Lignite Substrate and EC Modulates Positive Eustress in Cucumber at Hydroponic Cultivation
Abstract
:1. Introduction
2. Materials and Methods
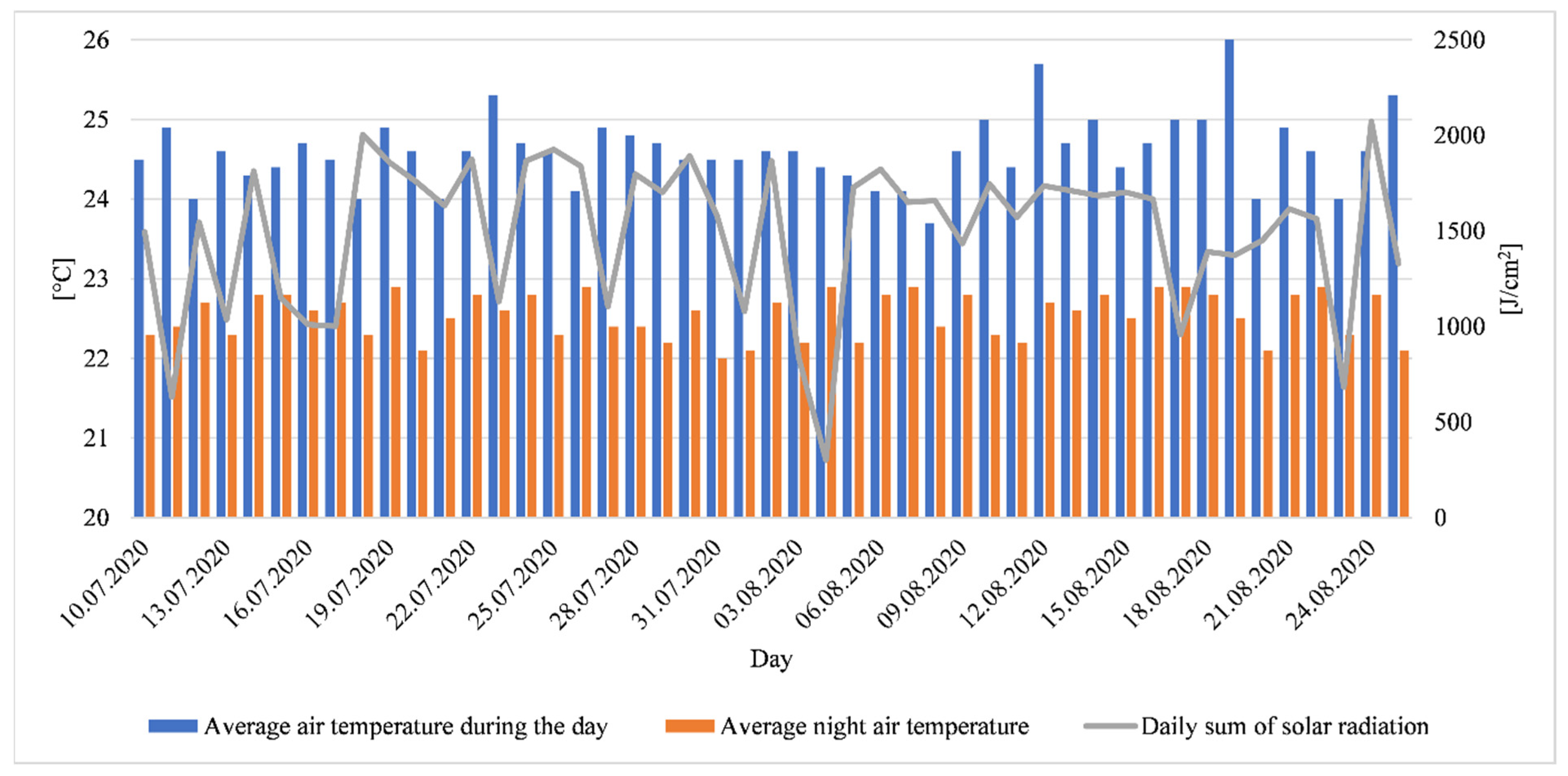
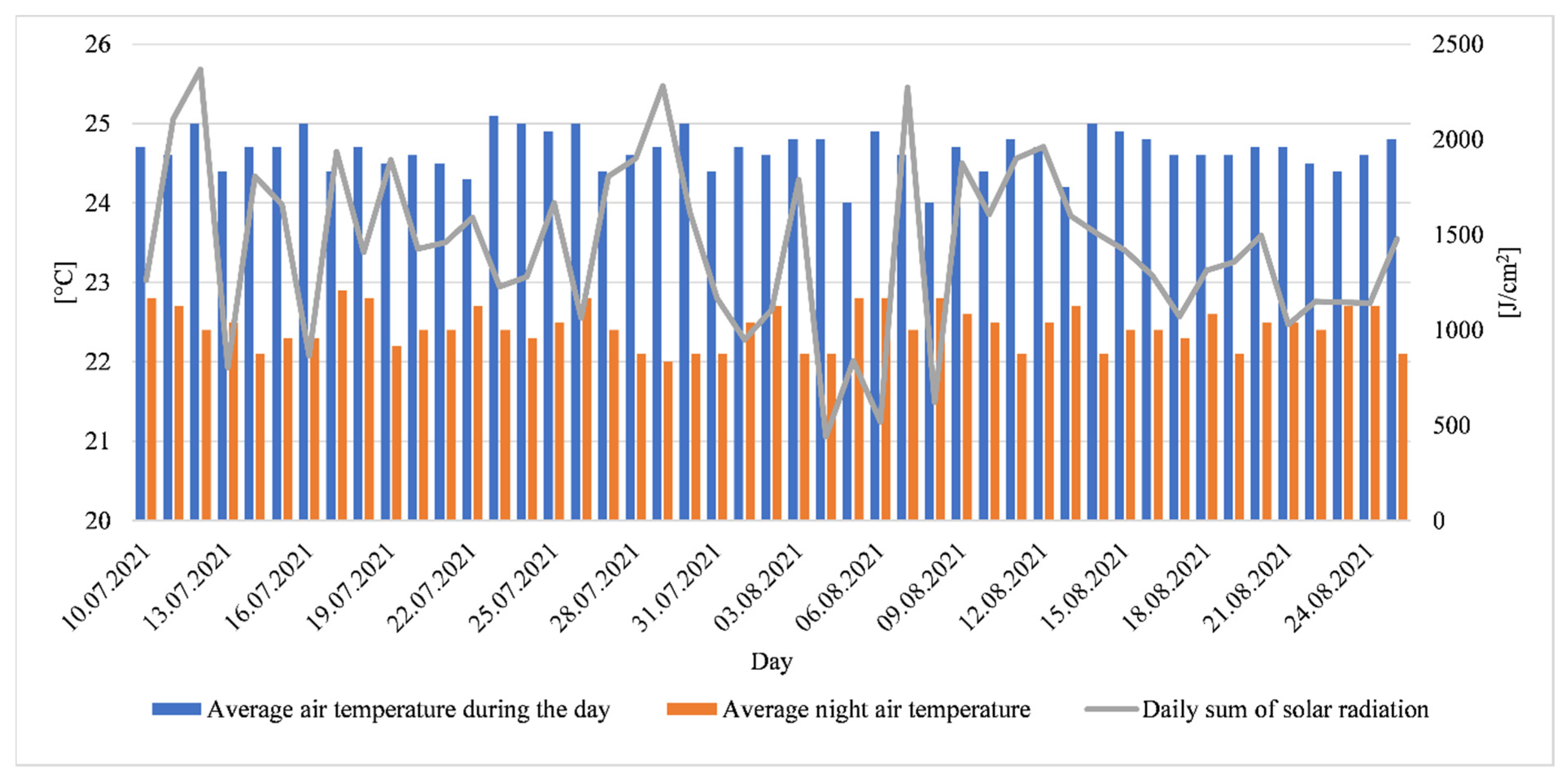
2.1. Plant Material, Location and Experimental Conditions
2.2. Morphological Measurements
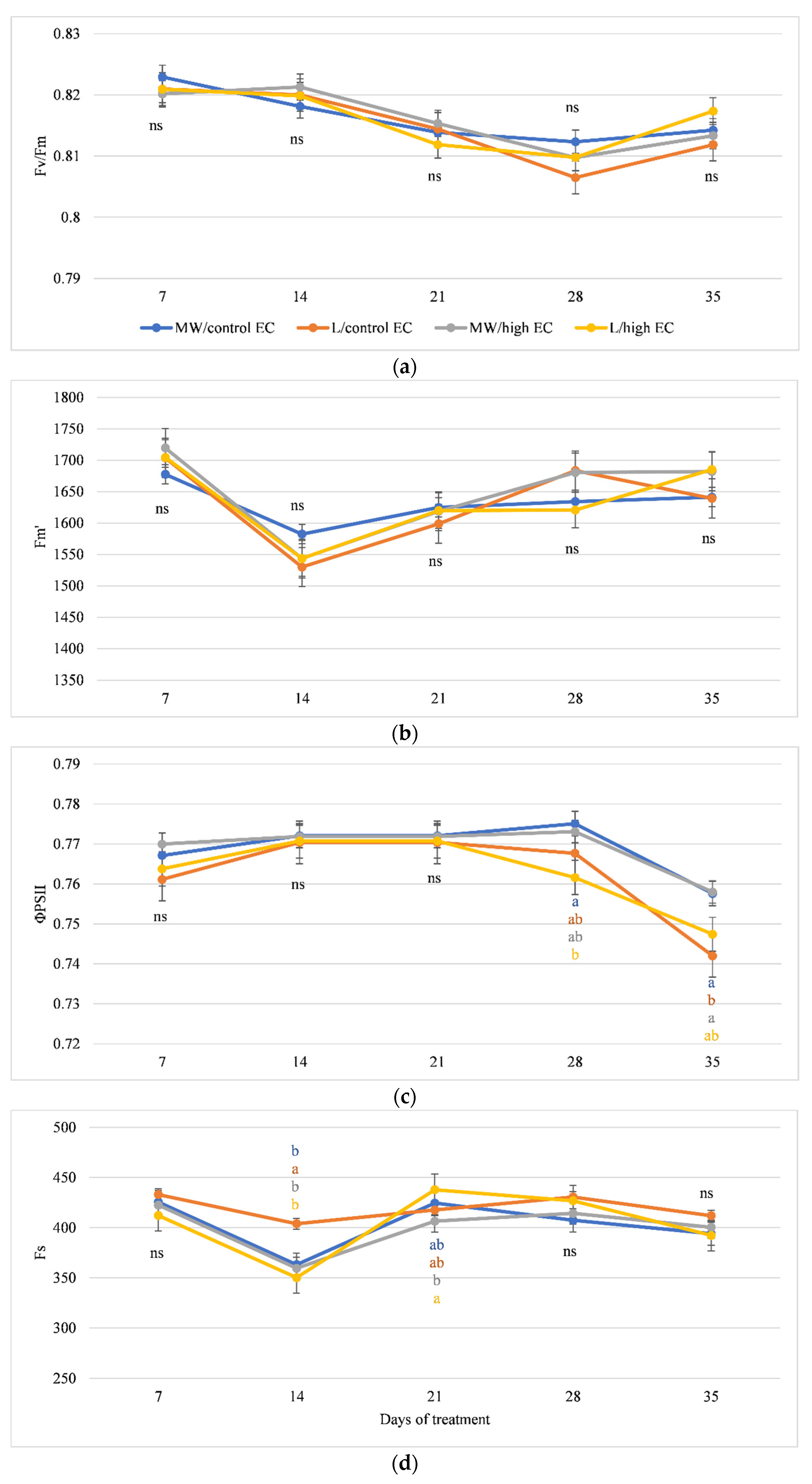
2.3. Gas Exchange and Chlorophyll Fluorescence
2.4. Contents of Dry Matter and Photosynthetic Pigments in Leaves
2.5. Yield Quantity and Fruit Quality
2.6. Statistical Analysis
3. Results
3.1. Morphological Parameters
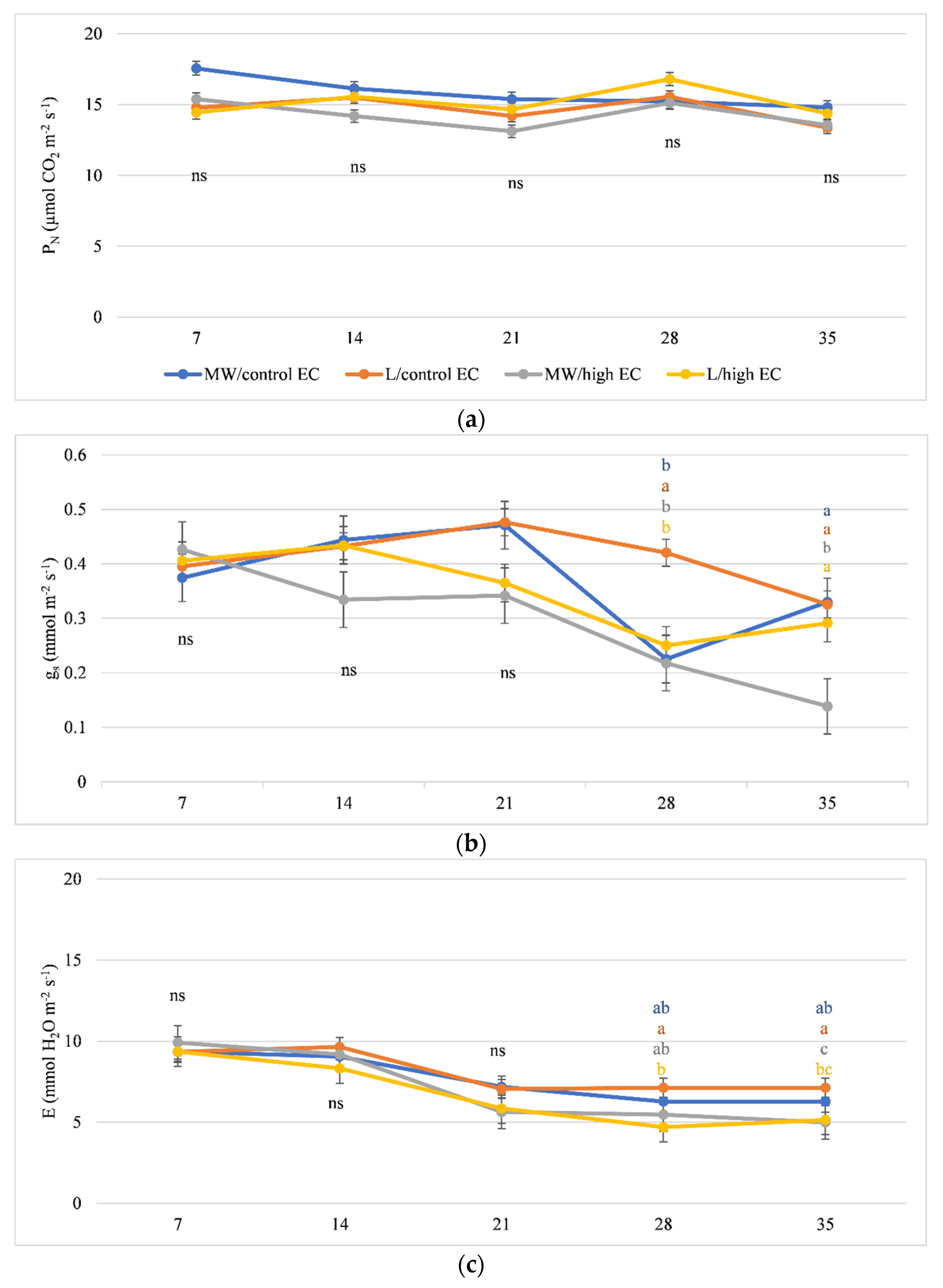
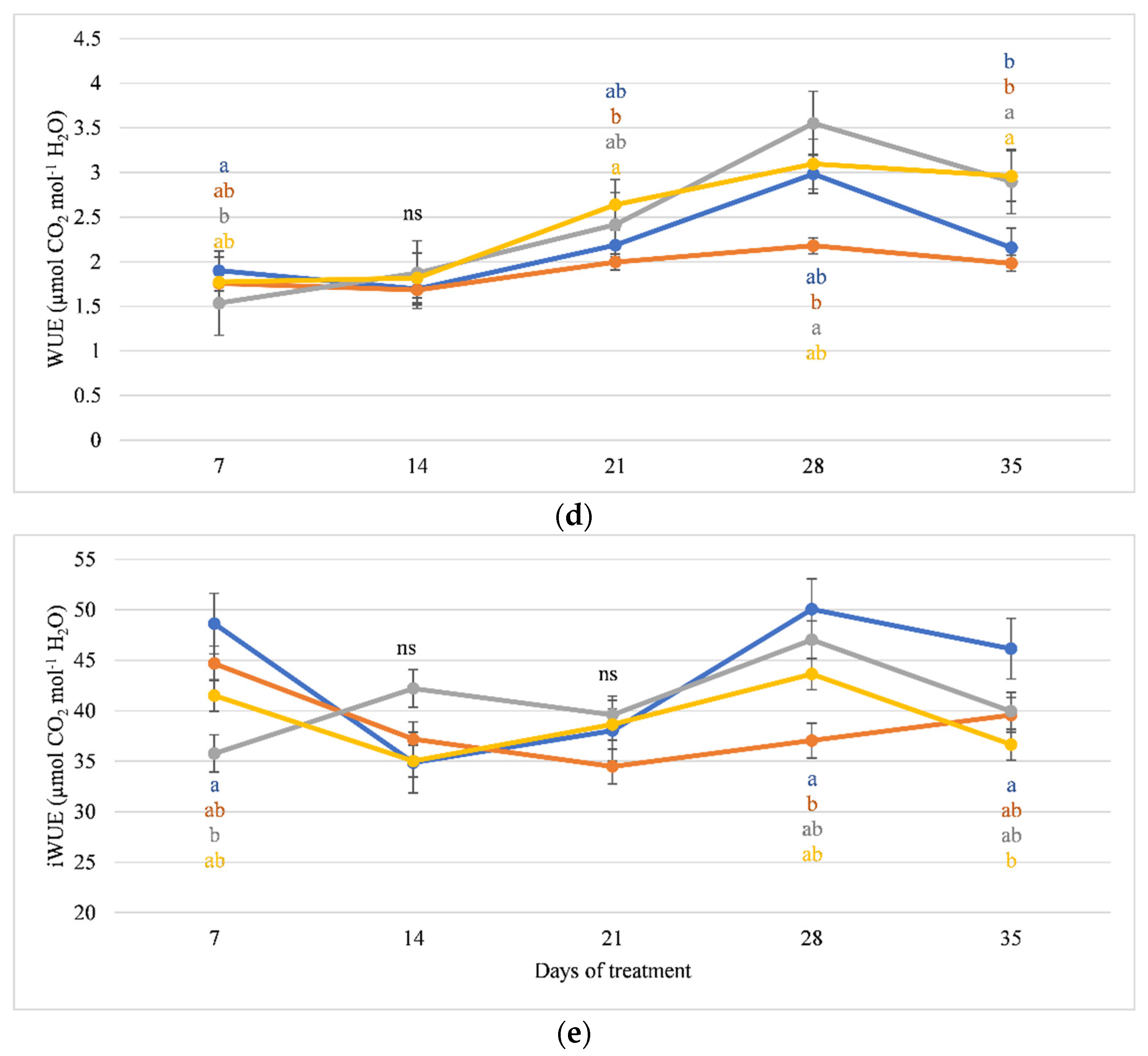
3.2. Gas Exchange and Chlorophyll Fluorescence
3.3. Photosynthetic Pigment Content and Leaf Dry Matter
3.4. Yield, Fruit Quality and Content of Biologically Active Compounds in Cucumber Fruit (Average from Two Years)
4. Discussion
4.1. Morphological Characteristics of Cucumber Plants Grown on Organic and Mineral Substrates at High EC
4.2. Variation in Photosynthetic Efficiency and Chlorophyll Fluorescence of Cucumber Grown on Organic and Mineral Substrates at High EC
4.3. Dry Matter and Photosynthetic Pigment Content of Cucumber Leaves Grown on Organic and Mineral Substrates at High EC
4.4. Yield, Quality, and Bioactive Compound Content of Cucumber Fruit Grown on Organic and Mineral Substrates at High EC
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kowalczyk, K.; Gajc-Wolska, J.; Bujalski, D.; Mirgos, M.; Niedzińska, M.; Mazur, K.; Niedzińska, P.; Szatkowski, D.; Cichoń, M.; Łęczycka, N. The effect of supplemental assimilation lighting with HPS and LED lamps on the cucumber yielding and fruit quality in autumn crop. Acta Sci. Pol. Hortorum Cultus 2018, 17, 193–200. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.-W.; Gomez Pineda, I.M.; Brand, A.M.; Stützel, H. Determining Ion Toxicity in Cucumber under Salinity Stress. Agronomy 2020, 10, 677. [Google Scholar] [CrossRef]
- Abdel-Farid, I.B.; Marghany, M.R.; Rowezek, M.M.; Sheded, M.G. Effect of Salinity Stress on Growth and Metabolomic Profiling of Cucumis sativus and Solanum lycopersicum. Plants 2020, 9, 1626. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-W.; Stützel, H.; Kahlen, K. High light aggravates functional limitations of cucumber canopy photosynthesis under salinity. Ann. Bot. 2018, 121, 797–807. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.Y.; Liu, J.L.; Wang, W.X.; Sun, Y. Exogenous melatonin improves growth and photosynthetic capacity of cucumber under salinity-induced stress. Photosynthetica 2016, 54, 19–27. [Google Scholar] [CrossRef]
- Mohsin, S.; Hasanuzzaman, M.; Bhuyan, M.; Parvin, K.; Fujita, M. Exogenous Tebuconazole and Trifloxystrobin Regulates Reactive Oxygen Species Metabolism Toward Mitigating Salt-Induced Damages in Cucumber Seedling. Plants 2019, 8, 428. [Google Scholar] [CrossRef] [Green Version]
