Humic Substances as Indicator of Degradation Rate of Chernozems in South-Eastern Poland
Abstract
:1. Introduction
2. Materials and Methods
2.1. Area of Study
2.2. Sample Preparations and Analysis
2.3. Statistical Analysis
3. Results
3.1. Basic Soil Characteristics and Properties
3.2. Soil Humic Fractions as Indicators of SOM Responses to Degradation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Daniels, W.L. The Nature and Properties of Soils, 15th ed.; Weil, R.R., Brady, N.C., Eds.; Pearson Press: Upper Saddle River, NJ, USA, 2017. [Google Scholar] [CrossRef]
- Kleber, M.; Eusterhues, K.; Keiluweit, M.; Mikutta, C.; Mikutta, R.; Nico, P.S. Mineral-Organic Associations: Formation, Properties, and Relevance in Soil Environments. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2015; Volume 130, pp. 1–140. [Google Scholar] [CrossRef]
- Lugato, E.; Lavallee, J.M.; Haddix, M.L.; Panagos, P.; Cotrufo, M.F. Different Climate Sensitivity of Particulate and Mineral-Associated Soil Organic Matter. Nat. Geosci. 2021, 14, 295–300. [Google Scholar] [CrossRef]
- Schmidt, M.W.I.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kögel-Knabner, I.; Lehmann, J.; Manning, D.A.C.; et al. Persistence of Soil Organic Matter as an Ecosystem Property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehmann, J.; Kleber, M. The Contentious Nature of Soil Organic Matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Cotrufo, M.F.; Ranalli, M.G.; Haddix, M.L.; Six, J.; Lugato, E. Soil Carbon Storage Informed by Particulate and Mineral-Associated Organic Matter. Nat. Geosci. 2019, 12, 989–994. [Google Scholar] [CrossRef]
- Chabbi, A.; Lehmann, J.; Ciais, P.; Loescher, H.W.; Cotrufo, M.F.; Don, A.; SanClements, M.; Schipper, L.; Six, J.; Smith, P.; et al. Aligning Agriculture and Climate Policy. Nat. Clim. Change 2017, 7, 307–309. [Google Scholar] [CrossRef]
- Lal, R. Restoring Soil Quality to Mitigate Soil Degradation. Sustainability 2015, 7, 5875–5895. [Google Scholar] [CrossRef] [Green Version]
- Obalum, S.E.; Chibuike, G.U.; Peth, S.; Ouyang, Y. Soil Organic Matter as Sole Indicator of Soil Degradation. Environ. Monit. Assess. 2017, 189, 176. [Google Scholar] [CrossRef]
- Jamroz, E.; Bekier, J.; Medyńska-Juraszek, A.; Kałuża-Haładyn, A.; Ćwieląg-Piasecka, I.; Bednik, M. The Contribution of Water Extractable Forms of Plant Nutrients to Evaluate MSW Compost Maturity: A Case Study. Sci. Rep. 2020, 10, 12842. [Google Scholar] [CrossRef]
