Identification and Fine Mapping of a Quantitative Trait Locus Controlling the Total Flower and Pod Numbers in Soybean
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Field Experiments
2.2. Construction of Linkage Map
2.3. QTL Analysis
2.4. Fine Mapping
2.5. Genome Resequencing Analysis
2.6. Identification of the Candidate Gene for qFPN4
2.7. Phenotype Statistics
3. Results
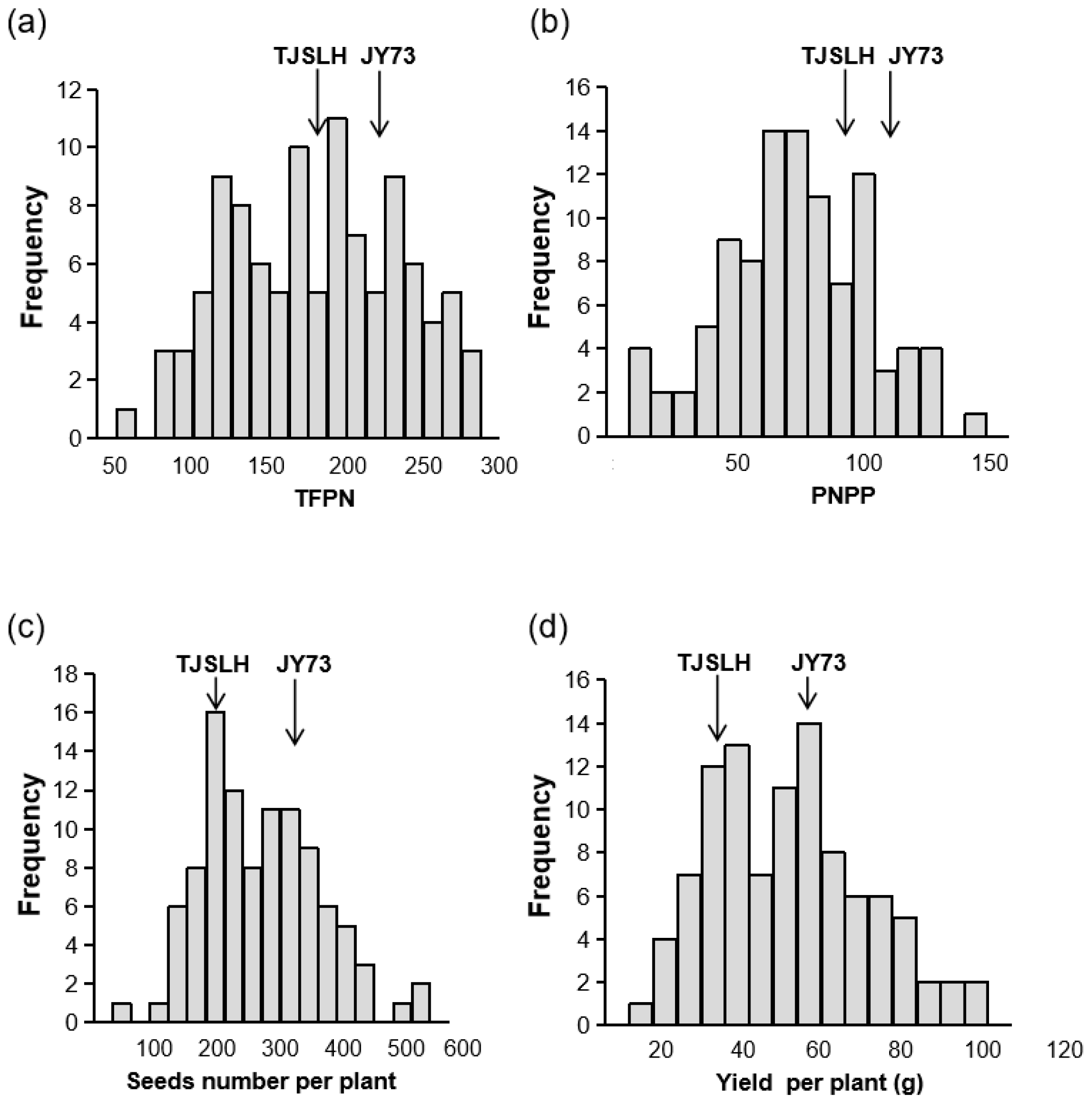
3.1. Phenotypic Identification of the Mapping Population
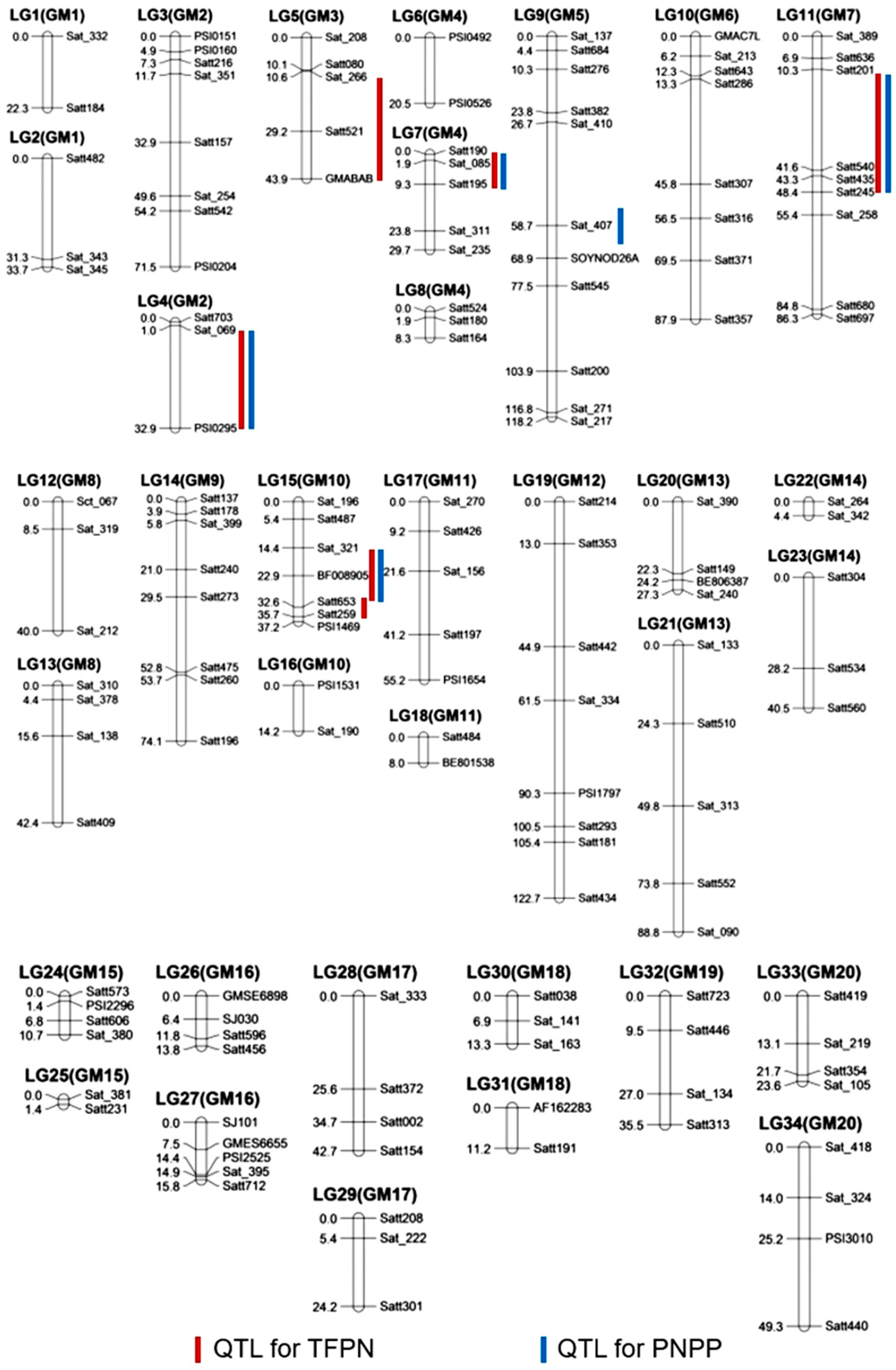
3.2. Multiple QTL Control TFPN and PNPP
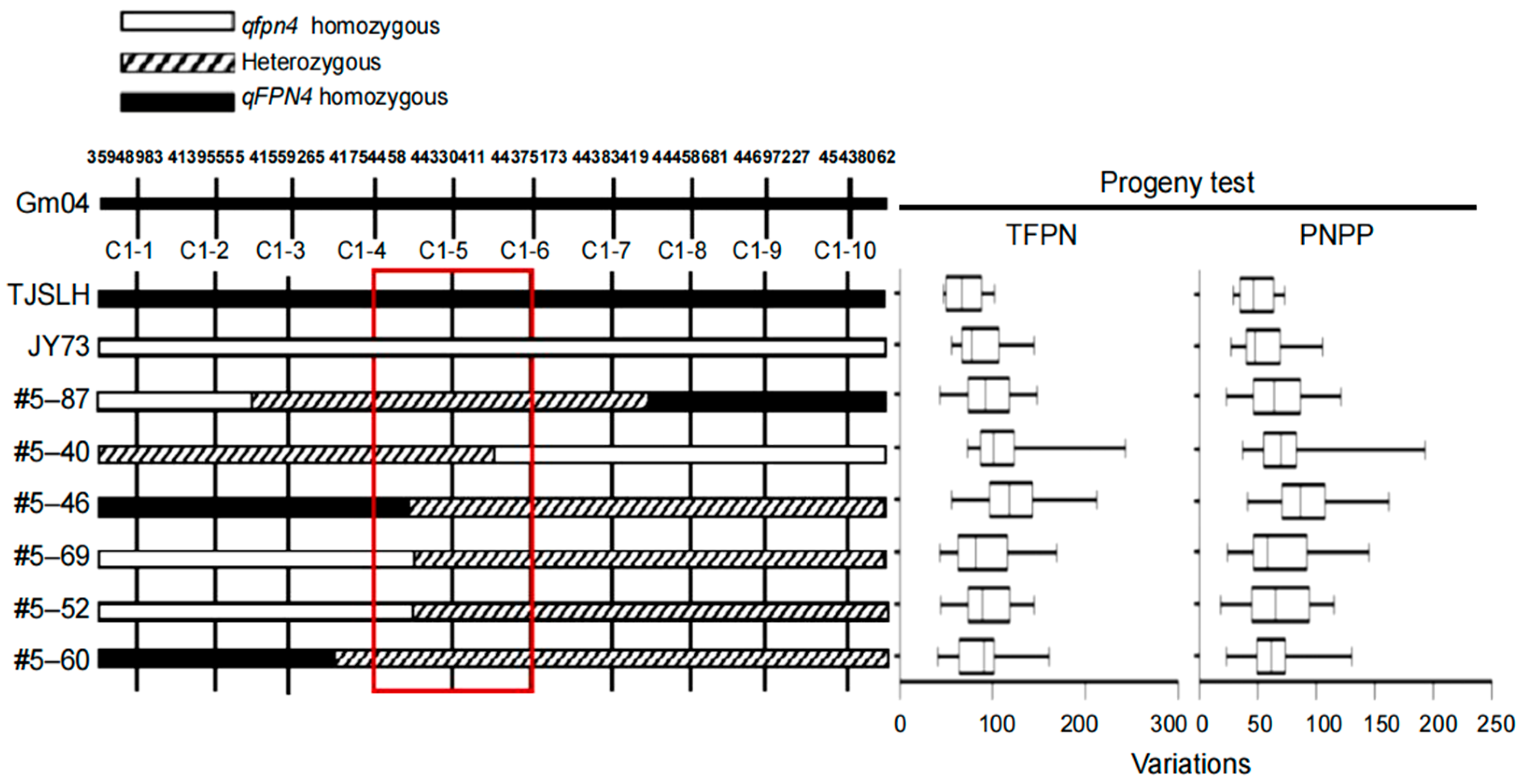
3.3. Fine Mapping of qFPN4
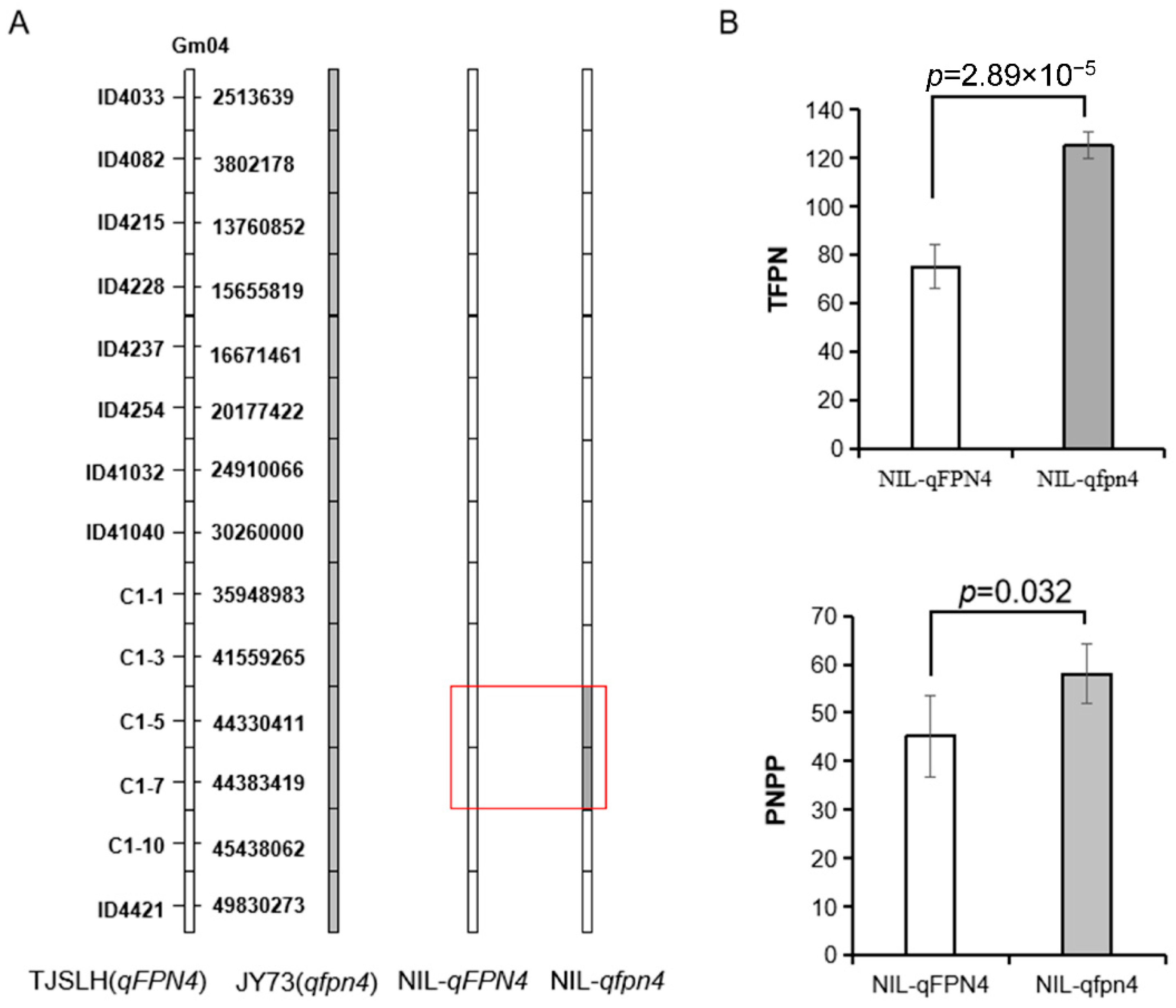
3.4. qFPN4 Controls Low Number of Flowers and Pods
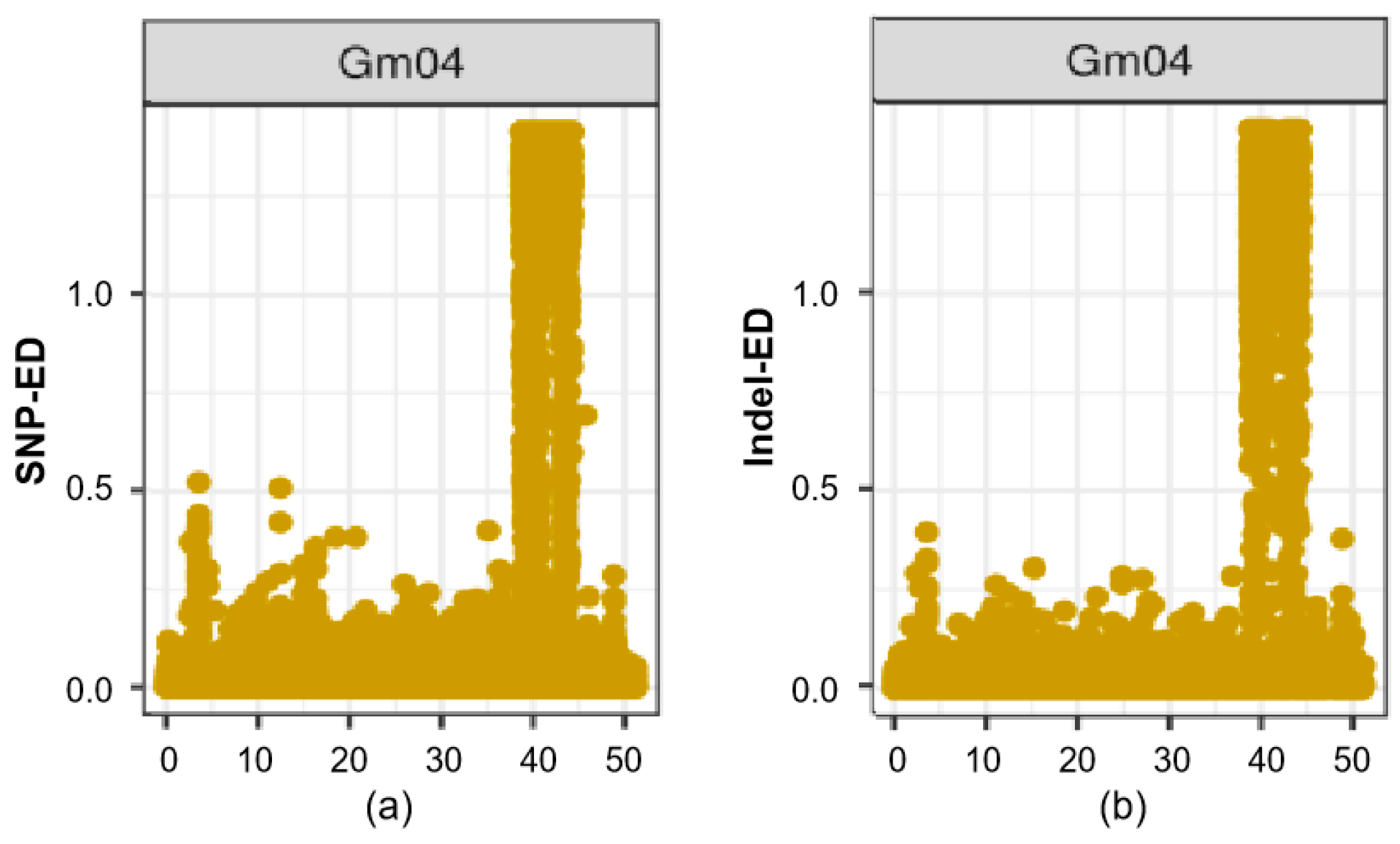
