Salinity Tolerance Characteristics of Marginally Located Rice Varieties in the Northernmost Rice-Growing Area in Europe
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment 1: Screening the Salinity Stress Tolerance at the Seedling Stage
2.2. Experiment 2: Screening the Constant Salinity Stress at the Reproductive Stage
3. Results
3.1. Seedling Stage
3.2. Reproductive Stage
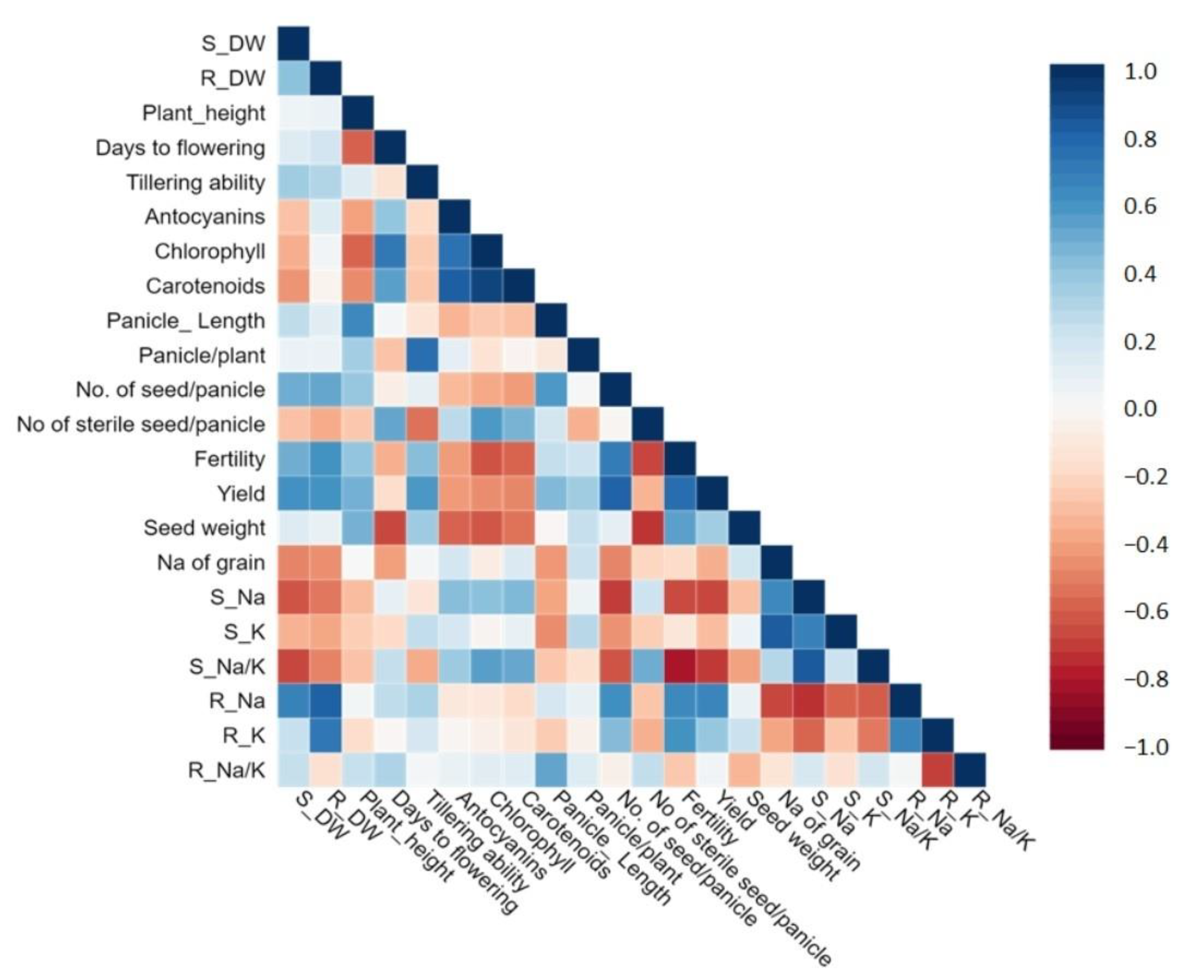
3.2.1. Morphophysiological Parameters
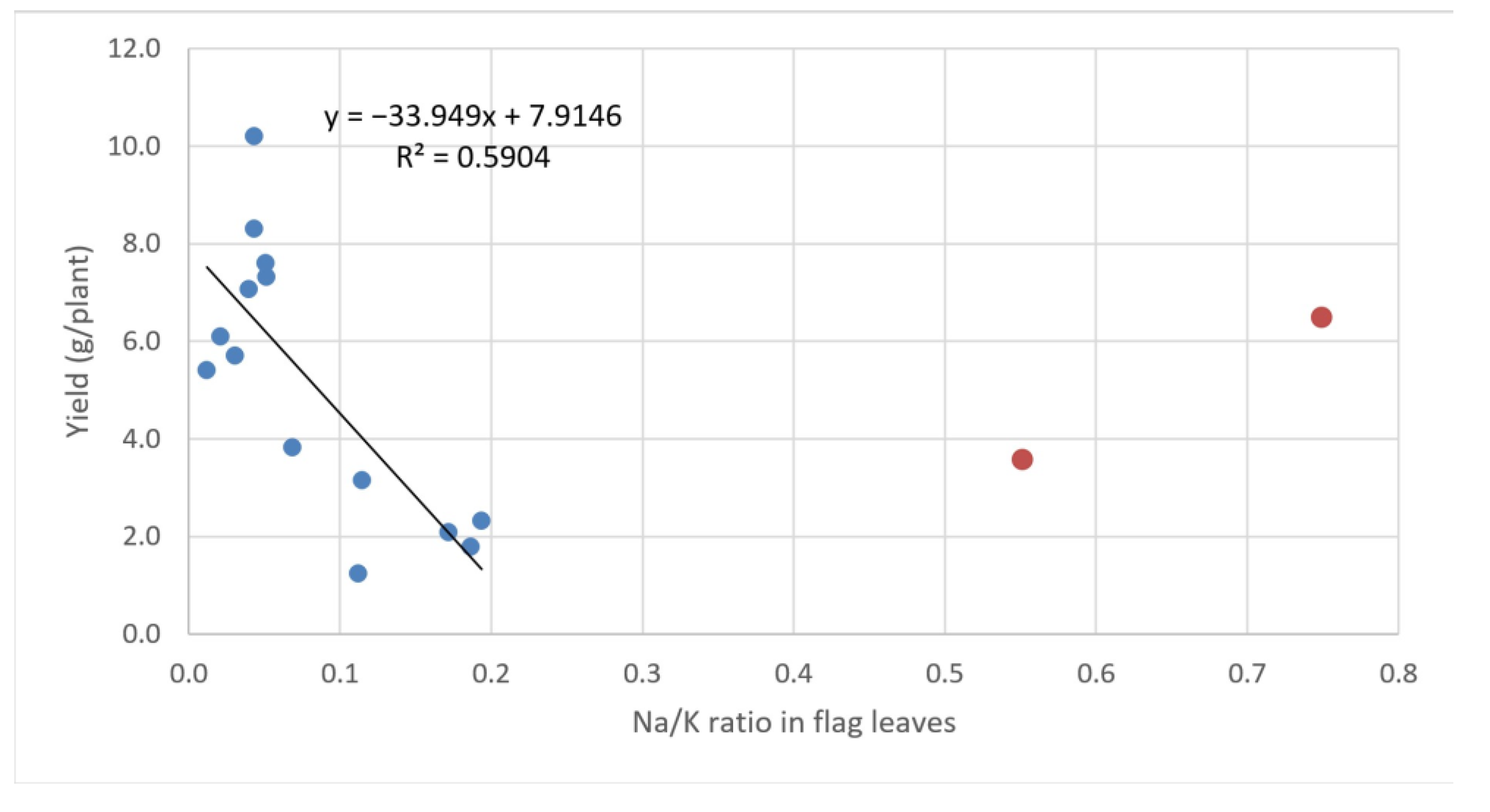
3.2.2. Yield Related Parameters
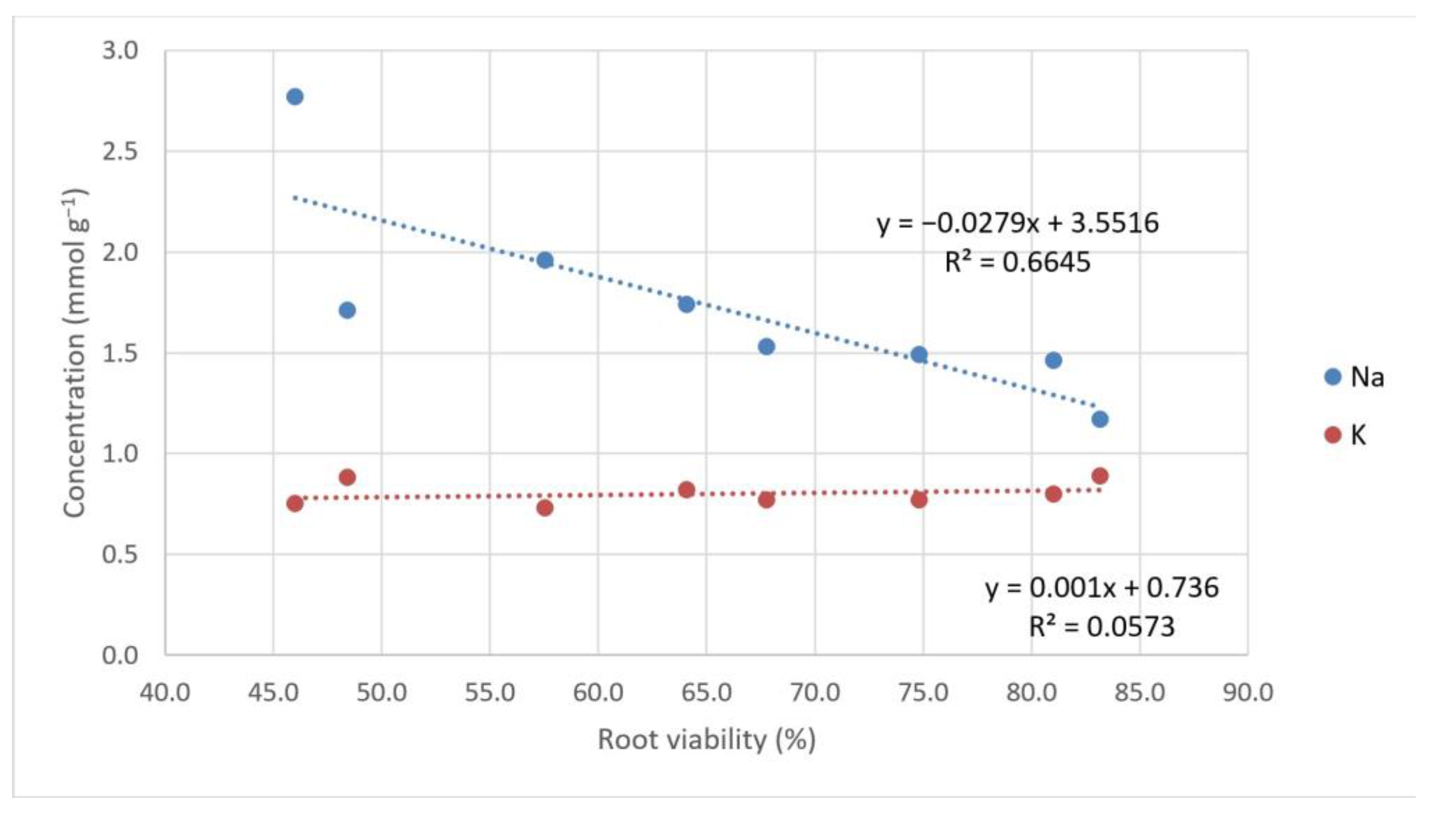
3.2.3. Sodium and Potassium Concentration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maas, E.V.; Hoffman, G.J. Crop Salt Tolerance—Current Assessment. J. Irrig. Drain. Div. 1977, 103, 115–134. [Google Scholar] [CrossRef]
- Ali, Y.; Aslam, Z.; Awan, A.R.; Hussain, F.; Cheema, A.A. Screening Rice (Oryza sativa L.) Lines/Cultivars Against Salinity in Relation to Morphological and Physiological Traits and Yield Components. Int. J. Agric. Biol. 2004, 6, 572–575. [Google Scholar]
- Negrão, S.; Schmöckel, S.M.; Tester, M. Evaluating Physiological Responses of Plants to Salinity Stress. Ann. Bot. 2017, 119, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.A.; Rafii, M.Y.; Yusoff, M.M.; Ali, N.S.; Yusuff, O.; Datta, D.R.; Anisuzzaman, M.; Ikbal, M.F. Advanced Breeding Strategies and Future Perspectives of Salinity Tolerance in Rice. Agronomy 2021, 11, 1631. [Google Scholar] [CrossRef]
- Asch, F.; Dingkuhn, M.; Dörffling, K.; Miezan, K. Leaf K/Na Ratio Predicts Salinity Induced Yield Loss in Irrigated Rice. Euphytica 2000, 113, 109. [Google Scholar] [CrossRef]
- Mohammadi, R.; Mendioro, M.; Diaz, G.; Gregorio, G.; Singh, R. Genetic Analysis of Salt Tolerance at Seedling and Reproductive Stages in Rice (Oryza sativa). Plant Breed. 2014, 133, 548–559. [Google Scholar] [CrossRef]