- Ouyang, W.; Wu, Y.; Hao, Z.; Zhang, Q.; Bu, Q.; Gao, X. Combined Impacts of Land Use and Soil Property Changes on Soil Erosion in a Mollisol Area under Long-Term Agricultural Development. Sci. Total Environ. 2018, 613–614, 798–809. [Google Scholar] [CrossRef]
- Kabała, C. Chernozem (Czarnoziem)—Soil of the Year 2019 in Poland. Origin, Classification and Use of Chernozems in Poland. Soil Sci. Annu. 2019, 70, 184–192. [Google Scholar] [CrossRef]
- Łabaz, B.; Kabała, C.; Dudek, M.; Waroszewski, J. Morphological Diversity of Chernozemic Soils in South-Western Poland. Soil Sci. Annu. 2019, 70, 211–224. [Google Scholar] [CrossRef] [Green Version]
- Łabaz, B.; Waroszewski, J.; Dudek, M.; Bogacz, A.; Kabała, C. Persistence of arable Chernozems and Chernic Rendzic Phaeozems in the eroded undulating loess plateau in Central Europe. Catena, 2021; submitted. [Google Scholar]
- Kleber, M.; Lehmann, J. Humic Substances Extracted by Alkali Are Invalid Proxies for the Dynamics and Functions of Organic Matter in Terrestrial and Aquatic Ecosystems. J. Environ. Qual. 2019, 48, 207–216. [Google Scholar] [CrossRef]
- Olk, D.C.; Bloom, P.R.; De Nobili, M.; Chen, Y.; McKnight, D.M.; Wells, M.J.M.; Weber, J. Using Humic Fractions to Understand Natural Organic Matter Processes in Soil and Water: Selected Studies and Applications. J. Environ. Qual. 2019, 48, 1633–1643. [Google Scholar] [CrossRef] [Green Version]
- Guimarães, D.V.; Gonzaga, M.I.S.; da Silva, T.O.; da Silva, T.L.; da Silva Dias, N.; Matias, M.I.S. Soil Organic Matter Pools and Carbon Fractions in Soil under Different Land Uses. Soil Tillage Res. 2013, 126, 177–182. [Google Scholar] [CrossRef] [Green Version]
- Jensen, J.L.; Schjønning, P.; Watts, C.W.; Christensen, B.T.; Obour, P.B.; Munkholm, L.J. Soil Degradation and Recovery—Changes in Organic Matter Fractions and Structural Stability. Geoderma 2020, 364, 114181. [Google Scholar] [CrossRef]
- Martinez-Salgado, M.M.; Gutiérrez-Romero, V.; Jannsens, M.; Ortega-Blu, R. Biological Soil Quality Indicators: A Review. Curr. Res. Technol. Educ. Top. Appl. Microbiol. 2010, 1, 319–328. [Google Scholar]
- Olk, D.C.; Bloom, P.R.; Perdue, E.M.; McKnight, D.M.; Chen, Y.; Farenhorst, A.; Senesi, N.; Chin, Y.-P.; Schmitt-Kopplin, P.; Hertkorn, N.; et al. Environmental and Agricultural Relevance of Humic Fractions Extracted by Alkali from Soils and Natural Waters. J. Environ. Qual. 2019, 48, 217–232. [Google Scholar] [CrossRef]
- Jamroz, E.; Jerzykiewicz, M. Humic Fractions as Indicators of Soil Organic Matter Responses to Clear-Cutting in the Mountain and Lowland Conditions of South-Western Poland. Land Degrad. Dev. 2021, 33, 368–378. [Google Scholar] [CrossRef]
