Waterlogging Stress Physiology in Barley
Abstract
1. Introduction
2. Barley Susceptibility to Waterlogging
3. Hypoxic Stress
3.1. Waterlogging and Oxygen Deprivation
3.2. Effects of Waterlogging on Plants
3.3. Plant Response to Waterlogging
3.4. Structural Changes
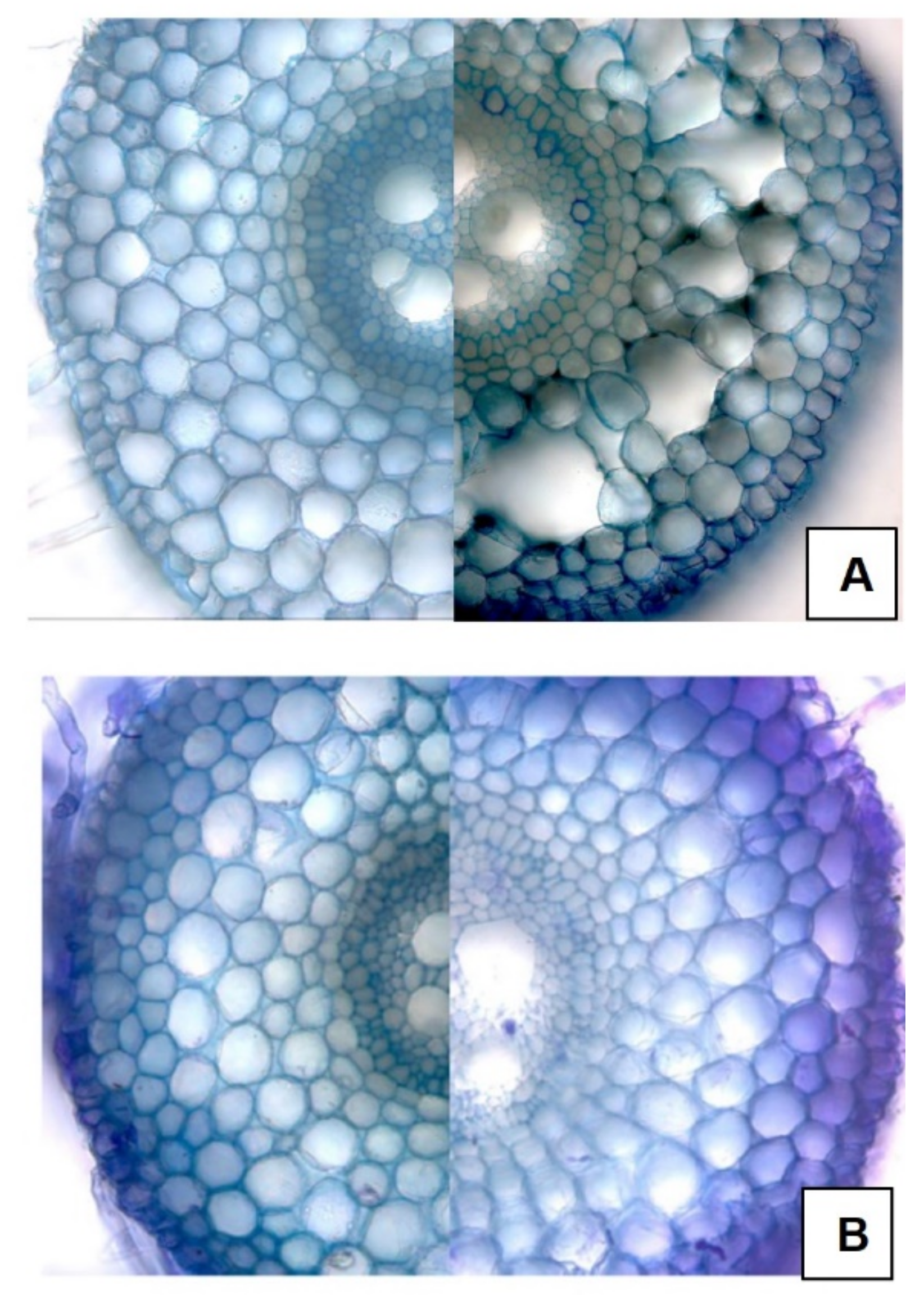
3.5. Physiological Changes
4. Nitric Oxide
4.1. Roles in Plant Development
4.2. Nitric Oxide Synthesis
4.3. Roles of Nitric Oxide in Biotic and Abiotic Stress
5. Reactive Oxygen Species
5.1. Deleterious Effects of Reactive Oxygen Species
5.2. Reactive Oxygen Species in Signaling and Stress Response
6. Phytoglobins
6.1. Classes of Phytoglobins