- Chakraborty, K.; Chattaopadhyay, K.; Nayak, L.; Ray, S.; Yeasmin, L.; Jena, P.; Gupta, S.; Mohanty, S.K.; Swain, P.; Sarkar, R.K. Ionic Selectivity and Coordinated Transport of Na+ and K+ in Flag Leaves Render Differential Salt Tolerance in Rice at the Reproductive Stage. Planta 2019, 250, 1637–1653. [Google Scholar] [CrossRef]
- Normand, S.; Treier, U.A.; Randin, C.; Vittoz, P.; Guisan, A.; Svenning, J.-C. Importance of Abiotic Stress as a Range-Limit Determinant for European Plants: Insights from Species Responses to Climatic Gradients. Glob. Ecol. Biogeogr. 2009, 18, 437–449. [Google Scholar] [CrossRef]
- Frouin, J.; Languillaume, A.; Mas, J.; Mieulet, D.; Boisnard, A.; Labeyrie, A.; Bettembourg, M.; Bureau, C.; Lorenzini, E.; Portefaix, M.; et al. Tolerance to Mild Salinity Stress in Japonica Rice: A Genome-Wide Association Mapping Study Highlights Calcium Signaling and Metabolism Genes. PLoS ONE 2018, 13, e0190964. [Google Scholar] [CrossRef]
- Wankhade, S.; Bahaji, A.; Mateu-Andrés, I.; Cornejo, M.-J. Phenotypic Indicators of NaCl Tolerance Levels in Rice Seedlings: Variations in Development and Leaf Anatomy. Acta Physiol. Plant. 2010, 32, 1161–1169. [Google Scholar] [CrossRef][Green Version]
- Hoang, T.M.L.; Tran, T.N.; Nguyen, T.K.T.; Williams, B.; Wurm, P.; Bellairs, S.; Mundree, S. Improvement of Salinity Stress Tolerance in Rice: Challenges and Opportunities. Agronomy 2016, 6, 54. [Google Scholar] [CrossRef]
- Abe, A.; Takagi, H.; Fujibe, T.; Aya, K.; Kojima, M.; Sakakibara, H.; Uemura, A.; Matsuoka, M.; Terauchi, R. OsGA20ox1, a Candidate Gene for a Major QTL Controlling Seedling Vigor in Rice. Theor. Appl. Genet. 2012, 125, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Pauk, J.; Jancsó, M.; Simon-Kiss, I. Rice Doubled Haploids and Breeding. In Advances in Haploid Production in Higher Plants; Touraev, A., Forster, B.P., Jain, S.M., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 189–197. ISBN 978-1-4020-8854-4. [Google Scholar]