- Spaccini, R.; Piccolo, A.; Haberhauer, G.; Gerzabek, M.H. Transformation of Organic Matter from Maize Residues into Labile and Humic Fractions of Three European Soils as Revealed by 13C Distribution and CPMAS-NMR Spectra. Eur. J. Soil Sci. 2000, 51, 583–594. [Google Scholar] [CrossRef]
- Loba, A.; Waroszewski, J.; Tikhomirov, D.; Calitri, F.; Christl, M.; Sykuła, M.; Egli, M. Tracing Erosion Rates in Loess Landscape of the Trzebnica Hills (Poland) over Time Using Fallout and Cosmogenic Nuclides. J. Soils Sediments 2021, 21, 2952–2968. [Google Scholar] [CrossRef]
- Krysiak, Z. Carpathian Foredeep; Neogene; Neotectonics; Mesostructural Analysis; Teledetection; Synsedimentary Faults; Strike-Slip; Faults; Tectonic Breccias. Geol. Q. 2000, 44, 137–156. [Google Scholar]
- Drużkowski, M.M. Różnorodność krajobrazu obszarów rolnictwa tradycyjnego. Studium geoekologiczne dla Płaskowyżu Proszowickiego. Stud. Geoekologiczne Dla Płaskowyżu Proszowickiego. Probl. Ekol. Kraj. 2004, 13, 8. [Google Scholar]
- FAO. World Reference Base for Soil Resources 2014: International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; FAO: Rome, Italy, 2014; ISBN 978-92-5-108369-7. [Google Scholar]
- Kobierski, M.; Kondratowicz-Maciejewska, K.; Banach-Szott, M.; Wojewódzki, P.; Peñas Castejón, J.M. Humic Substances and Aggregate Stability in Rhizospheric and Non-Rhizospheric Soil. J. Soils Sediments 2018, 18, 2777–2789. [Google Scholar] [CrossRef] [Green Version]
- Dziadowiec, H.; Gonet, S.S. Przewodnik Metodyczny Do Badań Materii Organicznej Gleb. Pr. Kom. Nauk. PTG 1999, 120, 31–33. [Google Scholar]
- Łabaz, B.; Bogacz, A.; Żymełka, R. Substancje humusowe i własciwości czarnych ziem występujących w obniżeniu milicko-głogowskim. Woda-Śr.-Obsz. Wiej. 2010, 10, 113–128. [Google Scholar]
- Swift, R. Fractionation of Soil Substances. Humic Subst. Soil Sediment Water 1985, 387–408. [Google Scholar]
- Dudek, M.; Łabaz, B.; Kabała, C. Comparison of Sequential Extraction Techniques and Fractional Composition of Humic Substances in Chernozems and Phaeozems of Poland—A Review. Soil Sci. Annu. 2021, 72, 132235. [Google Scholar] [CrossRef]
- Weber, J.; Jamroz, E.; Kocowicz, A.; Debicka, M.; Bekier, J.; Ćwieląg-Piasecka, I.; Ukalska-Jaruga, A.; Mielnik, L.; Bejger, R.; Jerzykiewicz, M. Optimized Isolation Method of Humin Fraction from Mineral Soil Material. Environ. Geochem. Health 2021, 1–10. [Google Scholar] [CrossRef]
- Rupnik, J.; Shawe-Taylor, J. Multi-View Canonical Correlation Analysis. Doctoral Dissertation, Jožef Stefan International Postgraduate School, Ljubljana, Slovenia, 17 June 2016. [Google Scholar]