3.5. Genome Sequencing Based BSA Analysis Confirmed qFPN4
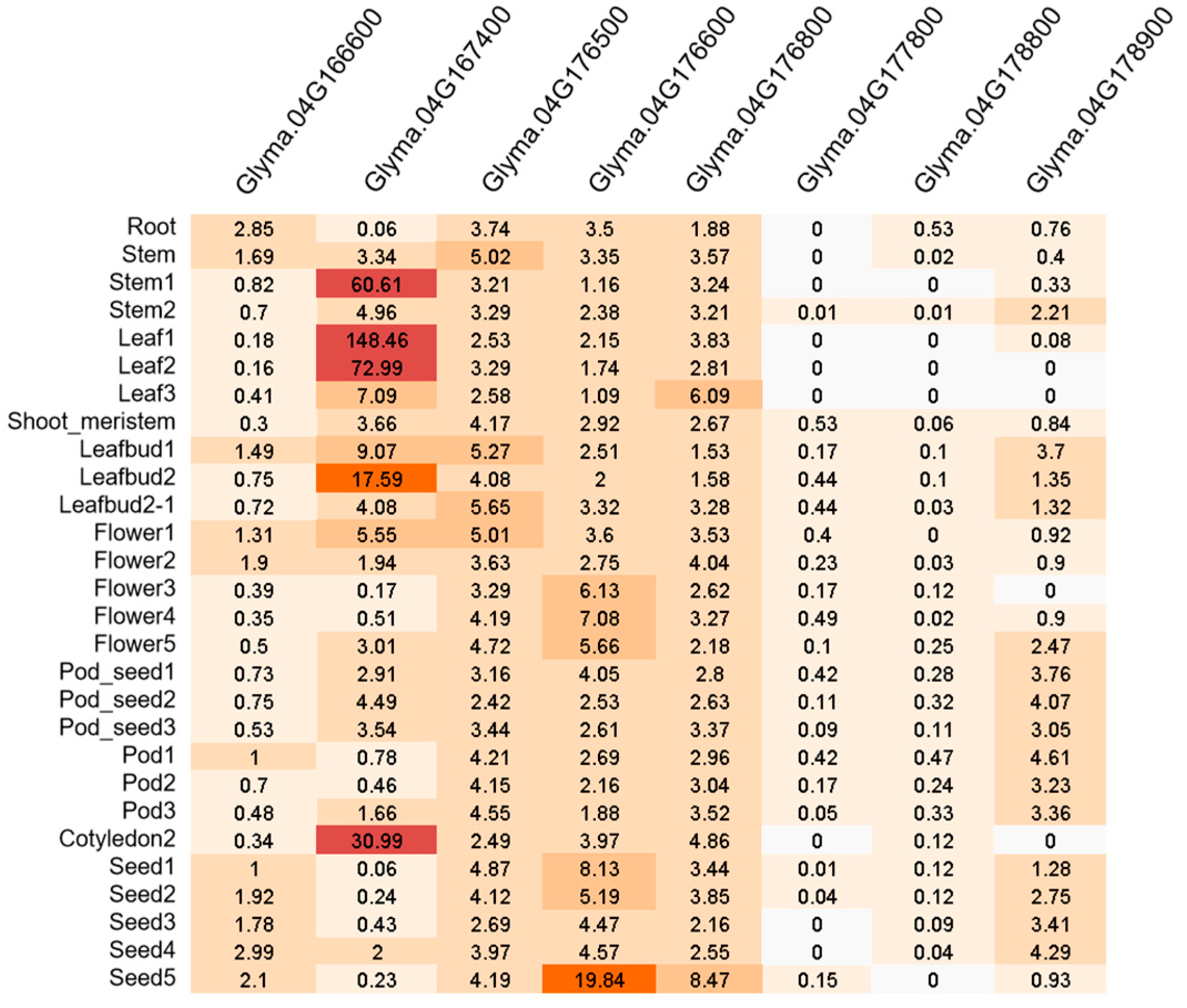
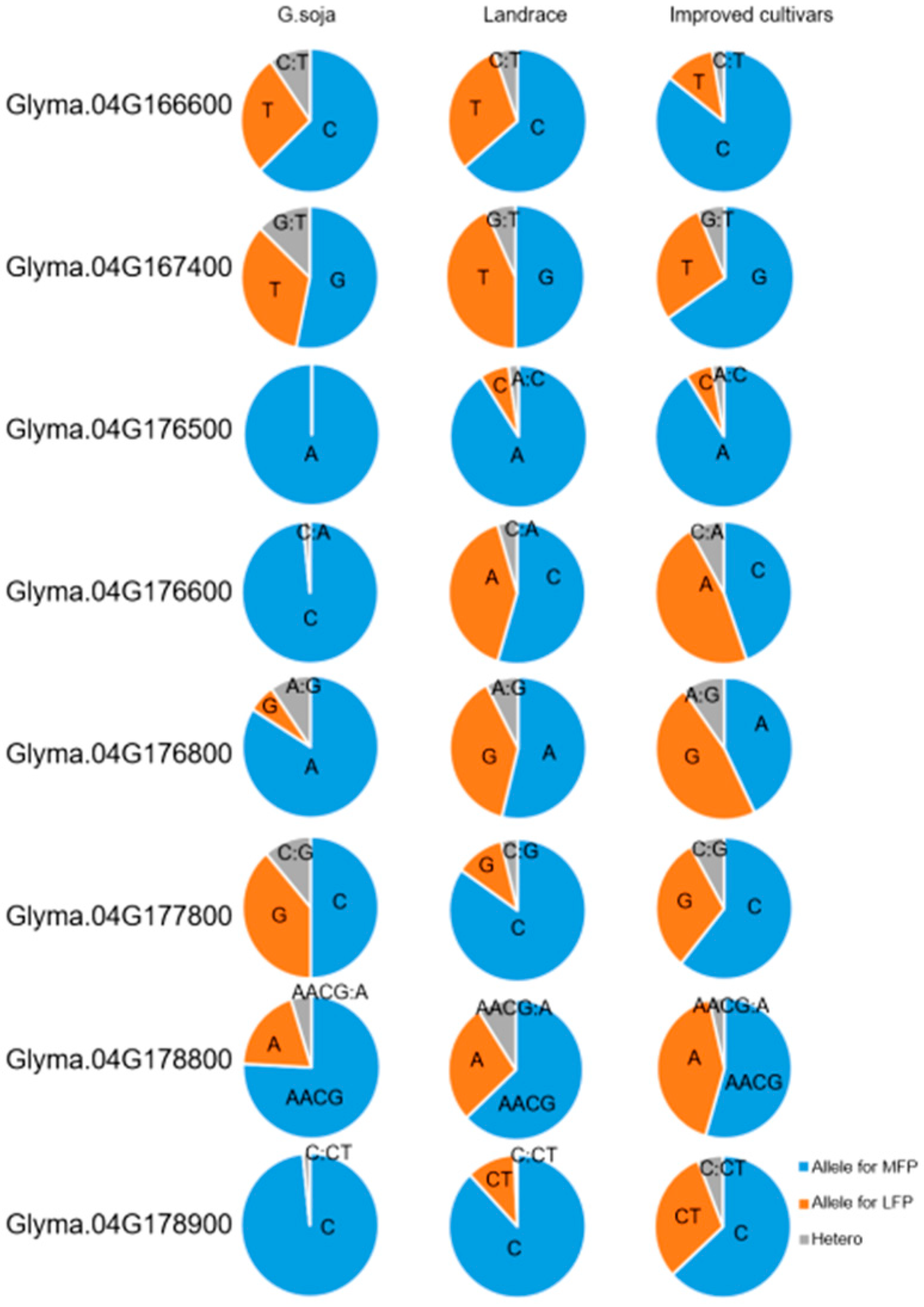
3.6. Promising Genes for qFPN4
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xavier, A.; Rainey, K.M. Quantitative genomic dissection of soybean yield components. G3 (Bethesda) 2020, 10, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Carter, T.E.; Nelson, R.L.