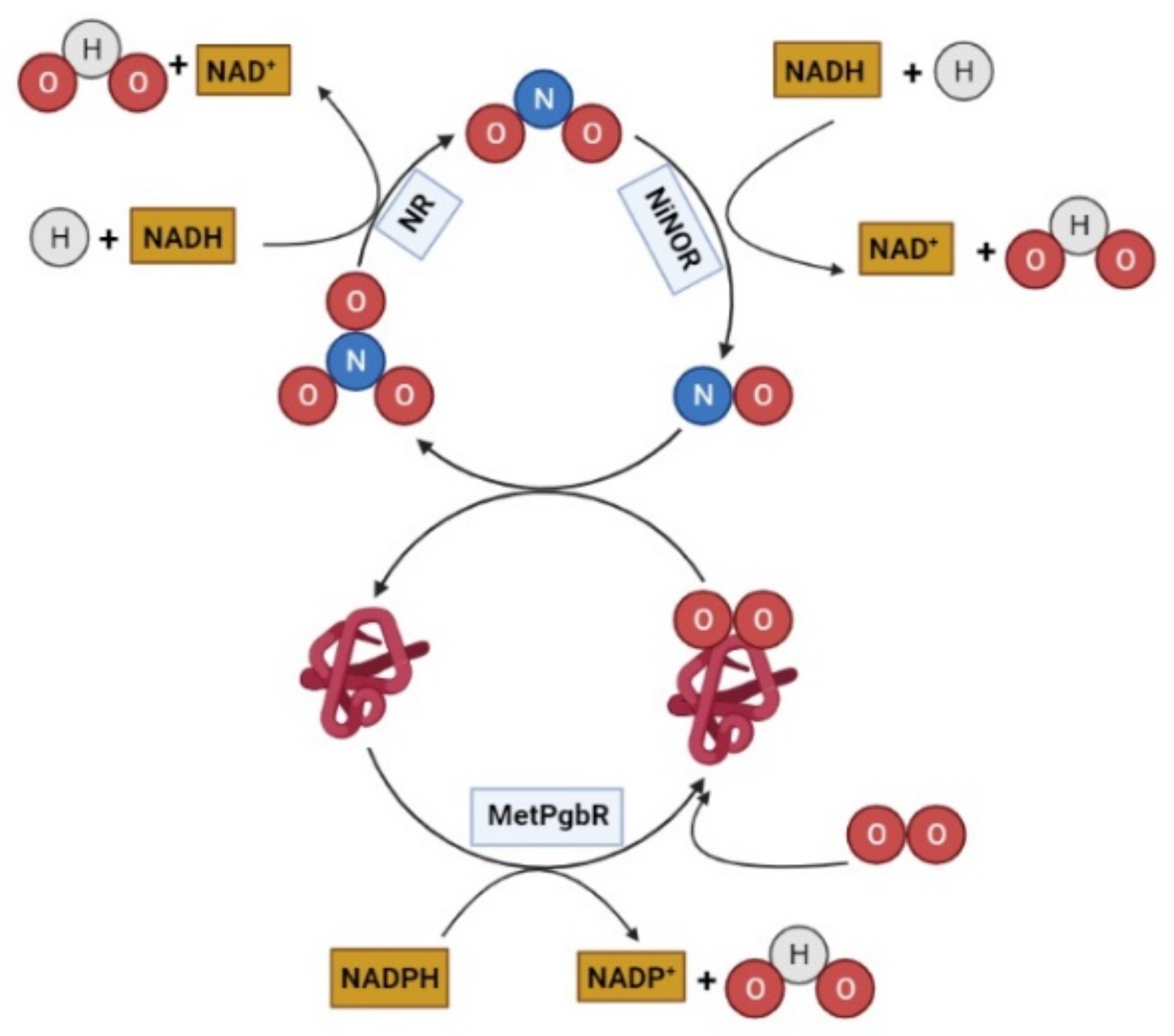
6.2. Class 1 Phytoglobin: Structure and Roles in Nitric Oxide Scavenging
6.3. Class 1 Phytoglobin and Stress Response
6.4. Nonsymbiotic Class 1 Phytoglobin in Barley
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#home (accessed on 31 January 2022).
- Giraldo, P.; Benavente, E.; Manzano-Agugliaro, F.; Gimenez, E. Worldwide Research Trends on Wheat and Barley: A Bibliometric Comparative Analysis. Agronomy 2019, 9, 352. [Google Scholar] [CrossRef]
- Malt_OEC—The Observatory of Economic Complexity. Available online: https://oec.world/en/profile/hs92/malt (accessed on 31 January 2022).