- Miljus-Djukic, J.; Stanisavljevic, N.; Radovic, S.; Jovanovic, Z.; Mikic, A.; Maksimovic, V. Differential response of three contrasting pea (“Pisum arvense, P. sativum” and P. ’fulvum’) species to salt stress: Assessment of variation in antioxidative defence and miRNA expression. Aust. J. Crop Sci. 2013, 7, 2145–2153. [Google Scholar]
- Abdul Qados, A.M.S. Effect of salt stress on plant growth and metabolism of bean plant Vicia faba (L.). J. Saudi Soc. Agric. Sci. 2011, 10, 7–15. [Google Scholar] [CrossRef] [Green Version]
- Kamran, M.; Parveen, A.; Ahmar, S.; Malik, Z.; Hussain, S.; Chattha, M.S.; Saleem, M.H.; Adil, M.; Heidari, P.; Chen, J.-T. An Overview of Hazardous Impacts of Soil Salinity in Crops, Tolerance Mechanisms, and Amelioration through Selenium Supplementation. Int. J. Mol. Sci. 2019, 21, 148. [Google Scholar] [CrossRef] [Green Version]
- Gengmao, Z.; Shihui, L.; Xing, S.; Yizhou, W.; Zipan, C. The role of silicon in physiology of the medicinal plant (Lonicera japonica L.) under salt stress. Sci. Rep. 2015, 5, 12696. [Google Scholar] [CrossRef] [Green Version]
- Mittal, S.; Kumari, N.; Sharma, V. Differential response of salt stress on Brassica juncea: Photosynthetic performance, pigment, proline, D1 and antioxidant enzymes. Plant Physiol. Biochem. 2012, 54, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Cao, Q.; Sun, L.; Yang, X.; Yang, W.; Zhang, H. Stomatal Conductance and Morphology of Arbuscular Mycorrhizal Wheat Plants Response to Elevated CO2 and NaCl Stress. Front. Plant Sci. 2018, 9, 1363. [Google Scholar] [CrossRef] [PubMed]
- Lotfi, R.; Ghassemi-Golezani, K.; Pessarakli, M. Salicylic acid regulates photosynthetic electron transfer and stomatal conductance of mung bean (Vigna radiata L.) under salinity stress. Biocatal. Agric. Biotechnol. 2020, 26, 101635. [Google Scholar] [CrossRef]
- Pérez-López, U.; Robredo, A.; Lacuesta, M.; Mena-Petite, A.; Muñoz-Rueda, A. Elevated CO2 reduces stomatal and metabolic limitations on photosynthesis caused by salinity in Hordeum vulgare. Photosynth. Res. 2012, 111, 269–283. [Google Scholar] [CrossRef]
- Zhang, R.H.; Li, J.; Guo, S.R.; Tezuka, T. Effects of exogenous putrescine on gas-exchange characteristics and chlorophyll fluorescence of NaCl-stressed cucumber seedlings. Photosynth. Res. 2009, 100, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Savvas, D.; Pappa, V.; Kotsiras, A.; Gizas, G. NaCl accumulation in a cucumber crop grown in a completely closed hydroponic system as influenced by NaCl concentration in irrigation water. Eur. J. Hortic. Sci. 2005, 70, 217–223. [Google Scholar]
- Dannehl, D.; Suhl, J.; Ulrichs, C.; Schmidt, U. Evaluation of substitutes for rock wool as growing substrate for hydroponic tomato production. J. Appl. Bot. Food Qual. 2015, 88, 68–77. [Google Scholar] [CrossRef]
- Kennard, N.; Stirling, R.; Prashar, A.; Lopez-Capel, E. Evaluation of Recycled Materials as Hydroponic Growing Media. Agronomy 2020, 10, 1092. [Google Scholar] [CrossRef]
