The Utilization of Phosphogypsum as a Sustainable Phosphate-Based Fertilizer by Aspergillus niger
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Fungal Strains
2.2. Medium Preparation
2.3. TCP, PG and Soil Collection
2.4. PG Fertilizer Preparation
2.5. Flask Experiment
2.6. Soil Incubation Experiment
2.7. Instrumentation
2.8. Statistical Analysis
3. Results
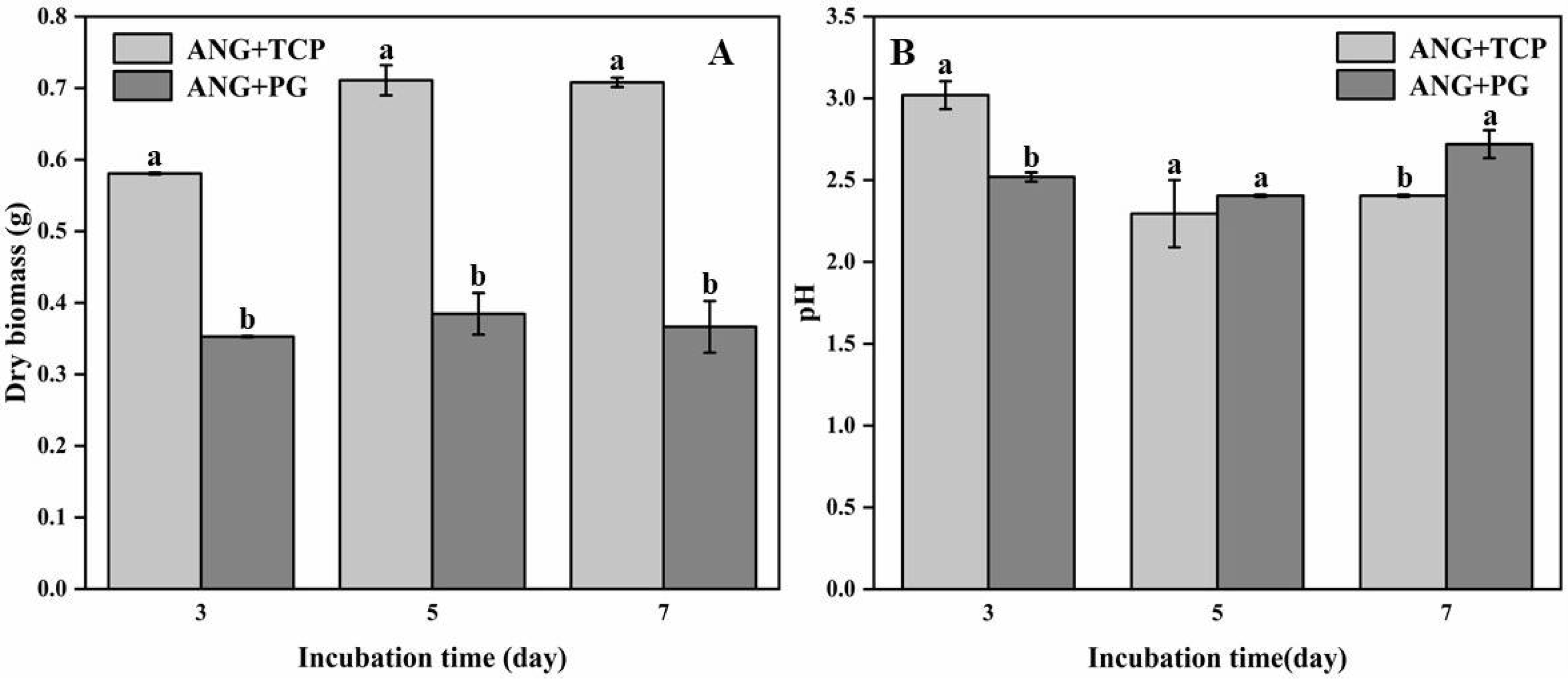
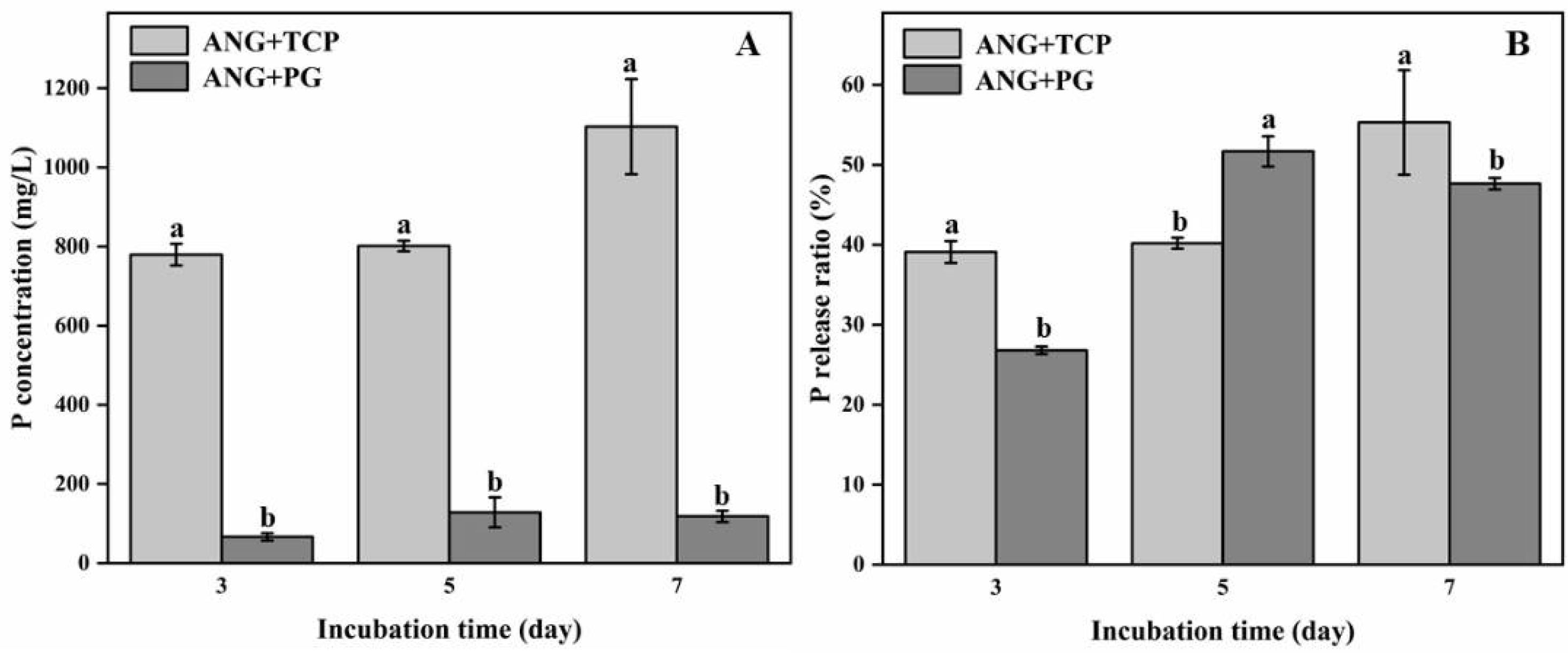
3.1. Fungal Biomass, pH, and P Content Released by A. niger in Flask Experiment
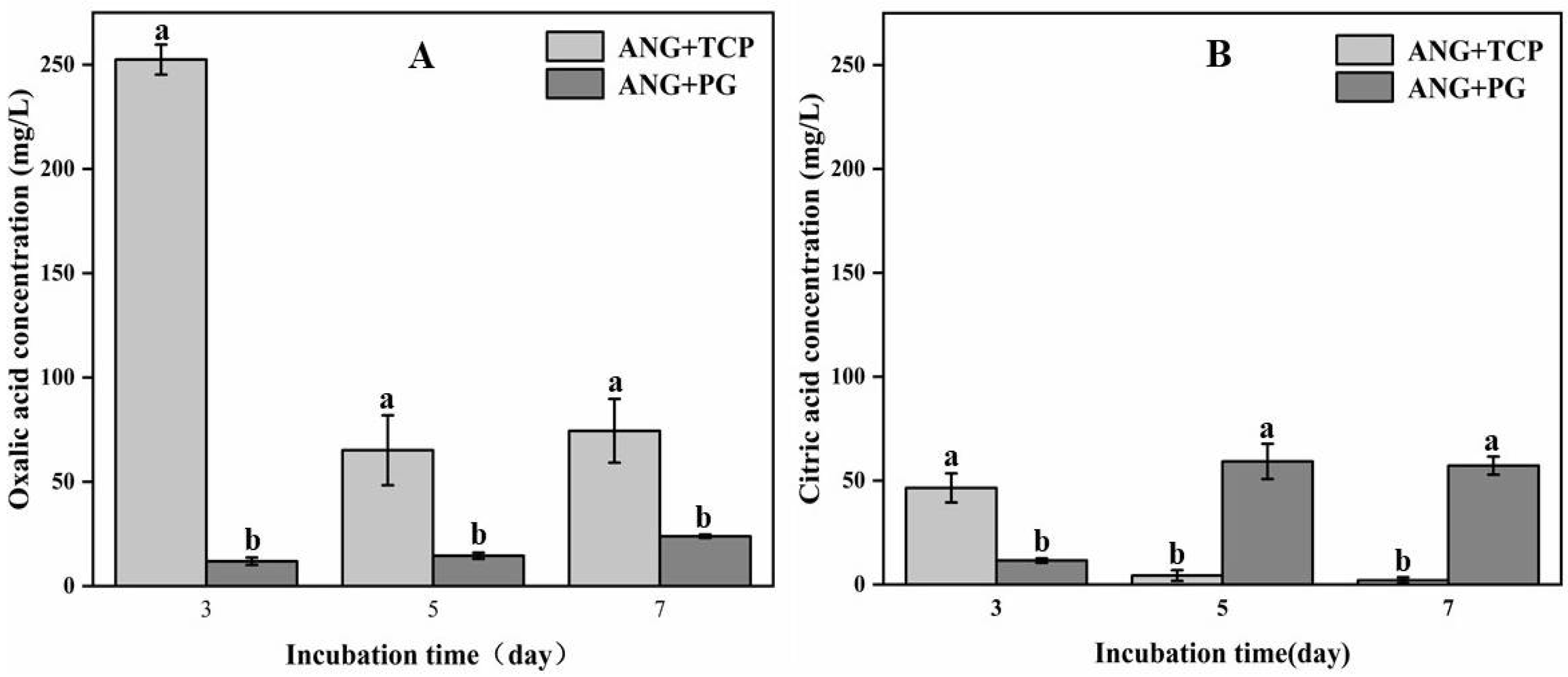
3.2. Secretion of Organic Acids by A. niger in Flask Experiment
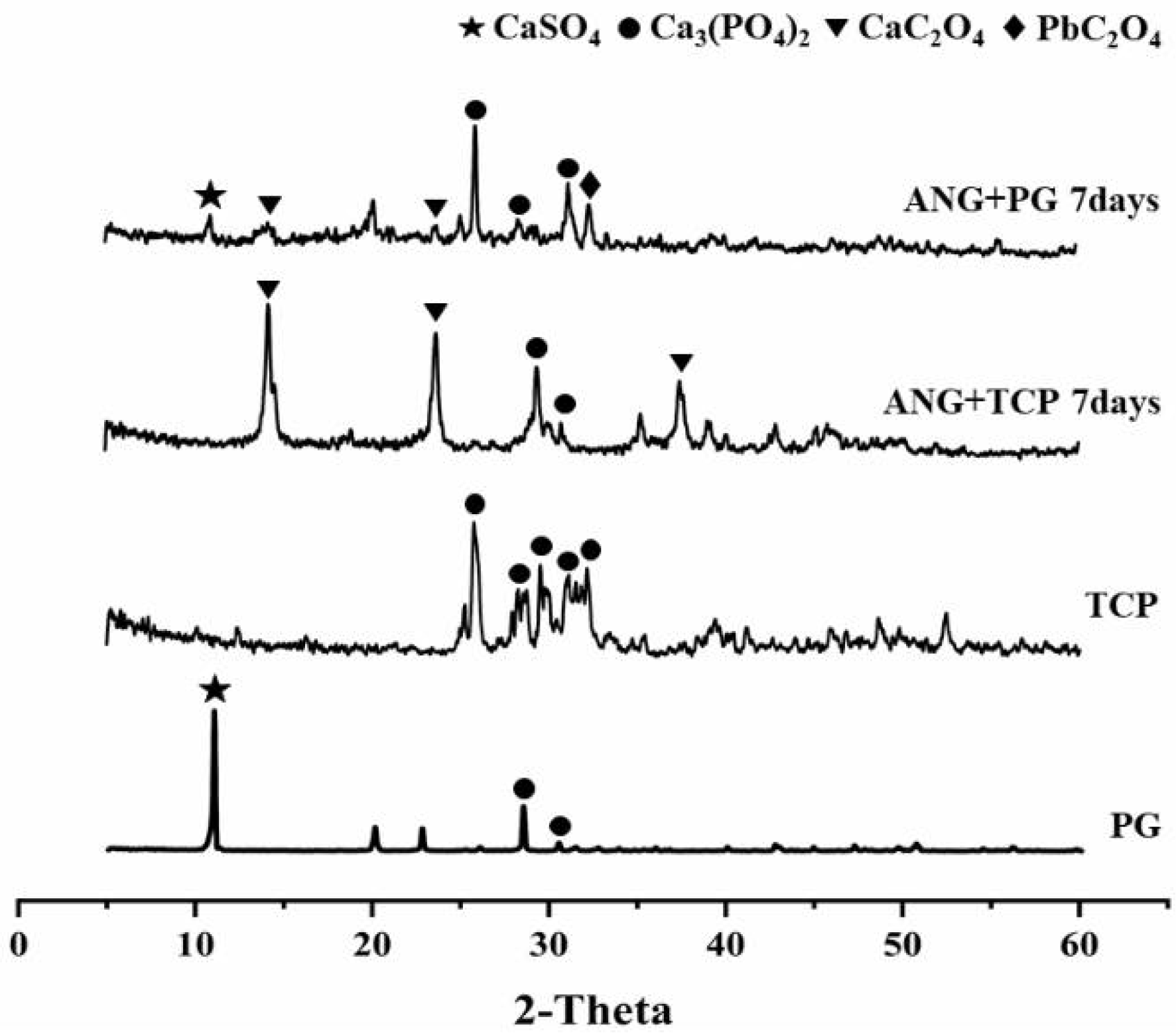
3.3. XRD Analysis
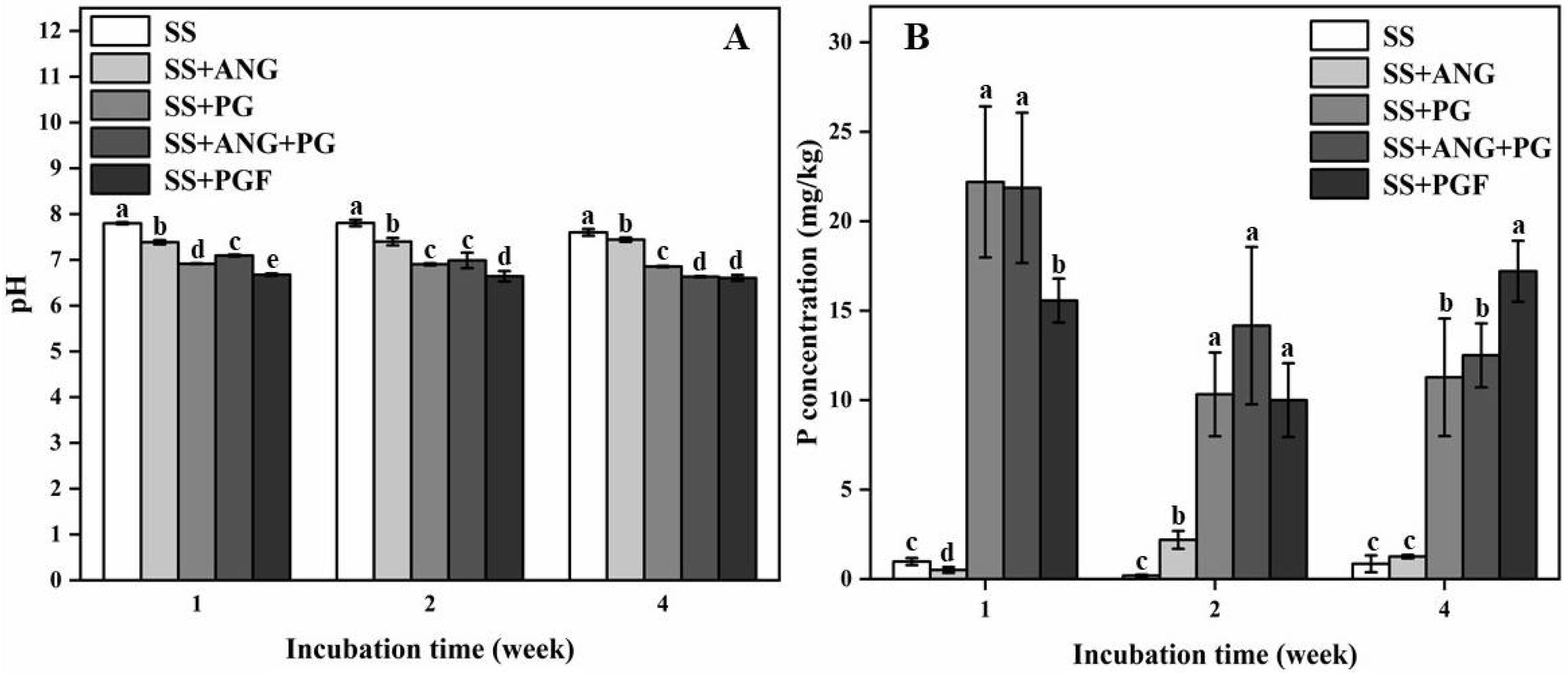
3.4. Soil pH and Soil Soluble P Content
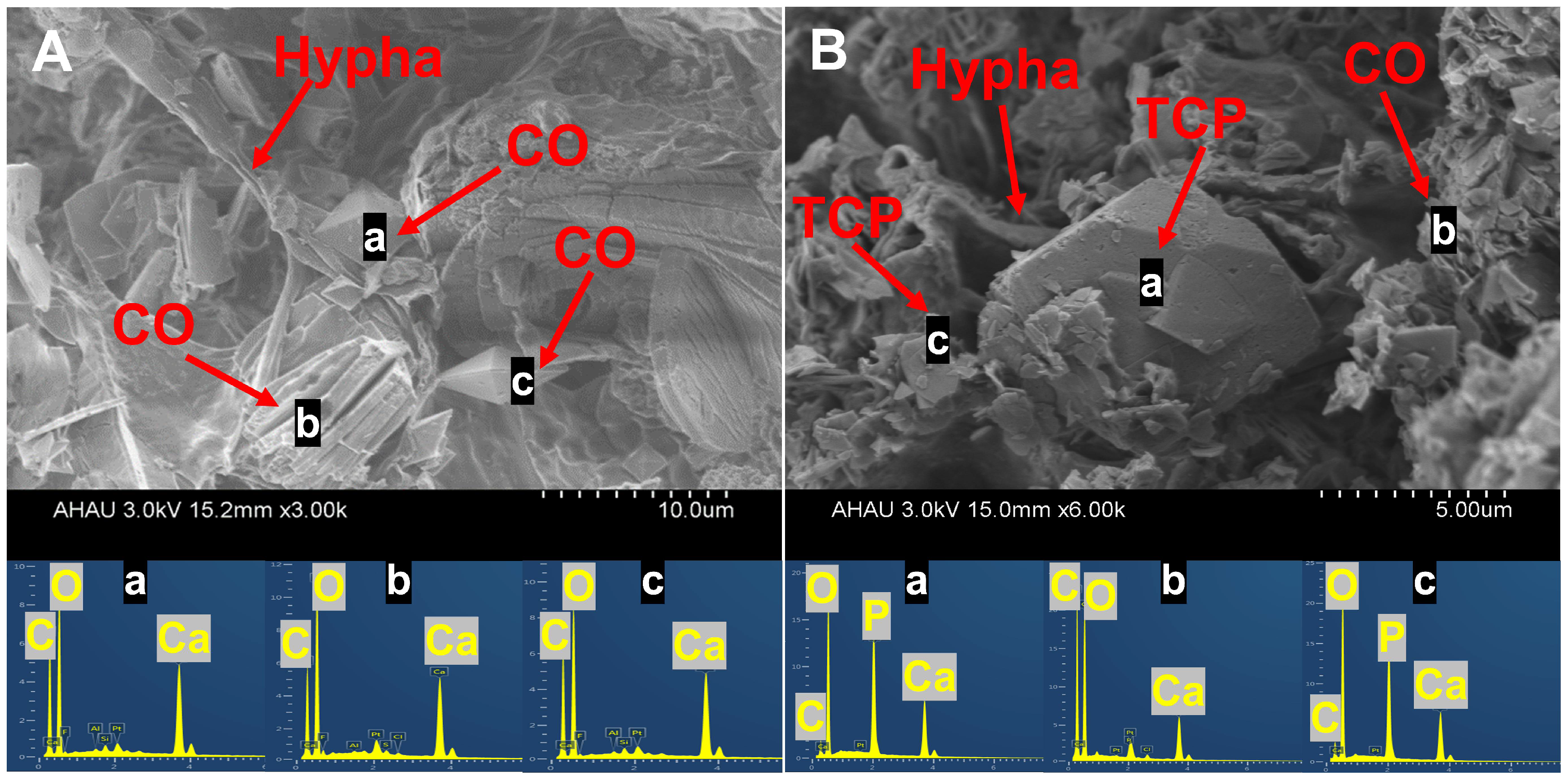
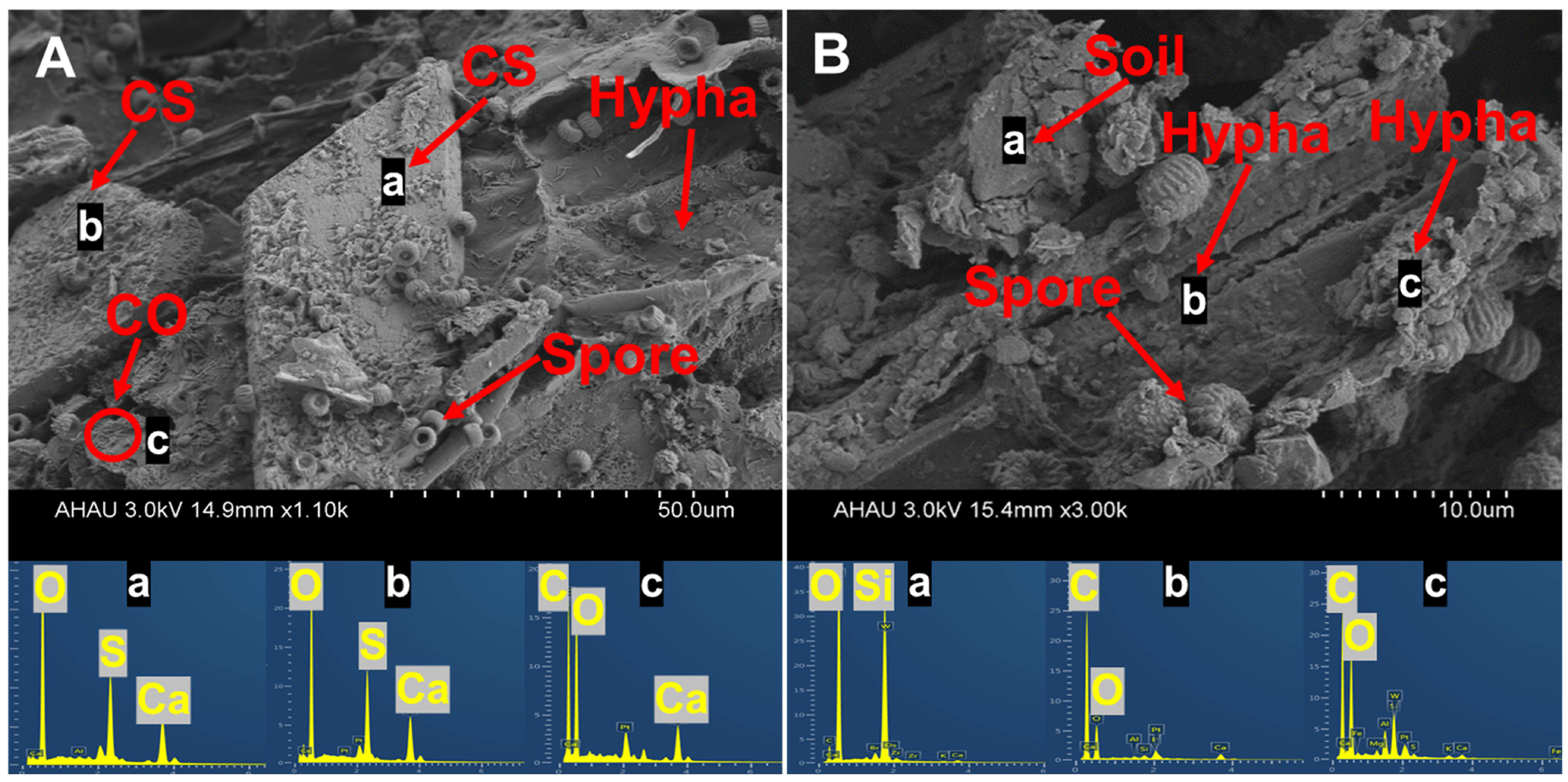
3.5. SEM-EDS Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gadd, G.M. Geomycology: Biogeochemical transformations of rocks, minerals, metals and radionuclides by fungi, bioweathering and bioremediation. Mycol. Prog. 2007, 111, 3–49. [Google Scholar] [CrossRef]
- Ruan, Y.Y.; He, D.S.; Chi, R. Review on beneficiation techniques and reagents used for phosphate ores. Minerals 2019, 9, 253. [Google Scholar] [CrossRef] [Green Version]