- Chillo, S.; Ranawana, D.V.; Pratt, M.; Henry, C.J.K. Glycemic response and glycemic index of semolina spaghetti enriched with barley β-glucan. Nutrition 2011, 27, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Ames, N.; Storsley, J. Effects of barley on post-prandial glycemic response. Diabesity 2015, 1, 21–23. [Google Scholar] [CrossRef][Green Version]
- Yaduvanshi, N.P.S.; Setter, T.L.; Sharma, S.K.; Singh, K.N.; Kulshreshtha, N. Influence of waterlogging on yield of wheat (Triticum aestivum), redox potentials, and concentrations of microelements in different soils in India and Australia. Soil Res. 2012, 50, 489–499. [Google Scholar] [CrossRef]
- Allen, D.M.; Buttle, J.M.; Allen, D.M.; Caissie, D.; Davison, B.; Peters, D.L.; Pomeroy, J.W.; Simonovic, S.; St-Hilaire, A.; Whitfield, P.H. Flood processes in Canada: Regional and special aspects. Can. Water Resour. J. Rev. Can. Res. Hydriques 2016, 41, 7–30. [Google Scholar] [CrossRef]
- Saskatchewan Crop Insurance Corporation—SCIC. Available online: https://www.scic.ca/ (accessed on 1 February 2022).
- Manitoba Agricultural Services Corporation (MASC). Available online: https://www.masc.mb.ca/masc.nsf/index.html?OpenPage (accessed on 31 January 2022).
- Westra, S.; Fowler, H.J.; Evans, J.P.; Alexander, L.V.; Berg, P.R.; Johnson, F.; Kendon, E.J.; Lenderink, G.; Roberts, N.M. Future changes to the intensity and frequency of short-duration extreme rainfall. Rev. Geophys. 2014, 52, 522–555. [Google Scholar] [CrossRef]
- Hirabayashi, Y.; Mahendran, R.; Koirala, S.; Konoshima, L.; Yamazaki, D.; Watanabe, S.; Kim, H.; Kanae, S. Global flood risk under climate change. Nat. Clim. Chang. 2013, 3, 816–821. [Google Scholar] [CrossRef]
- Province of Manitoba|Climate Change. Available online: https://www.gov.mb.ca/climateandgreenplan/climatechange.html (accessed on 31 January 2022).
- De San Celedonio, R.P.; Abeledo, L.G.; Miralles, D.J. Identifying the critical period for waterlogging on yield and its components in wheat and barley. Plant Soil 2014, 378, 265–277. [Google Scholar] [CrossRef]
- Setter, T.L.; Waters, I. Review of prospects for germplasm improvement for waterlogging tolerance in wheat, barley and oats. Plant Soil 2003, 253, 1–34. [Google Scholar] [CrossRef]
- Setter, T.L.; Burguess, P.; Waters, I.; Kuo, J. Genetic Diversity of Barley and Wheat for Waterlogging Tolerance in Western Australia. In Proceedings of the Australian Barley Technical Symposium, Melbourne, Australia, 12–16 September 1999. [Google Scholar]
- De San Celedonio, R.P.; Abeledo, L.G.; Miralles, D.J. Physiological traits associated with reductions in grain number in wheat and barley under waterlogging. Plant Soil 2018, 429, 469–481. [Google Scholar] [CrossRef]
- Borrego-Benjumea, A.; Carter, A.; Glenn, A.J.; Badea, A. Impact of excess moisture due to precipitation on barley grain yield in the Canadian Prairies. Can. J. Plant Sci. 2019, 99, 93–96. [Google Scholar] [CrossRef]
- Andrzejczak, O.A.; Havelund, J.F.; Wang, W.-Q.; Kovalchuk, S.; Hagensen, C.E.; Hasler-Sheetal, H.; Jensen, O.N.; Rogowska-Wrzesinska, A.; Møller, I.M.; Hebelstrup, K.H. The Hypoxic Proteome and Metabolome of Barley (Hordeum vulgare L.) with and without Phytoglobin Priming. Int. J. Mol. Sci. 2020, 21, 1546. [Google Scholar] [CrossRef]
- Ploschuk, R.A.; Miralles, D.J.; Colmer, T.D.; Ploschuk, E.L.; Striker, G.G. Waterlogging of Winter Crops at Early and Late Stages: Impacts on Leaf Physiology, Growth and Yield. Front. Plant Sci. 2018, 9, 1–15. [Google Scholar] [CrossRef]
- Voesenek, L.A.C.J.; Sasidharan, R. Ethylene—and oxygen signalling—drive plant survival during flooding. Plant Biol. 2013, 15, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Manik, S.M.N.; Pengilley, G.; Dean, G.; Field, B.; Shabala, S.; Zhou, M. Soil and Crop Management Practices to Minimize the Impact of Waterlogging on Crop Productivity. Front. Plant Sci. 2019, 10, 140. [Google Scholar] [CrossRef]
- MASC—AgriInsurance. Available online: https://www.masc.mb.ca/masc.nsf/program_agriinsurance.html (accessed on 31 January 2022).