- Kraska, T.; Kleinschmidt, B.; Weinand, J.; Pude, R. Cascading use of Miscanthus as growing substrate in soilless cultivation of vegetables (tomatoes, cucumbers) and subsequent direct combustion. Sci. Hortic. 2018, 235, 205–213. [Google Scholar] [CrossRef]
- Łaźny, R.; Mirgos, M.; Przybył, J.L.; Nowak, J.S.; Kunka, M.; Gajc-Wolska, J.; Kowalczyk, K. Effect of re-used lignite and mineral wool growing mats on plant growth, yield and fruit quality of cucumber and physical parameters of substrates in hydroponic cultivation. Agronomy 2021, 11, 998. [Google Scholar] [CrossRef]
- Gruda, N. Increasing Sustainability of Growing Media Constituents and Stand-Alone Substrates in Soilless Culture Systems. Agronomy 2019, 9, 298. [Google Scholar] [CrossRef] [Green Version]
- Czaplicka-Kolarz, K.; Burchart-Korol, D.; Krawczyk, P. Metodyka analizy ekoefektywności. J. Ecol. Health 2010, 14, 267–271. [Google Scholar]
- Vinci, G.; Rapa, M. Hydroponic cultivation: Life cycle assessment of substrate choice. Br. Food J. 2019, 121, 1801–1812. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; Rouphael, Y. Towards a new definition of quality for fresh fruits and vegetables. Sci. Hortic. 2018, 234, 463–469. [Google Scholar] [CrossRef]
- Rouphael, Y.; Petropoulos, S.A.; Cardarelli, M.; Colla, G. Salinity as eustressor for enhancing quality of vegetables. Sci. Hortic. 2018, 234, 361–369. [Google Scholar] [CrossRef]
- Baenas, N.; García-Viguera, C.; Moreno, D. Elicitation: A Tool for Enriching the Bioactive Composition of Foods. Molecules 2014, 19, 13541–13563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viacava, G.E.; Goyeneche, R.; Goñi, M.G.; Roura, S.I.; Agüero, M.V. Natural elicitors as preharvest treatments to improve postharvest quality of Butterhead lettuce. Sci. Hortic. 2018, 228, 145–152. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Jawad, R.; Kumar, P.; Rea, E.; Cardarelli, M. The effectiveness of grafting to improve NaCl and CaCl2 tolerance in cucumber. Sci. Hortic. 2013, 164, 380–391. [Google Scholar] [CrossRef]
- Giuffrida, F.; Cassaniti, C.; Malvuccio, A.; Leonardi, C. Effects of salt stress imposed during two growth phases on cauliflower production and quality. J. Sci. Food Agric. 2017, 97, 1552–1560. [Google Scholar] [CrossRef]
- Borgognone, D.; Cardarelli, M.; Rea, E.; Lucini, L.; Colla, G. Salinity source-induced changes in yield, mineral composition, phenolic acids and flavonoids in leaves of artichoke and cardoon grown in floating system. J. Sci. Food Agric. 2014, 94, 1231–1237. [Google Scholar] [CrossRef]
- Mayta, M.L.; Arce, R.C.; Zurbriggen, M.D.; Valle, E.M.; Hajirezaei, M.-R.; Zanor, M.I.; Carrillo, N. Expression of a Chloroplast-Targeted Cyanobacterial Flavodoxin in Tomato Plants Increases Harvest Index by Altering Plant Size and Productivity. Front. Plant Sci. 2019, 10, 1432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbagallo, R.N.; Di Silvestro, I.; Patanè, C. Yield, physicochemical traits, antioxidant pattern, polyphenol oxidase activity and total visual quality of field-grown processing tomato cv. Brigade as affected by water stress in Mediterranean climate. J. Sci. Food Agric. 2012, 93, 1449–1457. [Google Scholar] [CrossRef] [PubMed]