- Heszky, L.E.; Simon-Kiss, I.; Binh, D.Q. Release of the Rice Variety Dama Developed by Haploid Somaclone Breeding. In Somaclonal Variation in Crop Improvement II; Bajaj, Y.P.S., Ed.; Biotechnology in Agriculture and Forestry; Springer: Berlin/Heidelberg, Germany, 1996; pp. 46–54. ISBN 978-3-642-61081-3. [Google Scholar]
- Székely, Á.; Szaloki, T.; Ibadzade, M.; Pauk, J.; Lantos, C.; Jancsó, M. Germination Dynamics of European Rice Varieties under Salinity Stress. Pak. J. Agric. Sci. 2021, 58, 1–5. [Google Scholar] [CrossRef]
- Narale, R.P.; Subramanyam, T.K.; Mukherjee, R.K. Influence of Salinity on Germination, Vegetative Growth, and Grain Yield of Rice (Oryza sativa Var. Dular)1. Agron. J. 1969, 61, 341–344. [Google Scholar] [CrossRef]
- Gregorio, G.; Senadhira, D.; Mendoza, R. Screening Rice for Salinity Tolerance. In IRRI Discussion Paper Series; International Rice Research Institute: Los Baños, Philippine, 1997. [Google Scholar]
- Mishra, S.S.; Behera, P.K.; Kumar, V.; Lenka, S.K.; Panda, D. Physiological Characterization and Allelic Diversity of Selected Drought Tolerant Traditional Rice (Oryza sativa L.) Landraces of Koraput, India. Physiol. Mol. Biol. Plants 2018, 24, 1035–1046. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Relationships between Leaf Pigment Content and Spectral Reflectance across a Wide Range of Species, Leaf Structures and Developmental Stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Zeng, L. Exploration of Relationships between Physiological Parameters and Growth Performance of Rice (Oyza sativa L.) Seedlings under Salinity Stress Using Multivariate Analysis. Plant Soil 2005, 268, 51–59. [Google Scholar] [CrossRef]
- Qin, H.; Li, Y.; Huang, R. Advances and Challenges in the Breeding of Salt-Tolerant Rice. Int. J. Mol. Sci. 2020, 21, 8385. [Google Scholar] [CrossRef]
- Bado, S.; Forster, B.; Padilla Alvarez, R.; Resch, C.; Migliori, A.; Diawara, Y.; Jaksic, M.; Ali, A.; Ghanim, A.M.A.; Nielen, S.; et al. Prediction of Salt Tolerance in Rice (Oryza sativa) Based on Shoot Ion Content under Non-Stressed Conditions. J. Mater. Sci. Eng. A 2016, 6, 1–16. [Google Scholar] [CrossRef]