- Cisse, L.; Mrabet, T. World Phosphate Production: Overview and Prospects. Phosphorus Res. Bull. 2012, 15, 21–25. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Gao, H.; Luo, Y.; Gao, J. Status, trends and suggestions of phosphorus ore resources at home and abroad. China Min. Mag. 2015, 24, 6–10. [Google Scholar]
- Karamesouti, M.; Gasparatos, D. Sustainable Management of Soil Phosphorus in a Changing World. In Adaptive Soil Management: From Theory to Practices; Rakshit, A., Abhilash, P., Singh, H., Ghosh, S., Eds.; Springer: Singapore, 2017; pp. 189–214. ISBN 978-981-10-3637-8. [Google Scholar]
- Cordell, D.; Drangert, J.O.; White, S. The story of phosphorus: Global food security and food for thought. Global. Environ. Chang. 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Saadaoui, E.; Ghazel, N.; Ben Romdhane, C.; Massoudi, N. Phosphogypsum: Potential uses and problems—A review. Int. J. Environ. Stud. 2017, 74, 558–567. [Google Scholar] [CrossRef]
- Tayibi, H.; Choura, M.; Lopez, F.A.; Alguacil, F.J.; Lopez-Delgado, A. Environmental impact and management of phosphogypsum. J. Environ. Manag. 2009, 90, 2377–2386. [Google Scholar] [CrossRef] [Green Version]
- Singh, M. Treating waste phosphogypsum for cement and plaster manufacture. Cem. Concr. Res. 2002, 32, 1033–1038. [Google Scholar] [CrossRef]