- Dudek, M.; Kabała, C.; Łabaz, B.; Mituła, P.; Bednik, M.; Medyńska-Juraszek, A. Mid-Infrared Spectroscopy Supports Identification of the Origin of Organic Matter in Soils. Land 2021, 10, 215. [Google Scholar] [CrossRef]
- Ertlen, D.; Schwartz, D.; Trautmann, M.; Webster, R.; Brunet, D. Discriminating between Organic Matter in Soil from Grass and Forest by Near-Infrared Spectroscopy. Eur. J. Soil Sci. 2010, 61, 207–216. [Google Scholar] [CrossRef]
- Roth, P.M.; Hirzer, M.; Köstinger, M.; Beleznai, C.; Bischof, H. Mahalanobis Distance Learning for Person Re-Identification. In Person Re-Identification; Gong, S., Cristani, M., Yan, S., Loy, C.C., Eds.; Springer: London, UK, 2014; pp. 247–267. ISBN 978-1-4471-6295-7. [Google Scholar]
- Łabaz, B.; Musztyfaga, E.; Waroszewski, J.; Bogacz, A.; Jezierski, P.; Kabała, C. Landscape-Related Transformation and Differentiation of Chernozems—Catenary Approach in the Silesian Lowland, SW Poland. Catena 2018, 161, 63–76. [Google Scholar] [CrossRef]
- Ľubica, P.; Fasurová, N.; Barančíková, G.; Liptaj, T. Spectral Characteristics of Humic Acids Isolated from South Moravian Lignite and Soils. Pet. Coal 2008, 50, 30–36. [Google Scholar]
- Prikhod’ko, V.E.; Cheverdin, Y.I.; Titova, T.V. Changes in the Organic Matter Forms in Chernozems of the Kamennaya Steppe under Different Land Uses, Locations, and Hydromorphism Degrees. Eurasian Soil Sci. 2013, 46, 1230–1240. [Google Scholar] [CrossRef]
- Turski, R. The Influence of Erosion on the Organic Matter Compounds in the Brown Soils and Chernozems of the Lublin Upland. Soil Sci. Annu. 1964, 14, 345–352. [Google Scholar]
- Hladký, J.; Kynický, J.; Dvořáčková, H.; Elbl, J.; Brtnický, M. Effect of Long-Term Erosion on Humus Content and Quality on Chernozem Soils. Int. Multidiscip. Sci. GeoConf. 2017, 17, 179–184. [Google Scholar] [CrossRef]
- López, R.; Gondar, D.; Iglesias, A.; Fiol, S.; Antelo, J.; Arce, F. Acid Properties of Fulvic and Humic Acids Isolated from Two Acid Forest Soils under Different Vegetation Cover and Soil Depth. Eur. J. Soil Sci. 2008, 59, 892–899. [Google Scholar] [CrossRef]
- Horáček, J.; Novák, P.; Liebhard, P.; Strosser, E.; Babulicová, M. The Long-Term Changes in Soil Organic Matter Contents and Quality in Chernozems. Plant Soil Environ. 2017, 63, 8–13. [Google Scholar] [CrossRef] [Green Version]
- Dębska, B.; Banach-Szott, M.; Rosa, E.; Pakuła, J. The Effect of Soil Pollution by Selected PAHs on the Fractional Composition of Organic Matter. Soil Sci. Annu. 2012, 63, 13–17. [Google Scholar] [CrossRef] [Green Version]