- Ahmadi, N.; Negrão, S.; Katsantonis, D.; Frouin, J.; Ploux, J.; Letourmy, P.; Droc, G.; Babo, P.; Trindade, H.; Bruschi, G.; et al. Targeted Association Analysis Identified Japonica Rice Varieties Achieving Na+/K+ Homeostasis without the Allelic Make-up of the Salt Tolerant Indica Variety Nona Bokra. Theor. Appl. Genet. 2011, 123, 881–895. [Google Scholar] [CrossRef]
- Rohila, J.S.; Edwards, J.D.; Tran, G.D.; Jackson, A.K.; McClung, A.M. Identification of Superior Alleles for Seedling Stage Salt Tolerance in the USDA Rice Mini-Core Collection. Plants 2019, 8, 472. [Google Scholar] [CrossRef] [PubMed]
- Platten, J.D.; Egdane, J.A.; Ismail, A.M. Salinity Tolerance, Na+ Exclusion and Allele Mining of HKT1;5 in Oryza sativa and O. glaberrima: Many Sources, Many Genes, One Mechanism? BMC Plant Biol. 2013, 13, 32. [Google Scholar] [CrossRef] [PubMed]
- Pires, I.; Negrao, S.; Oliveira, M.; Purugganan, M. Comprehensive Phenotypic Analysis of Rice (Oryza sativa) Response to Salinity Stress. Physiol. Plant. 2015, 155, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Yeo, A.R.; Yeo, M.E.; Flowers, S.A.; Flowers, T.J. Screening of Rice (Oryza sativa L.) Genotypes for Physiological Characters Contributing to Salinity Resistance, and Their Relationship to Overall Performance. Theor. Appl. Genet. 1990, 79, 377–384. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Cotsaftis, O.; Plett, D.; Shirley, N.; Tester, M.; Hrmova, M. A Two-Staged Model of Na+ Exclusion in Rice Explained by 3D Modeling of HKT Transporters and Alternative Splicing. PLoS ONE 2012, 7, e39865. [Google Scholar] [CrossRef]
- Lee, K.-S.; Choi, W.-Y.; Ko, J.-C.; Kim, T.-S.; Gregorio, G. Salinity Tolerance of Japonica and Indica Rice (Oryza sativa L.) at the Seedling Stage. Planta 2003, 216, 1043–1046. [Google Scholar] [CrossRef]