- Canut, M.M.C.; Jacomino, V.M.F.; Bråtveit, K.; Gomes, A.M.; Yoshida, M.I. Microstructural analyses of phosphogypsum generated by Brazilian fertilizer industries. Mater. Charact. 2008, 59, 365–373. [Google Scholar] [CrossRef]
- Tian, D.; Li, Z.; O’Connor, D.; Shen, Z.; Hou, D. The need to prioritize sustainable phosphate-based fertilizers. Soil. Use. Manag. 2020, 36, 351–354. [Google Scholar] [CrossRef]
- Al-Enazy, A.R.; Al-Oud, S.S.; Al-Barakah, F.N.; Usman, A.R. Role of microbial inoculation and industrial by-product phosphogypsum in growth and nutrient uptake of maize (Zea mays L.) grown in calcareous soil. J. Sci. Food. Agric. 2017, 97, 3665–3674. [Google Scholar] [CrossRef]
- Rutherford, P.M.; Dudas, M.J.; Samek, R.A. Environmental impacts of phosphogypsum. Sci. Total Environ. 1994, 149, 2377–2386. [Google Scholar] [CrossRef]
- Peng, H.; Fang, S.X.; Yang, W.Q.; Hai-Tao, H.E.; Zong, S.R. Microwave Weight Method for Rapid Determination of Total Phosphorus in Phosphogypsum. Guangzhou Chem. Ind. 2014, 42, 124–153. [Google Scholar]
- Cao, J.-X. Study on existing form and distribution of the impurities in phosphogypsum. J. Guizhou Univ. Nat. Sci. Ed. 2009, 26, 95–99. [Google Scholar]
- Shen, Z.; Tian, D.; Zhang, X.; Tang, L.; Su, M.; Zhang, L.; Li, Z.; Hu, S.; Hou, D. Mechanisms of biochar assisted immobilization of Pb2+ by bioapatite in aqueous solution. Chemosphere 2018, 190, 260–266. [Google Scholar] [CrossRef]