- Cécillon, L.; Barthès, B.G.; Gomez, C.; Ertlen, D.; Genot, V.; Hedde, M.; Stevens, A.; Brun, J.J. Assessment and Monitoring of Soil Quality Using Near-Infrared Reflectance Spectroscopy (NIRS). Eur. J. Soil Sci. 2009, 60, 770–784. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Herbert, S.J.; Hashemi, A.M.; Zhang, X.; Ding, G. Effects of Agricultural Management on Soil Organic Matter and Carbon Transformation–A Review. Plant Soil Environ. 2011, 52, 531–543. [Google Scholar] [CrossRef] [Green Version]
- Mielnik, L.; Hewelke, E.; Weber, J.; Oktaba, L.; Jonczak, J.; Podlasiński, M. Changes in the Soil Hydrophobicity and Structure of Humic Substances in Sandy Soil Taken out of Cultivation. Agric. Ecosyst. Environ. 2021, 319, 107554. [Google Scholar] [CrossRef]
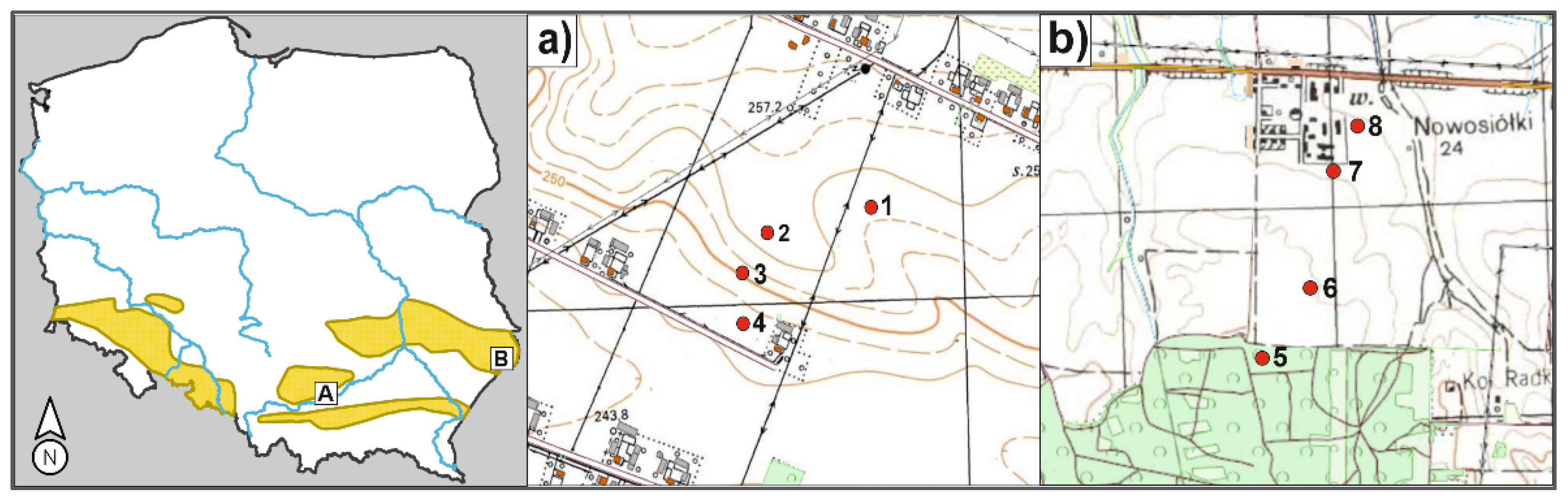


| Soil Classification | Altitude m.a.s.l. | Landscape Position | Land Use | Type of Degradation | |
|---|---|---|---|---|---|
| Profile 1 | Chernic Phaeozem | 254 | plain part of long slope | arable—wheat | non-eroded |
| Profile 2 | Calcic Chernozem | 257 | middle part of long slope | arable—wheat | erosion |
| Profile 3 | Haplic Calcisol | 251 | ridge in midslope | arable—wheat | erosion (strong) |
| Profile 4 | Calcic Chernozem | 247 | footslope below the ridge | arable—wheat | colluvial accumulation |
| Profile 5 | Haplic Chernozem | 252 | upper part of long slope | forest | acidification |
| Profile 6 | Haplic Chernozem | 245 | middle part of long slope | arable—oilseed rape | erosion |
| Profile 7 | Haplic Chernozem | 230 | plain part of slope | arable—oilseed rape | non-eroded |
| Profile 8 | Haplic Chernozem | 219 | footslope | arable—oilseed rape | colluvial accumulation |
| Horizon | Color | Depth cm | pH | CaCO3 % [m/m] | SOC % | TN % | Clay % | Texture Class |
|---|---|---|---|---|---|---|---|---|
| Profile 1 | ||||||||
| Ap | 10YR 3/1 | 0–25 | 7.60 | 0.42 | 1.94 | 0.16 | 17 | SiL |
| A2 | 10YR 2/1 | 25–40 | 7.88 | 0.21 | 1.64 | 0.12 | 16 | SiL |
| A3 | 10YR 2/1 | 40–75 | 7.96 | 0.30 | 1.99 | 0.12 | 22 | SiL |
| Profile 2 | ||||||||
| Apk | 10YR 2/2 | 0–35 | 8.10 | 6.36 | 1.44 | 0.13 | 15 | SiL |
| Profile 3 | ||||||||
| Ap | 10YR 4/2 | 0–30 | 8.18 | 16.7 | 1.03 | 0.07 | 17 | SiL |
| Profile 4 | ||||||||
| Ap | 10YR 3/2 | 0–25 | 8.10 | 8.48 | 1.48 | 0.14 | 18 | SiL |
| A2 | 10YR 3/2 | 25–48 | 8.05 | 9.54 | 1.31 | 0.14 | 17 | SiL |
| A3 | 10YR 3/2 | 48–54 | 8.23 | 5.09 | 1.52 | 0.12 | 23 | SiL |
| Profile 5 | ||||||||