- Reddy, I.N.B.L.; Kim, B.-K.; Yoon, I.-S.; Kim, K.-H.; Kwon, T.-R. Salt Tolerance in Rice: Focus on Mechanisms and Approaches. Rice Sci. 2017, 24, 123–144. [Google Scholar] [CrossRef]
- De Leon, T.B.; Linscombe, S.; Gregorio, G.; Subudhi, P.K. Genetic Variation in Southern USA Rice Genotypes for Seedling Salinity Tolerance. Front. Plant Sci. 2015, 6, 374. [Google Scholar] [CrossRef]
- Kranto, S.; Chankaew, S.; Monkham, T.; Theerakulpisut, P.; Sanitchon, J. Evaluation for Salt Tolerance in Rice Using Multiple Screening Methods. J. Agric. Sci. Technol. 2016, 18, 1921–1931. [Google Scholar]
- Zeng, L.; Shannon, M. Salinity Effects on Seedling Growth and Yield Components of Rice. Crop Sci. 2000, 40, 996–1003. [Google Scholar] [CrossRef]
- Rubel, M.; Hassan, L.; Islam, M.; Robin, A.; Alam, M. Evaluation of Rice Genotypes under Salt Stress at the Seedling and Reproductive Stages Using Phenotypic and Molecular Markers. Pak. J. Bot. 2014, 46, 423–432. [Google Scholar]
- Gerona, M.E.B.; Deocampo, M.P.; Egdane, J.A.; Ismail, A.M.; Dionisio-Sese, M.L. Physiological Responses of Contrasting Rice Genotypes to Salt Stress at Reproductive Stage. Rice Sci. 2019, 26, 207–219. [Google Scholar] [CrossRef]
- Sen, T.T.H.; Nhi, P.T.P.; Sen, T.T. Salinity Effect at Seedling and Flowering Stages of Some Rice Lines and Varieties (Oryza sativa L.). J. Agric. Sci. Technol. 2017, 7, 32–39. [Google Scholar] [CrossRef]
- Hakim, M.; Juraimi, A.; Hanafi, M.M.; Ismail, M.R.; Rafii, M.; Islam, M.; Selamat, A. The Effect of Salinity on Growth, Ion Accumulation and Yield of Rice Varieties. J. Anim. Plant Sci. 2014, 24, 874–885. [Google Scholar]
- Wang, H.; Takano, T.; Liu, S. Screening and Evaluation of Saline–Alkaline Tolerant Germplasm of Rice (Oryza sativa L.) in Soda Saline–Alkali Soil. Agronomy 2018, 8, 205. [Google Scholar] [CrossRef]
- Ahmadizadeh, M.; Vispo, N.A.; Calapit-Palao, C.D.O.; Pangaan, I.D.; Viña, C.D.; Singh, R.K. Reproductive Stage Salinity Tolerance in Rice: A Complex Trait to Phenotype. Ind. J. Plant Physiol. 2016, 21, 528–536. [Google Scholar] [CrossRef]