- Kpomblekou, A.K.; Tabatabai, M.A. Effect of low-molecular weight organic acids on phosphorus release and phytoavailabilty of phosphorus in phosphate rocks added to soils. Agric. Ecosyst. Environ. 2003, 100, 275–284. [Google Scholar] [CrossRef]
- Palmieri, F.; Estoppey, A.; House, G.L.; Lohberger, A.; Junier, P. Oxalic acid, a molecule at the crossroads of bacterial-fungal interactions—ScienceDirect. Adv. Appl. Microbiol. 2019, 106, 49–77. [Google Scholar]
- Coutinho, F.P.; Felix, W.P.; Yano-Melo, A.M. Solubilization of phosphates in vitro by Aspergillus spp. and Penicillium spp. Ecol. Eng. 2012, 42, 85–89. [Google Scholar] [CrossRef]
- Mendes, G.O.; Murta, H.M.; Valadares, R.V.; Silveira, W.B.; Silva, I.R.; Costa, M.D. Oxalic acid is more efficient than sulfuric acid for rock phosphate solubilization. Miner. Eng. 2020, 155, 106458. [Google Scholar] [CrossRef]
- Tian, D.; Wang, L.; Hu, J.; Zhang, L.; Zhou, N.; Xia, J.; Xu, M.; Yusef, K.K.; Wang, S.; Li, Z.; et al. A study of P release from Fe-P and Ca-P via the organic acids secreted by Aspergillus niger. J. Microbiol. 2021, 59, 819–826. [Google Scholar] [CrossRef]
- Da Silva, V.N.; de Souza Fernandes da Silva, L.E.; da Silva, A.J.N.; Stamford, N.P.; de Macedo, G.R. Solubility curve of rockpowder inoculated with microorganisms in the production of biofertilizers. Agric. Nat. Resour. 2017, 51, 142–147. [Google Scholar]
