 0.003 |
| 66 | TP | 0.0319 | 1.334 | 0.88 | <0.0001 | 0.03 |
| 66 | CC | 0.0151 | 3.503 | 0.83 | <0.0001 | <0.0001 |
| 100 | S | 0.0195 | 3.859 | 0.89 | <0.0001 | <0.0001 |
| 100 | TP | 0.0286 | 3.113 | 0.67 | <0.0001 | 0.02 |
| 100 | CC | 0.0099 | 5.214 | 0.58 | <0.0001 | <0.0001 |
| Height—Figure 3G–I | ||||||
| Prob. of F < 0.05 | ||||||
| RNH4+ | Medium | a | b | r2 | a | b |
| % | cm day−1 | cm | ||||
| 33 | S | 0.139 | 46.15 | 0.31 | 0.0004 | 0.0006 |
| 33 | TP | 0.247 | 43.71 | 0.72 | <0.0001 | 0.0009 |
| 33 | CC | 0.013 | 70.35 | 0.02 | n.s. | <0.0001 |
| 66 | S | 0.178 | 42.98 | 0.57 | <0.0001 | 0.0002 |
| 66 | TP | 0.241 | 37.90 | 0.55 | <0.0001 | 0.006 |
| 66 | CC | 0.050 | 71.08 | 0.07 | n.s. | <0.0001 |
| 100 | S | 0.247 | 32.89 | 0.61 | <0.0001 | 0.04 |
| 100 | TP | 0.172 | 51.21 | 0.36 | 0.002 | 0.007 |
| 100 | CC | 0.017 | 75.08 | 0.01 | n.s. | <0.0001 |
| Variable | N | P | K | Ca | Mg | Fe | Mn | Zn |
|---|---|---|---|---|---|---|---|---|
| g kg−1 dry weight | mg kg−1 dry weight | |||||||
| Date | ||||||||
| Spring | 19.3 a | 1.67 a | 5.16 a | 7.97 a | 1.67 a | 508 a | 35.0 a | 35.8 a |
| Summer | 17.4 b | 1.45 b | 4.80 a | 2.05 b | 0.46 b | 125 b | 27.6 b | 19.3 b |
| Autumn | 12.9 c | 1.12 c | 5.12 a | 2.59 b | 0.54 b | 47 b | 29.8 b | 27.9 ab |
| RNH4+ (%) | ||||||||
| 33 | 17.1 a | 1.46 a | 5.76 a | 4.48 a | 0.93 a | 220 a | 26.8 b | 23.9 a |
| 66 | 16.2 a | 1.39 a | 4.66 b | 3.83 a | 0.82 a | 388 a | 33.8 a | 23.8 a |
| 100 | 15.9 a | 1.39 a | 4.57 b | 4.16 a | 0.94 a | 219 a | 31.9 ab | 25.0 a |
| Growth medium | ||||||||
| Tuff/Peat mixture (TP) | 15.0 b | 1.24 c | 4.20 b | 3.81 b | 0.79 b | 254 a | 29.0 a | 26.9 a |
| Sandy soil (S) | 16.5 a | 1.39 b | 4.54 b | 3.44 b | 0.88 ab | 261 a | 33.0 a | 24.6 a |
| Calcareous clayey soil (CC) | 17.8 a | 1.61 a | 6.25 a | 5.23 a | 1.00 a | 311 a | 30.5 a | 21.3 a |
| Variable | Significance: F < 0.05 | |||||||
| RNH4+ | n.s. | n.s. | <0.0001 | n.s. | n.s. | 0.035 | 0.019 | n.s. |
| Medium | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.01 | n.s. | n.s. | n.s. |
| Block | n.s. | n.s. | n.s. | n.s. | n.s. | 0.039 | n.s. | n.s. |
| Date | <0.0001 | <0.0001 | n.s. | <0.0001 | <0.0001 | <0.0001 | 0.014 | <0.0001 |
| RNH4+ × Medium | 0.001 | 0.003 | <0.0001 | 0.004 | 0.0004 | n.s. | n.s. | n.s. |
| RNH4+ × Date | n.s. | n.s. | 0.017 | n.s. | 0.04 | 0.006 | n.s. | n.s. |
| Medium × Date | n.s. | n.s. | 0.0002 | n.s. | n.s. | n.s. | <0.0001 | n.s. |
| RNH4+ × Medium×Date | n.s. | n.s. | 0.002 | n.s. | n.s. | n.s. | n.s. | n.s. |
| Spring (16 May 2017) | ||||||||
| RNH4+ (%) | ||||||||