- Kulapichitr, F.; Borompichaichartkul, C.; Fang, M.; Suppavorasatit, I.; Cadwallader, K.R. Effect of post-harvest drying process on chlorogenic acids, antioxidant activities and CIE-Lab color of Thai Arabica green coffee beans. Food Chem. 2022, 366, 130504. [Google Scholar] [CrossRef] [PubMed]
- López Camelo, A.F.; Gómez, P.A. Comparison of color indexes for tomato ripening. Hortic. Bras. 2004, 22, 534–537. [Google Scholar] [CrossRef]
- Rajendran, K.; Tester, M.; Roy, S.J. Quantifying the three main components of salinity tolerance in cereals. Plant. Cell Environ. 2009, 32, 237–249. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Ahmadi, F.I.; Karimi, K.; Struik, P.C. Effect of exogenous application of methyl jasmonate on physiological and biochemical characteristics of Brassica napus L. cv. Talaye under salinity stress. S. Afr. J. Bot. 2018, 115, 5–11. [Google Scholar] [CrossRef]
- Mallahi, T.; Saharkhiz, M.J.; Javanmardi, J. Salicylic acid changes morpho-physiological attributes of feverfew (Tanacetum parthenium L.) under salinity stress. Acta Ecol. Sin. 2018, 38, 351–355. [Google Scholar] [CrossRef]
- Schwarz, D.; Kuchenbuch, R. Water uptake by tomato plants grown in closed hydroponic systems dependent on the EC-level. Acta Hortic. 1998, 458, 323–328. [Google Scholar] [CrossRef]
- Ding, X.; Jiang, Y.; Zhao, H.; Guo, D.; He, L.; Liu, F.; Zhou, Q.; Nandwani, D.; Hui, D.; Yu, J. Electrical conductivity of nutrient solution influenced photosynthesis, quality, and antioxidant enzyme activity of pakchoi (Brassica campestris L. ssp. Chinensis) in a hydroponic system. PLoS ONE 2018, 13, e0202090. [Google Scholar] [CrossRef]
- Van Ieperen, W. Effects of different day and night salinity levels on vegetative growth, yield and quality of tomato. J. Hortic. Sci. 1996, 71, 99–111. [Google Scholar] [CrossRef]
- Gorbe, E.; Calatayud, Á. Optimization of nutrition in soilless systems: A Review. In Advances in Botanical Research; Academic Press: New York, NY, USA, 2010; Volume 53, pp. 193–245. [Google Scholar]
- Van der Sleen, P.; Groenendijk, P.; Vlam, M.; Anten, N.P.R.; Boom, A.; Bongers, F.; Pons, T.L.; Terburg, G.; Zuidema, P.A. No growth stimulation of tropical trees by 150 years of CO2 fertilization but water-use efficiency increased. Nat. Geosci. 2014, 8, 24–28. [Google Scholar] [CrossRef]
- Santos, M.G.; Ribeiro, R.V.; Machado, E.C.; Pimentel, C. Photosynthetic parameters and leaf water potential of five common bean genotypes under mild water deficit. Biol. Plant. 2009, 53, 229–236. [Google Scholar] [CrossRef]
- Gong, H.; Chen, K. The regulatory role of silicon on water relations, photosynthetic gas exchange, and carboxylation activities of wheat leaves in field drought conditions. Acta Physiol. Plant. 2012, 34, 1589–1594. [Google Scholar] [CrossRef]