- Tian, D.; Su, M.; Zou, X.; Zhang, L.; Li, Z. Influences of phosphate addition on fungal weathering of carbonate in the red soil from karst region. Sci. Total Environ. 2021, 755, 142570. [Google Scholar] [CrossRef]
- Su, M.; Meng, L.Z.; Zhao, L.; Tang, Y.K.; Qiu, J.J.; Tian, D.; Li, Z. Phosphorus deficiency in soils with red color: Insights from the Interactions between minerals and microorganisms. Geoderma 2021, 404, 115311. [Google Scholar] [CrossRef]
- Yin, Z.; Shi, F.; Jiang, H.; Roberts, D.P.; Chen, S.; Fan, B. Phosphate solubilization and promotion of maize growth by penicillium oxalicum P4 and Aspergillus niger P85 in a calcareous soil. Can. J. Microbiol. 2015, 61, 913–923. [Google Scholar] [CrossRef]
- Li, Z.; Bai, T.; Dai, L.; Wang, F.; Tao, J.; Meng, S.; Hu, Y.; Wang, S.; Hu, S. A study of organic acid production in contrasts between two phosphate solubilizing fungi: Penicillium oxalicum and Aspergillus niger. Sci. Rep. 2016, 6, 25313. [Google Scholar] [CrossRef]
- Li, X.; Luo, L.; Yang, J.; Li, B.; Yuan, H. Mechanisms for solubilization of various insoluble phosphates and activation of immobilized phosphates in different soils by an efficient and salinity-tolerant Aspergillus niger strain An2. Appl. Biochem. Biotechnol. 2015, 175, 2755–2768. [Google Scholar] [CrossRef]