| 33 | 19.7 a | 1.62 a | 5.41 a | 8.36 a | 1.67 a | 462 a | 29.3 a | 32.6 a |
| 66 | 19.8 a | 1.76 a | 5.50 a | 7.69 a | 1.58 a | 734 a | 39.0 a | 35.5 a |
| 100 | 18.7 a | 1.70 a | 4.79 a | 8.20 a | 1.83 a | 469 a | 40.5 a | 32.2 a |
| Growth medium | ||||||||
| Tuff/Peat mixture (TP) | 17.2 b | 1.50 b | 4.40 b | 7.23 b | 1.42 b | 656 a | 36.8 a | 33.5 a |
| Sandy soil (S) | 19.7 ab | 1.74 ab | 5.32 ab | 6.84 b | 1.66 ab | 523 a | 48.9 a | 33.8 a |
| Calcareous clayey soil (CC) | 20.9 a | 1.83 a | 5.97 a | 10.20 a | 1.81 a | 488 a | 22.9 b | 33.0 a |
| Variable | Significance: F < 0.05 | |||||||
| RNH4+ | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| Growth medium | 0.01 | 0.013 | 0.008 | 0.001 | 0.008 | n.s. | 0.0002 | n.s. |
| Block | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | 0.04 |
| RNH4+ × Growth medium | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| Summer (17 July 2017) | ||||||||
| RNH4+ (%) | ||||||||
| 33 | 18.3 a | 1.57 a | 5.56 a | 2.40 a | 25.3 a | 24.8 a | ||
| 66 | 16.6 a | 1.35 a | 4.33 b | 1.86 a | 29.0 a | 12.6 a | ||
| 100 | 17.2 a | 1.45 a | 4.53 b | 1.93 a | 29.2 a | 19.8 a | ||
| Growth medium | ||||||||
| Tuff/Peat mixture (TP) | 15.6 b | 1.26 b | 4.14 b | 1.57 b | 25.0 a | 14.9 a | ||
| Sandy soil (S) | 17.5 ab | 1.46 ab | 4.24 b | 1.50 b | 24.9 a | 32.7 a | ||
| Calcareous clayey soil (CC) | 18.9 a | 1.65 a | 6.03 a | 3.12 a | 33.5 a | 9.57 a | ||
| Variable | Significance: F < 0.05 | |||||||
| RNH4+ | n.s. | n.s. | 0.005 | n.s. | n.s. | n.s. | ||
| Growth medium | 0.006 | 0.015 | <0.0001 | <0.0001 | n.s. | n.s. | ||
| Block | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | ||
| RNH4+ × Growth medium | n.s. | n.s. | n.s. | n.s. | 0.0008 | <0.0001 | n.s. | n.s. |
| Autumn (8 November 2017) | ||||||||
| RNH4+ (%) | ||||||||
| 33 | 41.9 a | 25.3 b | 36.7 a | |||||
| 66 | 55.8 a | 34.1 a | 27.8 a | |||||
| 100 | 42.5 a | 31.3 ab | 26.3 a | |||||
| Growth medium | ||||||||
| Tuff/Peat mixture (TP) | 53.3 a | 31.0 ab | 42.6 a | |||||
| Sandy soil (S) | 38.3 a | 25.9 b | 22.9 a | |||||
| Calcareous clayey soil (BS) | 48.6 a | 33.9 a | 25.3 a | |||||
| Variable | Significance: F < 0.05 | |||||||
| RNH4+ | n.s. | 0.016 | n.s. | |||||
| Growth medium | n.s. | 0.027 | n.s. | |||||
| Block | n.s. | 0.001 | n.s. | |||||
| RNH4+ × Growth medium | 0.0007 | 0.002 | <0.0001 | 0.018 | 0.003 | n.s. | n.s. | n.s. |
| Summer (17 July 2017) | |||||
| Mg | Fe | ||||
| g kg−1 dry weight | mg kg−1 dry weight | ||||
| Tuff/Peat mixture (TP) | |||||
| RNH4+ (%) | |||||
| 33 | 0.33 a | 78.8 a | |||
| 66 | 0.43 a | 84.8 a | |||