- Rosenzweig, C.; Tubiello, F.N.; Goldberg, R.; Mills, E.; Bloomfield, J. Increased crop damage in the US from excess precipitation under climate change. Glob. Environ. Chang. 2002, 12, 197–202. [Google Scholar] [CrossRef]
- Borrego-Benjumea, A.; Carter, A.; Zhu, M.; Tucker, J.R.; Zhou, M.; Badea, A. Genome-Wide Association Study of Waterlogging Tolerance in Barley (Hordeum vulgare L.) Under Controlled Field Conditions. Front. Plant Sci. 2021, 12, 10–16. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, G.; Shabala, S.; Koutoulis, A.; Shabala, L.; Johnson, P.; Li, C.; Zhou, M. Identification of aerenchyma formation-related QTL in barley that can be effective in breeding for waterlogging tolerance. Theor. Appl. Genet. 2016, 129, 1167–1177. [Google Scholar] [CrossRef]
- Gill, M.B.; Zeng, F.; Shabala, L.; Zhang, G.; Fan, Y.; Shabala, S.; Zhou, M. Cell-Based Phenotyping Reveals QTL for Membrane Potential Maintenance Associated with Hypoxia and Salinity Stress Tolerance in Barley. Front. Plant Sci. 2017, 8, 1–9. [Google Scholar] [CrossRef]
- Gill, M.B.; Zeng, F.; Shabala, L.; Zhang, G.; Yu, M.; Demidchik, V.; Shabala, S.; Zhou, M. Identification of QTL Related to ROS Formation under Hypoxia and Their Association with Waterlogging and Salt Tolerance in Barley. Int. J. Mol. Sci. 2019, 20, 699. [Google Scholar] [CrossRef]
- Zhang, X.; Shabala, S.; Koutoulis, A.; Shabala, L.; Zhou, M. Meta-analysis of major QTL for abiotic stress tolerance in barley and implications for barley breeding. Planta 2017, 245, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Luan, H.; Shen, H.; Pan, Y.; Guo, B.; Lv, C.; Xu, R. Elucidating the hypoxic stress response in barley (Hordeum vulgare L.) during waterlogging: A proteomics approach. Sci. Rep. 2018, 8, 9655. [Google Scholar] [CrossRef]
- Borrego-Benjumea, A.; Carter, A.; Tucker, J.R.; Yao, Z.; Xu, W.; Badea, A. Genome-wide analysis of gene expression provides new insights into waterlogging responses in barley (Hordeum vulgare L.). Plants 2020, 9, 240. [Google Scholar] [CrossRef]
- Tong, C.; Hill, C.B.; Zhou, G.; Zhang, X.-Q.; Jia, Y.; Li, C. Opportunities for Improving Waterlogging Tolerance in Cereal Crops—Physiological Traits and Genetic Mechanisms. Plants 2021, 10, 1560. [Google Scholar] [CrossRef]
- Pradet, A.; Bomsel, J. Energy Metabolism in Plants under Hypoxia and Anoxia. In Plant Life in Anaerobic Environments; Hook, D.D., Crawford, R., Eds.; Ann. Arbor Science: Ann Arbor, MI, USA, 1978; pp. 89–118. [Google Scholar]
- Sasidharan, R.; Bailey-Serres, J.; Ashikari, M.; Atwell, B.J.; Colmer, T.D.; Fagerstedt, K.; Fukao, T.; Geigenberger, P.; Hebelstrup, K.H.; Hill, R.D.; et al. Community recommendations on terminology and procedures used in flooding and low oxygen stress research. New Phytol. 2017, 214, 1403–1407. [Google Scholar] [CrossRef]
- Loreti, E.; Poggi, A.; Novi, G.; Alpi, A.; Perata, P. A Genome-Wide Analysis of the Effects of Sucrose on Gene Expression in Arabidopsis Seedlings under Anoxia. Plant Physiol. 2005, 137, 1130–1138. [Google Scholar] [CrossRef]
- Drew, M.C. Oxygen deficiency and root metabolism: Injury and acclimation under hypoxia and anoxia. Annu. Rev. Plant Biol. 1997, 48, 223–250. [Google Scholar] [CrossRef]
- Armstrong, W.; Drew, M. Root Growth and Metabolism under Oxygen Deficiency. In Plant Roots; Waisel, Y., Eshel, A., Beeckman, T., Kafkafi, U., Eds.; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Barrett-Lennard, E.G. The Interaction between Waterlogging and Salinity in Higher Plants: Causes, Consequences and Implications the Interaction between Waterlogging and Salinity in Higher Plants: Causes, Consequences and Implications. Plant Soil 2003, 253, 35–54. [Google Scholar] [CrossRef]
- Evans, D.E. Aerenchyma formation. New Phytol. 2004, 161, 35–49. [Google Scholar] [CrossRef]