- Tian, D.; Jiang, Z.; Jiang, L.; Su, M.; Feng, Z.; Zhang, L.; Wang, S.; Li, Z.; Hu, S. A new insight into lead (II) tolerance of environmental fungi based on a study of Aspergillus niger and Penicillium oxalicum. Environ. Microbiol. 2019, 21, 471–479. [Google Scholar] [CrossRef] [Green Version]
- Fomina, M.; Hillier, S.; Charnock, J.M.; Melville, K.; Alexander, I.J.; Gadd, G.M. Role of oxalic acid overexcretion in transformations of toxic metal minerals by Beauveria caledonica. Appl. Environ. Microbiol. 2005, 71, 371–381. [Google Scholar] [CrossRef] [Green Version]
- Shao, X.; Hao, W.; Konhauser, K.O.; Gao, Y.; Tang, L.; Su, M.; Li, Z. The dissolution of fluorapatite by phosphate-solubilizing fungi: A balance between enhanced phosphorous supply and fluorine toxicity. Environ. Sci. Pollut. Res. 2021, 28, 69393–69400. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Lai, Z.; Zou, X.; Guo, C.; Tang, L.; Su, M.; Li, Z.; Hu., S. A contrast of lead immobilization via bioapatite under elevated CO2 between acidic and alkaline soils. Soil. Use Manag. 2018, 34, 542–544. [Google Scholar] [CrossRef]
- Liang, H.; Zhang, P.; Jin, Z.; DePaoli, D. Rare-earth leaching from Florida phosphate rock in wet-process phosphoric acid production. Miner. Metall. Process. 2017, 34, 146–153. [Google Scholar] [CrossRef]