- Wu, Y.; Jin, X.; Liao, W.; Hu, L.; Dawuda, M.M.; Zhao, X.; Tang, Z.; Gong, T.; Yu, J. 5-Aminolevulinic Acid (ALA) Alleviated Salinity Stress in Cucumber Seedlings by Enhancing Chlorophyll Synthesis Pathway. Front. Plant. Sci. 2018, 9, 635. [Google Scholar] [CrossRef] [Green Version]
- Talebnejad, R.; Sepaskhah, A.R. Physiological characteristics, gas exchange, and plant ion relations of quinoa to different saline groundwater depths and water salinity. Arch. Agron. Soil Sci. 2016, 62, 1347–1367. [Google Scholar] [CrossRef]
- Stirbet, A.; Govindjee. On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and Photosystem II: Basics and applications of the OJIP fluorescence transient. J. Photochem. Photobiol. B Biol. 2011, 104, 236–257. [Google Scholar] [CrossRef]
- Christou, A.; Manganaris, G.A.; Papadopoulos, I.; Fotopoulos, V. Hydrogen sulfide induces systemic tolerance to salinity and non-ionic osmotic stress in strawberry plants through modification of reactive species biosynthesis and transcriptional regulation of multiple defence pathways. J. Exp. Bot. 2013, 64, 1953–1966. [Google Scholar] [CrossRef]
- Jiang, J.-L.; Tian, Y.; Li, L.; Yu, M.; Hou, R.-P.; Ren, X.-M. H2S Alleviates Salinity Stress in Cucumber by Maintaining the Na+/K+ Balance and Regulating H2S Metabolism and Oxidative Stress Response. Front. Plant. Sci. 2019, 10, 678. [Google Scholar] [CrossRef] [Green Version]
- Hussain, S.; Bai, Z.; Huang, J.; Cao, X.; Zhu, L.; Zhu, C.; Khaskheli, M.A.; Zhong, C.; Jin, Q.; Zhang, J. 1-Methylcyclopropene Modulates Physiological, Biochemical, and Antioxidant Responses of Rice to Different Salt Stress Levels. Front. Plant. Sci. 2019, 10, 124. [Google Scholar] [CrossRef] [Green Version]
- Lee, G.; Carrow, R.N.; Duncan, R.R. Photosynthetic responses to salinity stress of halophytic seashore paspalum ecotypes. Plant. Sci. 2004, 166, 1417–1425. [Google Scholar] [CrossRef]
- Siler, B.; Misic, D.; Filipovic, B.; Popovic, Z.; Cvetic, T.; Mijovic, A. Effects of salinity on in vitro growth and photosynthesis of common centaury (Centaurium erythraea Rafn.). Arch. Biol. Sci. 2007, 59, 129–134. [Google Scholar] [CrossRef]
- Albornoz, F.; Lieth, J.H. Over fertilization limits lettuce productivity because of osmotic stress. Chil. J. Agric. Res. 2015, 75, 284–290. [Google Scholar] [CrossRef] [Green Version]
- Ouzounidou, G.; Giannakoula, A.; Ilias, I.; Zamanidis, P. Alleviation of drought and salinity stresses on growth, physiology, biochemistry and quality of two Cucumis sativus L. cultivars by Si application. Braz. J. Bot. 2016, 39, 531–539. [Google Scholar] [CrossRef]
- Dorai, M.; Papadopoulos, A.; Gosselin, A.; Dorais, M.; Papadopoulos, A.P. Influence of electric conductivity management on greenhouse tomato yield and fruit quality. Agronomie 2001, 21, 367–383. [Google Scholar] [CrossRef] [Green Version]
- Rosadi, R.A.B.; Senge, M.; Suhandy, D.; Tusi, A. The Effect of EC Levels of Nutrient Solution on the Growth, Yield, and Quality of Tomatoes (Solanum lycopersicum) under the Hydroponic System. J. Agric. Eng. Biotechnol. 2014, 2, 7–12. [Google Scholar] [CrossRef]