- Tan, X.; Xu, H.; Khan, S.; Equiza, M.A.; Lee, S.H.; Vaziriyeganeh, M.; Zwiazek, J.J. Plant water transport and aquaporins in oxygen-deprived environments. J. Plant Physiol. 2018, 227, 20–30. [Google Scholar] [CrossRef]
- Mira, M.M.; Hill, R.D.; Stasolla, C. Phytoglobins Improve Hypoxic Root Growth by Alleviating Apical Meristem Cell Death. Plant Physiol. 2016, 172, 2044–2056. [Google Scholar] [CrossRef] [PubMed]
- Bai, T.; Li, C.; Li, C.; Liang, D.; Ma, F. Contrasting hypoxia tolerance and adaptation inMalusspecies is linked to differences in stomatal behavior and photosynthesis. Physiol. Plant. 2013, 147, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Johnson, J.W.; Nesmith, S.; Bridges, D.C. Growth, physiological and anatomical responses of two wheat genotypes to waterlogging and nutrient supply. J. Exp. Bot. 1994, 45, 193–202. [Google Scholar] [CrossRef]
- Justin, S.H.F.W.; Armstrong, W. The Anatomical Characteristics of Roots and Plant Response to Soil Flooding. New Phytol. 1987, 106, 465–495. [Google Scholar] [CrossRef]
- Gibbs, J.; Greenway, H. Review: Mechanisms of anoxia tolerance in plants. I. Growth, survival and anaerobic catabolism. Funct. Plant Biol. 2003, 30, 1–47. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Colmer, T.D. Plant tolerance of flooding stress—recent advances. Plant Cell Environ. 2014, 37, 2211–2215. [Google Scholar] [CrossRef]
- Clark, L.H.; Harris, W.H. Observations on the Root Anatomy of Rice (Oryza sativa L.). Am. J. Bot. 1981, 68, 154–161. [Google Scholar]
- Armstrong, W. Aeration in Higher Plants. Adv. Bot. Res. 1980, 7, 226–332. [Google Scholar]
- Yu, F.; Han, X.; Geng, C.; Zhao, Y.; Zhang, Z.; Qiu, F. Comparative proteomic analysis revealing the complex network associated with waterlogging stress in maize (Zea mays L.) seedling root cells. Proteomics 2015, 15, 135–147. [Google Scholar] [CrossRef]
- Qi, X.; Li, Q.; Ma, X.; Qian, C.; Wang, H.; Ren, N.; Shen, C.; Huang, S.; Xu, X.; Xu, Q.; et al. Waterlogging-induced adventitious root formation in cucumber is regulated by ethylene and auxin through reactive oxygen species signalling. Plant Cell Environ. 2017, 42, 1458–1470. [Google Scholar] [CrossRef]
- De Castro, J. Role of Phytoglobin1 in Waterlogging Stress Response in Barley (Hordeum vulgare L.). Master’s Thesis, University of Manitoba, Winnipeg, MB, Canada, 2022. (In preparation). [Google Scholar]
- Sauter, M. Root responses to flooding. Curr. Opin. Plant Biol. 2013, 16, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Voesenek, L.A.C.J.; Bailey-Serres, J. Flood adaptive traits and processes: An overview. New Phytol. 2015, 206, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, D.W.; Shah, J.K.; Hebelstrup, K.H.; Igamberdiev, A.U. Expression of phytoglobin affects nitric oxide metabolism and energy state of barley plants exposed to anoxia. Plant Sci. 2017, 265, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Groß, F.; Durner, J.; Gaupels, F. Nitric oxide, antioxidants and prooxidants in plant defence responses. Front. Plant Sci. 2013, 4, 419. [Google Scholar] [CrossRef]
- Youssef, M.S.; Mira, M.M.; Renault, S.; Hill, R.D.; Stasolla, C. Phytoglobin expression influences soil flooding response of corn plants. Ann. Bot. 2016, 118, 919–931. [Google Scholar] [CrossRef]
- Yordanova, R.Y.; Christov, K.N.; Popova, L.P. Antioxidative enzymes in barley plants subjected to soil flooding. Environ. Exp. Bot. 2004, 51, 93–101. [Google Scholar] [CrossRef]
- Hill, R.D. Non-symbiotic haemoglobins—What’s happening beyond nitric oxide scavenging? AoB PLANTS 2012, 12, pls004. [Google Scholar] [CrossRef]
- Sasidharan, R.; Voesenek, L.A.C.J. Ethylene-Mediated Acclimations to Flooding Stress. Plant Physiol. 2015, 169, 3–12. [Google Scholar] [CrossRef]
- McDonald, M.P.; Galwey, N.W.; Colmer, T.D. Waterlogging tolerance in the tribe Triticeae: The adventitious roots of Critesion marinum have a relatively high porosity and a barrier to radial oxygen loss. Plant Cell Environ. 2001, 24, 585–596. [Google Scholar] [CrossRef]