| A1 | 10YR 3/2 | 0–35 | 4.55 | 0.59 | 1.82 | 0.15 | 12 | SiL |
| A2 | 10YR 3/1 | 35–65 | 5.14 | 0.51 | 0.83 | 0.06 | 23 | SiL |
| Profile 6 | ||||||||
| Ap | 10YR 2/1 | 0–31 | 7.16 | 3.13 | 3.29 | 0.30 | 14 | SiL |
| Profile 7 | ||||||||
| Ap1 | 10YR 2/2 | 0–20 | 7.13 | 0.05 | 1.44 | 0.11 | 13 | SiL |
| Ap2 | 10YR 1/1 | 20–40 | 7.08 | 0.08 | 1.49 | 0.12 | 11 | SiL |
| Ahk | 10YR 3/2 | 40–65 | 7.18 | 1.08 | 1.04 | 0.07 | 16 | SiL |
| Profile 8 | ||||||||
| Ap | 10YR 1/1 | 0–45 | 7.97 | 0.25 | 2.75 | 0.18 | 10 | SiL |
| A | 10YR 2/1 | 45–65 | 8.06 | 0.17 | 1.57 | 0.11 | 20 | SiL |
| Tyurin | IHSS | |||||
|---|---|---|---|---|---|---|
| HA | FA | HA/FA | HA | FA | HA/FA | |
| % of SOC | % of SOC | |||||
| Profile 1 | ||||||
| Ap | 33.5 | 26.3 | 1.3 | 23.9 | 14.8 | 1.6 |
| A2 | 40.8 | 20.1 | 2.0 | 24.6 | 15.0 | 1.6 |
| A3 | 42.7 | 26.0 | 1.6 | 35.2 | 19.9 | 1.8 |
| Profile 2 | ||||||
| Apk | 27.8 | 25.4 | 1.1 | 25.9 | 21.4 | 1.2 |
| Profile 3 | ||||||
| Ap | 22.3 | 34.1 | 0.7 | 14.6 | 26.7 | 0.5 |
| Profile 4 | ||||||
| Ap | 32.4 | 32.1 | 1.0 | 23.8 | 23.2 | 1.0 |
| A2 | 38.1 | 32.5 | 1.2 | 20.6 | 22.3 | 0.9 |
| A3 | 41.4 | 27.9 | 1.5 | 22.3 | 21.8 | 1.0 |
| Profile 5 | ||||||
| A1 | 29.6 | 39.9 | 0.7 | 18.3 | 30.5 | 0.6 |
| A2 | 30.1 | 48.8 | 0.6 | 15.5 | 31.1 | 0.5 |
| Profile 6 | ||||||
| Ap | 40.1 | 21.1 | 1.9 | 27.8 | 13.3 | 2.1 |
| Profile 7 | ||||||
| Ap1 | 38.9 | 35.8 | 1.1 | 31.9 | 22.7 | 1.4 |
| Ap2 | 39.6 | 38.9 | 1.0 | 31.0 | 18.6 | 1.7 |
| Ahk | 57.7 | 26.0 | 2.2 | 50.0 | 17.8 | 2.8 |
| Profile 8 | ||||||
| Ap | 49.1 | 16.7 | 2.9 | 37.1 | 15.1 | 2.5 |
| A | 53.5 | 22.9 | 2.3 | 34.9 | 18.1 | 1.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dudek, M.; Łabaz, B.; Bednik, M.; Medyńska-Juraszek, A. Humic Substances as Indicator of Degradation Rate of Chernozems in South-Eastern Poland. Agronomy 2022, 12, 733. https://doi.org/10.3390/agronomy12030733
Dudek M, Łabaz B, Bednik M, Medyńska-Juraszek A. Humic Substances as Indicator of Degradation Rate of Chernozems in South-Eastern Poland. Agronomy. 2022; 12(3):733. https://doi.org/10.3390/agronomy12030733
Chicago/Turabian StyleDudek, Michał, Beata Łabaz, Magdalena Bednik, and Agnieszka Medyńska-Juraszek. 2022. "Humic Substances as Indicator of Degradation Rate of Chernozems in South-Eastern Poland" Agronomy 12, no. 3: 733. https://doi.org/10.3390/agronomy12030733
APA StyleDudek, M., Łabaz, B., Bednik, M., & Medyńska-Juraszek, A. (2022). Humic Substances as Indicator of Degradation Rate of Chernozems in South-Eastern Poland. Agronomy, 12(3), 733. https://doi.org/10.3390/agronomy12030733