- Gao, Y.X.; Ran, J.G.; Li, G.; Wang, F.H. Preparation of bioactive hydroxyapatite by a modifed method. Mater. Sci. Technol. 2004, 12, 156–159. [Google Scholar]
- Yang, M. Solubility of phosphogypsum in pure water and lime solution. Adv. Mater. Res. 2011, 250, 131–135. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, L.; Lin, Q.; Cao, J.X. Analysis of phosphogypsum’s physical and chemical properties and making of anhydrite cement from phosphogypsum. Appl. Mech. Mater. 2013, 275–277, 2131–2135. [Google Scholar] [CrossRef]
- Li, Z.; Wang, F.; Bai, T.; Tao, J.; Guo, J.; Yang, M.; Wang, S.; Hu, S. Lead immobilization by geological fluorapatite and fungus Aspergillus niger. J. Hazard. Mater. 2016, 320, 386–392. [Google Scholar] [CrossRef]
- Partridge, E.P.; White, A.H. The solubility of calcium sulfate from 0 to 200°. J. Am. Chem. Soc. 2002, 51, 360–370. [Google Scholar] [CrossRef]
- Tao, B.R.; Shao, L.P.; Ming-Ying, F.U.; Zhang, Y.; Zhou, X.S.; Zhu, L.Q. Effects of the Straw Concentrated Buried Return on Nitrogen and Phosphorus Leaching in Wheat Fields. Chin. J. Soil Sci. 2013, 44, 945–951. [Google Scholar]
- Wang, X.; Wang, C.; Sui, J.; Liu, Z.; Li, Q.; Ji, C.; Song, X.; Hu, Y.; Wang, C.; Sa, R. Isolation and characterization of phosphofungi, and screening of their plant growth-promoting activities. Amb. Express. 2018, 8, 63. [Google Scholar] [CrossRef]