- Mano, Y.; Omori, F. Relationship between constitutive root aerenchyma formation and flooding tolerance in Zea nicaraguensis. Plant Soil 2013, 370, 447–460. [Google Scholar] [CrossRef]
- Kamal, A.H.M.; Komatsu, S. Jasmonic acid induced protein response to biophoton emissions and flooding stress in soybean. J. Proteom. 2016, 133, 33–47. [Google Scholar] [CrossRef]
- Haque, E.H.; Abe, F.; Mori, M.; Oyanagi, A.; Komatsu, S.; Kawaguchi, K. Characterization of a wheat pathogenesis-related protein, TaBWPR-1.2, in seminal roots in response to waterlogging stress. J. Plant Physiol. 2014, 171, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Hsu, F.-C.; Chou, M.-Y.; Peng, H.-P.; Chou, S.-J.; Shih, M.-C. Insights into Hypoxic Systemic Responses Based on Analyses of Transcriptional Regulation in Arabidopsis. PLoS ONE 2011, 6, e28888. [Google Scholar] [CrossRef]
- Luan, H.; Guo, B.; Pan, Y.; Lv, C.; Shen, H.; Xu, R. Morpho-anatomical and physiological responses to waterlogging stress in different barley (Hordeum vulgare L.) genotypes. Plant Growth Regul. 2018, 85, 399–409. [Google Scholar] [CrossRef]
- Neill, S.J.; Desikan, R.; Hancock, J.T. Nitric oxide signalling in plants. New Phytol. 2003, 159, 11–35. [Google Scholar] [CrossRef]
- Gupta, K.J.; Hebelstrup, K.H.; Mur, L.A.; Igamberdiev, A.U.J. Plant hemoglobins: Important players at the crossroads between oxygen and nitric oxide. FEBS Lett. 2011, 585, 3843–3849. [Google Scholar] [CrossRef]
- Dordas, C. Nonsymbiotic hemoglobins and stress tolerance in plants. Plant Sci. 2009, 176, 433–440. [Google Scholar] [CrossRef]
- Hufton, C.A.; Besford, R.T.; Wellburn, A.R. Effects of NO (+NO2) pollution on growth, nitrate reductase activities and associated protein contents in glasshouse lettuce grown hydroponically in winter with CO2 enrichment. New Phytol. 1996, 133, 495–501. [Google Scholar] [CrossRef]
- Leshem, Y.Y.; Haramaty, E. The Characterization and Contrasting Effects of the Nitric Oxide Free Radical in Vegetative Stress and Senescence of Pisum sativum Linn. Foliage. J. Plant Physiol. 1996, 148, 258–263. [Google Scholar] [CrossRef]
- Guo, F.-Q.; Okamoto, M.; Crawford, N.M. Identification of a Plant Nitric Oxide Synthase Gene Involved in Hormonal Signaling. Science 2003, 302, 100–103. [Google Scholar] [CrossRef]
- Kim, P.K.M.; Zamora, R.; Petrosko, P.; Billiar, T.R. The regulatory role of nitric oxide in apoptosis. Int. Immunopharmacol. 2001, 1, 1421–1441. [Google Scholar] [CrossRef] [PubMed]
- Igamberdiev, A.U.; Hill, R.D. Nitrate, NO and Haemoglobin in Plant Adaptation to Hypoxia: An Alternative to Classic Fermentation Pathways. J. Exp. Bot. 2004, 55, 2473–2482. [Google Scholar] [CrossRef] [PubMed]
- Stoimenova, M.; Igamberdiev, A.U.; Gupta, K.J.; Hill, R.D. Nitrite-driven anaerobic ATP synthesis in barley and rice root mitochondria. Planta 2007, 226, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.; Hargrove, M.; Arredondo-Peter, R. Phytoglobin: A novel nomenclature for plant globins accepted by the globin community at the 2014 XVIII conference on Oxygen-Binding and Sensing Proteins. F1000Research 2016, 5, 212. [Google Scholar] [CrossRef]
- Igamberdiev, A.U.; Bykova, N.V.; Shah, J.K.; Hill, R.D. Anoxic nitric oxide cycling in plants: Participating reactions and possible mechanisms. Physiol. Plant. 2010, 138, 393–404. [Google Scholar] [CrossRef]
- García-Mata, C.; LaMattina, L. Nitric Oxide Induces Stomatal Closure and Enhances the Adaptive Plant Responses against Drought Stress. Plant Physiol. 2001, 126, 1196–1204. [Google Scholar] [CrossRef]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef]
- Mhamdi, A.; Van Breusegem, F. Reactive oxygen species in plant development. Development 2018, 145, dev164376. [Google Scholar] [CrossRef]