- Scuderi, D.; Restuccia, C.; Chisari, M.; Barbagallo, R.N.; Caggia, C.; Giuffrida, F. Salinity of nutrient solution influences the shelf-life of fresh-cut lettuce grown in floating system. Postharvest Biol. Technol. 2011, 59, 132–137. [Google Scholar] [CrossRef]
- Abou-Hadid, A.F.; Abd-Elmoniem, E.M.; El-Shinawy, M.Z.; Abou-Elsoud, M. Electrical conductivity effect on growth and mineral composition of lettuce plants in hydroponic system. Acta Hortic. 1996, 434, 59–66. [Google Scholar] [CrossRef]
- Rubio, J.S.; García-Sánchez, F.; Rubio, F.; Martínez, V. Yield, blossom-end rot incidence, and fruit quality in pepper plants under moderate salinity are affected by K+ and Ca2+ fertilization. Sci. Hortic. 2009, 119, 79–87. [Google Scholar] [CrossRef]
- Coolong, T.W.; Randle, W.M.; Wicker, L. Structural and chemical differences in the cell wall regions in relation to scale firmness of three onion (Allium cepa L.) selections at harvest and during storage. J. Sci. Food Agric. 2008, 88, 1277–1286. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; Rouphael, Y.; Colla, G.; Zrenner, R.; Schwarz, D. Vegetable Grafting: The Implications of a Growing Agronomic Imperative for Vegetable Fruit Quality and Nutritive Value. Front. Plant. Sci. 2017, 8, 741. [Google Scholar] [CrossRef]
- Navarro, J.M.; Garrido, C.; Carvajal, M.; Martinez, V. Yield and fruit quality of pepper plants under sulphate and chloride salinity. J. Hortic. Sci. Biotechnol. 2002, 77, 52–57. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Rea, E.; Cardarelli, M. Grafting cucumber plants enhance tolerance to sodium chloride and sulfate salinization. Sci. Hortic. 2012, 135, 177–185. [Google Scholar] [CrossRef]
- Sublett, W.; Barickman, T.; Sams, C. The Effect of Environment and Nutrients on Hydroponic Lettuce Yield, Quality, and Phytonutrients. Horticulturae 2018, 4, 48. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-J.; Fonseca, J.M.; Choi, J.-H.; Kubota, C.; Kwon, D.Y. Salt in Irrigation Water Affects the Nutritional and Visual Properties of Romaine Lettuce (Lactuca sativa L.). J. Agric. Food Chem. 2008, 56, 3772–3776. [Google Scholar] [CrossRef] [PubMed]
- Pérez-López, U.; Miranda-Apodaca, J.; Lacuesta, M.; Mena-Petite, A.; Muñoz-Rueda, A. Growth and nutritional quality improvement in two differently pigmented lettuce cultivars grown under elevated CO2 and/or salinity. Sci. Hortic. 2015, 195, 56–66. [Google Scholar] [CrossRef]
- Colla, G.; Kim, H.-J.; Kyriacou, M.C.; Rouphael, Y. Nitrate in fruits and vegetables. Sci. Hortic. 2018, 237, 221–238. [Google Scholar] [CrossRef]
| Parameter | Unit | Combination | |||||
|---|---|---|---|---|---|---|---|
| MW/ Control EC | L/ Control EC | MW/ High EC | L/ High EC | ||||
| Shoot | Weekly increase in length | cm | 57.9 ± 1.9 ns | 62.2 ± 1.7 ns | 57.6 ± 1.9 ns | 60.0 ± 1.7 ns | |