- Aslam, M.; Qureshi, R.H.; Ahmed, N. A Rapid Screening Technique for Salt Tolerance in Rice (Oryza sativa L.). Plant Soil 1993, 150, 99–107. [Google Scholar] [CrossRef]
- Singh, R.; Flowers, T.J. The Physiology and Molecular Biology of the Effects of Salinity on Rice. In Handbook of Plant and Crop Stress; CRC Press: Boca Raton, FL, USA, 2010; pp. 901–942. [Google Scholar]
- Kibria, M.G.; Hossain, M.; Murata, Y.; Hoque, M.A. Antioxidant Defense Mechanisms of Salinity Tolerance in Rice Genotypes. Rice Sci. 2017, 24, 155–162. [Google Scholar] [CrossRef]


| Water | Soil | ||||
|---|---|---|---|---|---|
| EC25 (dS m−1) | EC25 (dS m−1) | Sodium Content (mg kg−1) | |||
| Control | Saline | Control | Saline | Control | Saline |
| 1.41 | 4.27 | 3.80 | 8.39 | 344 | 1000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Székely, Á.; Szalóki, T.; Pauk, J.; Lantos, C.; Ibadzade, M.; Jancsó, M. Salinity Tolerance Characteristics of Marginally Located Rice Varieties in the Northernmost Rice-Growing Area in Europe. Agronomy 2022, 12, 652. https://doi.org/10.3390/agronomy12030652
Székely Á, Szalóki T, Pauk J, Lantos C, Ibadzade M, Jancsó M. Salinity Tolerance Characteristics of Marginally Located Rice Varieties in the Northernmost Rice-Growing Area in Europe. Agronomy. 2022; 12(3):652. https://doi.org/10.3390/agronomy12030652
Chicago/Turabian StyleSzékely, Árpád, Tímea Szalóki, János Pauk, Csaba Lantos, Marks Ibadzade, and Mihály Jancsó. 2022. "Salinity Tolerance Characteristics of Marginally Located Rice Varieties in the Northernmost Rice-Growing Area in Europe" Agronomy 12, no. 3: 652. https://doi.org/10.3390/agronomy12030652
APA StyleSzékely, Á., Szalóki, T., Pauk, J., Lantos, C., Ibadzade, M., & Jancsó, M. (2022). Salinity Tolerance Characteristics of Marginally Located Rice Varieties in the Northernmost Rice-Growing Area in Europe. Agronomy, 12(3), 652. https://doi.org/10.3390/agronomy12030652