- Triantaphylidès, C.; Havaux, M. Singlet oxygen in plants: Production, detoxification and signaling. Trends Plant Sci. 2009, 14, 219–228. [Google Scholar] [CrossRef]
- Yang, C.; Li, W.; Cao, J.; Meng, F.; Yu, Y.; Huang, J.; Jiang, L.; Liu, M.; Zhang, Z.; Chen, X.; et al. Activation of ethylene signaling pathways enhances disease resistance by regulating ROS and phytoalexin production in rice. Plant J. 2017, 89, 338–353. [Google Scholar] [CrossRef]
- Lee, S.; Seo, P.J.; Lee, H.-J.; Park, C.-M. A NAC transcription factor NTL4 promotes reactive oxygen species production during drought-induced leaf senescence in Arabidopsis. Plant J. 2012, 70, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Ahammed, G.J.; He, B.-B.; Qian, X.-J.; Zhou, Y.-H.; Shi, K.; Zhou, J.; Yu, J.-Q.; Xia, X.-J. 24-Epibrassinolide alleviates organic pollutants-retarded root elongation by promoting redox homeostasis and secondary metabolism in Cucumis sativus L. Environ. Pollut. 2017, 229, 922–931. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Rajhi, I.; Nakazono, M. Lysigenous aerenchyma formation in maize root is confined to cortical cells by regulation of genes related to generation and scavenging of reactive oxygen species. Plant Signal. Behav. 2011, 6, 759–761. [Google Scholar] [CrossRef]
- Shiono, K.; Ejiri, M.; Shimizu, K.; Yamada, S. Improved waterlogging tolerance of barley (Hordeum vulgare) by pretreatment with ethephon. Plant Prod. Sci. 2019, 22, 285–295. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.; Tenhaken, R.; Dixon, R.; Lamb, C. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 1994, 79, 583–593. [Google Scholar] [CrossRef]
- Steffens, B.; Geske, T.; Sauter, M. Aerenchyma formation in the rice stem and its promotion by H2O2. New Phytol. 2011, 190, 369–378. [Google Scholar] [CrossRef]
- Yamauchi, T.; Yoshioka, M.; Fukazawa, A.; Mori, H.; Nishizawa, N.K.; Tsutsumi, N.; Yoshioka, H.; Nakazono, M. An NADPH Oxidase RBOH Functions in Rice Roots during Lysigenous Aerenchyma Formation under Oxygen-Deficient Conditions. Plant Cell 2017, 29, 775–790. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Torres, M.A.; Dangl, J.L. Functions of the respiratory burst oxidase in biotic interactions, abiotic stress and development. Curr. Opin. Plant Biol. 2005, 8, 397–403. [Google Scholar] [CrossRef]
- Suzuki, N.; Miller, G.; Morales, J.; Shulaev, V.; Torres, M.A.; Mittler, R. Respiratory burst oxidases: The engines of ROS signaling. Curr. Opin. Plant Biol. 2011, 14, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Kubo, H. Hemeprotein from the Root Nodules of Legumes. Acta Phytochim. 1939, 11, 195–200. [Google Scholar]
- Jackson, M.B.; Drew, M.C. Effects of Flooding on Growth and Metabolism of Herbaceous Plants. Flooding Plant Growth 1984, 47–128. [Google Scholar]
- Landsmann, J.; Dennis, E.S.; Higgins, T.J.V.; Appleby, C.A.; Kortt, A.A.; Peacock, W.J. Common evolutionary origin of legume and non-legume plant haemoglobins. Nature 1986, 324, 166–168. [Google Scholar] [CrossRef]
- Wittenberg, J.B.; Bolognesi, M.; Wittenberg, B.A.; Guertin, M. Truncated Hemoglobins: A New Family of Hemoglobins Widely Distributed in Bacteria, Unicellular Eukaryotes, and Plants. J. Biol. Chem. 2002, 277, 871–874. [Google Scholar] [CrossRef]
- Watts, R.A.; Hunt, P.W.; Hvitved, A.N.; Hargrove, M.S.; Peacock, W.J.; Dennis, E.S. A hemoglobin from plants homologous to truncated hemoglobins of microorganisms. Proc. Natl. Acad. Sci. USA 2001, 98, 10119–10124. [Google Scholar] [CrossRef]
- Duff, S.M.G.; Wittenberg, J.B.; Hill, R.D. Expression, Purification, and Properties of Recombinant Barley (Hordeum sp.) Hemoglobin: Optical spectra and reactions with gaseous ligands. J. Biol. Chem. 1997, 272, 16746–16752. [Google Scholar] [CrossRef]
- Appleby, C.A. Leghemoglobin and Rhizobium Respiration. Annu. Rev. Plant Physiol. 1984, 35, 443–478. [Google Scholar]
- Appleby, C.A. The Origin and Functions of Haemoglobin in Plants. Sci. Prog. 1992, 76, 365–398. [Google Scholar]