| Total length | 276.8 ± 3.0 ab | 286.9 ± 1.4 a | 269.7 ± 3.8 b | 280.4 ± 4.4 a | |||
| Diameter under 5th leaf | mm | 6.6 ± 0.1 a | 6.5 ± 0.1 ab | 6.2 ± 0.1 b | 6.4 ± 0.1 ab | ||
| Diameter under 10th leaf | 7.7 ± 0.1 a | 8.0 ± 0.1 a | 7.3 ± 0.1 b | 7.6 ±0.1 ab | |||
| Leaf | Number per week | pcs plant−1 | 4.1 ± 0.1 ns | 4.1 ± 0.1 ns | 4.0 ± 0.1 ns | 4.1 ± 0.1 ns | |
| 5th leaf | Length | cm | 20.3 ± 0.4 ns | 21.2 ± 0.4 ns | 20.3 ± 0.3 ns | 20.0 ± 0.4 ns | |
| Width | 23. 8 ± 0.4 ns | 24.8 ± 0.5 ns | 24.2 ± 0.4 ns | 23.7 ± 0.4 ns | |||
| Petiole length | 14.0 ± 0.4 ns | 14.2 ± 0.4 ns | 13.6 ± 0.3 ns | 13.6 ± 0.3 ns | |||
| Chlorophyll content | SPAD unit | 41.4 ± 0.7 ns | 41.2 ± 0.6 ns | 42.0 ± 0.7 ns | 42.3 ± 0.7 ns | ||
| 10th leaf | Length | cm | 25.3 ± 0.5 ns | 25.6 ± 0.6 ns | 24.0 ± 0.5 ns | 24.8 ± 0.5 ns | |
| Width | 31.5 ± 0.6 a | 31.5 ± 0.6 a | 30.7 ± 0.6 ab | 29.2 ± 0.6 b | |||
| Petiole length | 18.2 ± 0.4 ns | 18.0 ± 0.4 ns | 17.7 ± 0.3 ns | 17.0 ± 0.3 ns | |||
| Chlorophyll content | SPAD unit | 44.3 ± 0.4 ns | 44.8 ±0.4 ns | 44.8 ± 0.7 ns | 44.9 ± 0.4 ns | ||
| Total leaf and shoot weight | g plant−1 | 1267.8 ± 28.6 a | 1195.7 ± 22.2 a | 1164.0 ± 28.5 a | 934.9 ± 28.5 b | ||
| Number of Leaf | Parameter | Unit | Combination | |||
|---|---|---|---|---|---|---|
| MW/Control EC | L/Control EC | MW/High EC | L/High EC | |||
| 5th | Dry matter | % | 11.4 ± 0.1 a * | 11.0 ± 0.1 a | 11.5 ± 0.2 a | 11.4 ± 0.1 a |
| β-carotene | mg 100 g−1 FW | 16.6 ± 0.1 c | 20.5 ± 0.6 b | 20.4 ± 0.5 b | 22.4 ± 0.3 a | |
| Lutein | 10.6 ± 0.2 c | 14.8 ± 0.3 a | 13.6 ± 0.4 ab | 13.2 ± 0.4 b | ||
| Chlorophyll a | 124.4 ± 1.2 c | 142.9 ± 2.2 b | 139.7 ± 4.0 b | 155.0 ± 4.2 a | ||
| Chlorophyll b | 39.6 ± 0.4 c | 49.8 ± 1.1 a | 45.8 ± 1.1 b | 46.0 ± 0.7 b | ||
| Total chlorophyll a + b | 164.0 ± 1.6 c | 204.8 ± 5.3 a | 185.5 ± 5.1 b | 188.9 ± 2.9 b | ||
| 10th | Dry matter | % | 11.8 ± 0.1 b | 12.4 ± 0.2 ab | 13.1 ± 0.3 a | 13.2 ± 0.3 a |
| β-carotene | mg 100 g−1 FW | 14.9 ± 0.1 ab | 16.2 ± 0.5 ab | 14.7 ± 0.3 c | 16.4 ± 0.4 a | |
| Lutein | 9.7 ± 0.1 ab | 10.3 ± 0.1 ab | 9.5± 0.4 b | 10.4 ± 0.03 a | ||
| Chlorophyll a | 114.4 ± 0.9 ab | 116.9± 2.7 ab | 109.9 ± 4.7 b | 122.6 ± 1.4 a | ||
| Chlorophyll b | 42.0 ± 0.5 a | 38.1 ± 1.0 ab | 36.9 ± 1.5 b | 40.8 ± 0.9 ab | ||
| Total chlorophyll a + b | 156.5 ± 1.0 ab | 155.0 ± 3.7 ab | 146.8 ± 6.2 b | 163.4 ± 2.3 a | ||
| Harvested Fruit | Unit | Combination | ||||
|---|---|---|---|---|---|---|
| MW/ Control EC | L/ Control EC | MW/ High EC | L/ High EC | |||
| Weight of fruit | Total fruit | g plant−1 | 6503.2 ± 131.1 b | 7004.5 ± 102.1 a | 4766.0 ± 202.7 d | 5471.4 ± 145.3 c |
| Marketable fruit | 6190.3 ± 113.5 b | 6834.2 ± 83.7 a | 3486.5 ± 217.8 d | 4311.9 ± 150.2 c | ||
| Unmarketable fruit | 312.9 ± 71.9 b | 170.3 ± 60.7 b | 1279.5 ± 108.7 a | 1159.5 ± 0.8 a | ||
| Number of fruit | Total fruit | pcs plant−1 | 29.5 ± 0.8 b | 32.0 ± 0.6 a | 22.9 ± 0.4 d | 26.1 ± 0.4 c |