- Wu, H. Effects of soil amendment Phosphogypsum on physical and chemical properties of and wheat growth in coastal saline soil in Rudong, Jiangsu. Acta. Pedol. Sin. 2012, 49, 1262–1266. [Google Scholar]
- Nayak, A.K.; Mishra, V.K.; Sharma, D.K.; Jha, S.K.; Singh, C.S.; Shahabuddin, M.; Shahid, M. Efficiency of Phosphogypsum and Mined Gypsum in Reclamation and Productivity of Rice–Wheat Cropping System in Sodic Soil. Commun. Soil Sci. Plant. Anal. 2013, 44, 909–921. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, D.; Xia, J.; Zhou, N.; Xu, M.; Li, X.; Zhang, L.; Du, S.; Gao, H. The Utilization of Phosphogypsum as a Sustainable Phosphate-Based Fertilizer by Aspergillus niger. Agronomy 2022, 12, 646. https://doi.org/10.3390/agronomy12030646
Tian D, Xia J, Zhou N, Xu M, Li X, Zhang L, Du S, Gao H. The Utilization of Phosphogypsum as a Sustainable Phosphate-Based Fertilizer by Aspergillus niger. Agronomy. 2022; 12(3):646. https://doi.org/10.3390/agronomy12030646
Chicago/Turabian StyleTian, Da, Jingjing Xia, Ningning Zhou, Meiyue Xu, Xiang Li, Liangliang Zhang, Shuhua Du, and Hongjian Gao. 2022. "The Utilization of Phosphogypsum as a Sustainable Phosphate-Based Fertilizer by Aspergillus niger" Agronomy 12, no. 3: 646. https://doi.org/10.3390/agronomy12030646