- Arredondo-Peter, A.R.; Hargrove, M.S.; Sarath, G.; Moran, J.F.; Olson, J.S.; Klucas, R.; Physiology, S.P.; Nov, N.; Arredondo-peter, R.; Hargrove, M.S.; et al. Rice Hemoglobins: Gene Cloning, Analysis, and O2;-Binding Kinetics of a Recombinant Protein Synthesized in Escherichia coli. Plant Physiol. 1997, 115, 1259–1266. [Google Scholar]
- Smagghe, B.J.; Hoy, J.A.; Percifield, R.; Kundu, S.; Hargrove, M.S.; Sarath, G.; Hilbert, J.-L.; Watts, R.A.; Dennis, E.S.; Peacock, W.J.; et al. Review: Correlations between oxygen affinity and sequence classifications of plant hemoglobins. Biopolymers 2009, 91, 1083–1096. [Google Scholar] [CrossRef] [PubMed]
- Vigeolas, H.; Hühn, D.H.; Geigenberger, P. Nonsymbiotic Hemoglobin-2 Leads to an Elevated Energy State and to a Combined Increase in Polyunsaturated Fatty Acids and Total Oil Content When Overexpressed in Developing Seeds of Transgenic Arabidopsis Plants. Plant Physiol. 2011, 155, 1435–1444. [Google Scholar] [CrossRef] [PubMed]
- Hunt, P.W.; Klok, E.J.; Trevaskis, B.; Watts, R.A.; Ellis, M.H.; Peacock, W.J.; Dennis, E.S. Increased level of hemoglobin 1 enhances survival of hypoxic stress and promotes early growth in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2002, 99, 17197–17202. [Google Scholar] [CrossRef]
- Taylor, E.R.; Nie, X.Z.; MacGregor, A.W.; Hill, R.D. A cereal haemoglobin gene is expressed in seed and root tissues under anaerobic conditions. Plant Mol. Biol. 1994, 24, 853–862. [Google Scholar] [CrossRef]
- Garrocho-Villegas, V.; Gopalasubramaniam, S.K.; Arredondo-Peter, R. Plant hemoglobins: What we know six decades after their discovery. Gene 2007, 398, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.-L.; Zhong, N.-Q.; Wang, H.-Y.; Chen, A.-P.; Jian, G.-L.; Xia, G.-X. Ectopic Expression of the Cotton Non-symbiotic Hemoglobin Gene GhHbd1 Triggers Defense Responses and Increases Disease Tolerance in Arabidopsis. Plant Cell Physiol. 2006, 47, 1058–1068. [Google Scholar] [CrossRef]
- Igamberdiev, A.U.; Serege’lyes, C.; Manac’H, N.; Hill, R.D. NADH-dependent metabolism of nitric oxide in alfalfa root cultures expressing barley hemoglobin. Planta 2004, 219, 95–102. [Google Scholar] [CrossRef]
- Sowa, A.W.; Duff, S.M.G.; Guy, P.A.; Hill, R.D. Altering hemoglobin levels changes energy status in maize cells under hypoxia. Proc. Natl. Acad. Sci. USA 1998, 95, 10317–10321. [Google Scholar] [CrossRef]
- Bahmani, R.; Kim, D.G.; Na, J.D.; Hwang, S. Expression of the Tobacco Non-symbiotic Class 1 Hemoglobin Gene Hb1 Reduces Cadmium Levels by Modulating Cd Transporter Expression Through Decreasing Nitric Oxide and ROS Level in Arabidopsis. Front. Plant Sci. 2019, 10, 201. [Google Scholar] [CrossRef]
- Mira, M.M.; Asmundson, B.; Renault, S.; Hill, R.D.; Stasolla, C. Suppression of the soybean (Glycine max) Phytoglobin GmPgb1 improves tolerance to iron stress. Acta Physiol. Plant. 2021, 43, 1–14. [Google Scholar] [CrossRef]
- Zafari, S.; Hebelstrup, K.H.; Igamberdiev, A.U. Transcriptional and Metabolic Changes Associated with Phytoglobin Expression during Germination of Barley Seeds. Int. J. Mol. Sci. 2020, 21, 2796. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Castro, J.; Hill, R.D.; Stasolla, C.; Badea, A. Waterlogging Stress Physiology in Barley. Agronomy 2022, 12, 780. https://doi.org/10.3390/agronomy12040780
De Castro J, Hill RD, Stasolla C, Badea A. Waterlogging Stress Physiology in Barley. Agronomy. 2022; 12(4):780. https://doi.org/10.3390/agronomy12040780
Chicago/Turabian StyleDe Castro, James, Robert D. Hill, Claudio Stasolla, and Ana Badea. 2022. "Waterlogging Stress Physiology in Barley" Agronomy 12, no. 4: 780. https://doi.org/10.3390/agronomy12040780
APA StyleDe Castro, J., Hill, R. D., Stasolla, C., & Badea, A. (2022). Waterlogging Stress Physiology in Barley. Agronomy, 12(4), 780. https://doi.org/10.3390/agronomy12040780