| 100 | 0.39 a | 117 a | |||
| Variable | Significance: F < 0.05 | ||||
| RNH4+ (%) | n.s. | n.s | |||
| Sandy soil (S) | |||||
| RNH4+ (%) | |||||
| 33 | 0.41 a | 119 b | |||
| 66 | 0.51 a | 101 b | |||
| 100 | 0.40 a | 302 a | |||
| Variable | Significance: F < 0.05 | ||||
| RNH4+ (%) | n.s. | 0.025 | |||
| Calcareous clayey soil (CC) | |||||
| RNH4+ (%) | |||||
| 33 | 0.77 a | 128 a | |||
| 66 | 0.55 ab | 166 a | |||
| 100 | 0.38 b | 108 a | |||
| Variable | Significance: F < 0.05 | ||||
| RNH4+ (%) | 0.009 | n.s. | |||
| Autumn (8 November 2017) | |||||
| N | P | K | Ca | Mg | |
| g kg−1 dry weight | |||||
| Tuff/Peat mixture (TP) | |||||
| RNH4+ (%) | |||||
| 33 | 11.1 b | 0.90 a | 4.23 a | 2.11 a | 0.49 a |
| 66 | 13.0 a | 0.98 a | 4.53 a | 2.71 a | 0.52 a |
| 100 | 12.6 a | 0.97 a | 3.71 a | 2.34 a | 0.52 a |
| Variable | Significance: F < 0.05 | ||||
| RNH4+ (%) | 0.001 | n.s. | n.s. | n.s. | n.s. |
| Sandy soil (S) | |||||
| RNH4+ (%) | |||||
| 33 | 12.1 a | 0.95 a | 4.41 a | 2.16 a | 0.43 b |
| 66 | 13.0 a | 1.03 a | 3.91 a | 2.60 a | 0.47 ab |
| 100 | 12.6 a | 0.96 a | 3.82 a | 2.13 a | 0.53 a |
| Variable | Significance: F < 0.05 | ||||
| RNH4+ (%) | n.s. | n.s. | n.s. | n.s. | 0.045 |
| Calcareous clayey soil (CC) | |||||
| RNH4+ (%) | |||||
| 33 | 17.4 a | 1.82 a | 10.5 a | 4.05 a | 0.91 a |
| 66 | 12.8 b | 1.30 b | 4.88 b | 2.35 b | 0.50 b |
| 100 | 12.2 b | 1.18 b | 5.87 b | 3.02 ab | 0.53 ab |
| Variable | Significance: F < 0.05 | ||||
| RNH4+ (%) | 0.013 | 0.018 | <0.0001 | 0.05 | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamir, G.; Eli, D.; Zilkah, S.; Bar-Tal, A.; Dai, N. Improving Rabbiteye Blueberry Performance in a Calcareous Soil by Growing Plants in Pits Filled with Low-CaCO3 Growth Media. Agronomy 2022, 12, 574. https://doi.org/10.3390/agronomy12030574
Tamir G, Eli D, Zilkah S, Bar-Tal A, Dai N. Improving Rabbiteye Blueberry Performance in a Calcareous Soil by Growing Plants in Pits Filled with Low-CaCO3 Growth Media. Agronomy. 2022; 12(3):574. https://doi.org/10.3390/agronomy12030574
Chicago/Turabian StyleTamir, Guy, Dagan Eli, Shmuel Zilkah, Asher Bar-Tal, and Nir Dai. 2022. "Improving Rabbiteye Blueberry Performance in a Calcareous Soil by Growing Plants in Pits Filled with Low-CaCO3 Growth Media" Agronomy 12, no. 3: 574. https://doi.org/10.3390/agronomy12030574
APA StyleTamir, G., Eli, D., Zilkah, S., Bar-Tal, A., & Dai, N. (2022). Improving Rabbiteye Blueberry Performance in a Calcareous Soil by Growing Plants in Pits Filled with Low-CaCO3 Growth Media. Agronomy, 12(3), 574. https://doi.org/10.3390/agronomy12030574