| Marketable fruit | 27.9 ± 0.5 b | 31.2 ± 0.3 a | 16.0 ± 0.9 d | 19.8 ± 0.7 c | ||
| Unmarketable fruit | 1.6 ± 0.3 b | 0.8 ± 0.3 c | 6.9 ± 0.6 a | 6.3 ± 0.5 a | ||
| Aborded fruit | 0 | 0 | 7.3 ± 0.4 a | 6.4 ± 0.3 a | ||
| HI index | 0.84 ± 0.02 a | 0.85 ± 0.02 a | 0.80 ± 0.02 b | 0.85 ± 0.02 a | ||
| Combination | |||||
|---|---|---|---|---|---|
| Parameter | Unit | MW/ Control EC | L/ Control EC | MW/ High EC | L/ High EC |
| Firmness | HPE | 63.1 ± 0.8 ns | 63.7 ± 0.5 ns | 61.8 ± 0.4 ns | 63.9 ± 0.6 ns |
| Colour | a* | −7.2 ± 0.3 ns | −7.0 ± 0.2 ns | −6.7 ± 0.2 ns | −6.6 ± 0.2 ns |
| b* | 12.7 ± 0.7 ab | 12.9 ± 0.5 a | 11.2 ± 0.3 ab | 10.9 ± 0.3 b | |
| L* | 32.1 ± 1.1 ns | 33.4 ± 0.9 ns | 31.7 ± 0.3 ns | 32.0 ± 0.8 ns | |
| C* | 14.6 ± 0.8 ns | 14.7 ± 0.6 ns | 13.0 ± 0.4 ns | 12.7 ± 0.4 ns | |
| H* | 124.8 ± 2.1 ns | 128.0 ± 1.2 ns | 122.2 ± 1.6 ns | 122.1 ± 1.0 ns | |
| a*/b* | −0.57 ± 0.02 ab | −0.54 ± 0.01 b | −0.60 ± 0.01 a | −0.60 ± 0.01 a | |
| Combination | |||||
|---|---|---|---|---|---|
| Parameter | Unit | MW/ Control EC | L/ Control EC | MW/ High EC | L/ High EC |
| Dry matter | % | 3.8 ± 0.1 c | 4.0 ± 0.1 bc | 4.6 ± 0.2 a | 4.4 ± 0.1 ab |
| TSS | 3.8 ± 0.1 ns | 4.0 ± 0.2 ns | 4.3 ± 0.1 ns | 4.3 ± 0.2 ns | |
| β-carotene | mg 100 g−1 FW | 0.2 ± 0.01 b | 0.3 ± 0.01 a | 0.2 ± 0.01 b | 0.3 ± 0.01 a |
| Lutein | 0.4 ± 0.01 b | 0.5 ± 0.03 a | 0.3 ± 0.01 b | 0.5 ± 0.01 a | |
| Chlorophyll a | 3.7 ± 0.1 b | 4.6 ± 0.2 a | 3.0 ± 0.2 c | 4.2 ± 0.09 a | |
| Chlorophyll b | 1.7 ± 0.07 bc | 2.2 ± 0.02 a | 1.4 ± 0.07 c | 1.9 ± 0.04 ab | |
| Total chlorophyll a + b | 5.4 ± 0.2 b | 6.7 ± 0.3 a | 4.4 ± 0.2 c | 6.0 ± 0.1 a | |
| Nitrates | mg NO3 kg−1 FW | 28.2 ± 1.6 c | 15.5 ± 0.6 d | 89.9 ± 0.4 a | 45.0 ± 1.3 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łaźny, R.; Mirgos, M.; Przybył, J.L.; Niedzińska, M.; Gajc-Wolska, J.; Kowalczyk, W.; Nowak, J.S.; Kalisz, S.; Kowalczyk, K. Lignite Substrate and EC Modulates Positive Eustress in Cucumber at Hydroponic Cultivation. Agronomy 2022, 12, 608. https://doi.org/10.3390/agronomy12030608
Łaźny R, Mirgos M, Przybył JL, Niedzińska M, Gajc-Wolska J, Kowalczyk W, Nowak JS, Kalisz S, Kowalczyk K. Lignite Substrate and EC Modulates Positive Eustress in Cucumber at Hydroponic Cultivation. Agronomy. 2022; 12(3):608. https://doi.org/10.3390/agronomy12030608
Chicago/Turabian StyleŁaźny, Radosław, Małgorzata Mirgos, Jarosław L. Przybył, Monika Niedzińska, Janina Gajc-Wolska, Waldemar Kowalczyk, Jacek S. Nowak, Stanisław Kalisz, and Katarzyna Kowalczyk. 2022. "Lignite Substrate and EC Modulates Positive Eustress in Cucumber at Hydroponic Cultivation" Agronomy 12, no. 3: 608. https://doi.org/10.3390/agronomy12030608
APA StyleŁaźny, R., Mirgos, M., Przybył, J. L., Niedzińska, M., Gajc-Wolska, J., Kowalczyk, W., Nowak, J. S., Kalisz, S., & Kowalczyk, K. (2022). Lignite Substrate and EC Modulates Positive Eustress in Cucumber at Hydroponic Cultivation. Agronomy, 12(3), 608. https://doi.org/10.3390/agronomy12030608