; Sneller, C.H.; Cui, Z.; Boerma, H.R.; Specht, J.E. Genetic diversity in soybean. In Soybeans: Improvement, Production, and Uses, 3rd ed.; American Society of Agronomy: Madison, WI, USA, 2004; Volume 16, pp. 303–396. [Google Scholar]
- Mikel, M.A.; Diers, B.W.; Nelson, R.L.; Smith, H.H. Genetic diversity and agronomic improvement of North American soybean germplasm. Crop Sci. 2010, 50, 1219–1229. [Google Scholar] [CrossRef]
- Rincker, K.; Nelson, R.; Specht, J.; Sleper, D.; Cary, T.; Cianzio, S.R.; Casteel, S.; Conley, S.; Chen, P.; Davis, V.; et al. Genetic improvement of US soybean in maturity groups II, III, and IV. Crop Sci. 2014, 54, 1419–1432. [Google Scholar] [CrossRef]
- Specht, J.E.; Hume, D.J.; Kumudini, S.V. Soybean yield potential: A genetic and physiological perspective. Crop Sci. 1999, 39, 1560–1570. [Google Scholar] [CrossRef]
- Ning, H.; Yuan, J.; Dong, Q.; Li, W.; Xue, H.; Wang, Y.; Tian, Y.; Li, W.X. Identification of QTLs related to the vertical distribution and seed-set of pod number in soybean Glycine max (L.) Merri. PLoS ONE 2018, 13, e0195830. [Google Scholar] [CrossRef]
- Machado, B.Q.V.; Nogueira, A.P.O.; Hamawaki, O.T.; Rezende, G.F.; Jorge, G.L.; Silveira, I.C.; Medeiros, L.A.; Hamawaki, R.L.; Hamawaki, C.D.L. Phenotypic and genotypic correlations between soybean agronomic traits and path analysis. Genet. Mol. Res. 2017, 16, gmr16029696. [Google Scholar] [CrossRef]
- Raza, M.A.; Feng, L.Y.; van der Werf, W.; Iqbal, N.; Khalid, M.H.B.; Chen, Y.K.; Wasaya, A.; Ahmed, S.; Ud Din, A.M.; Khan, S.A.; et al. Maize leaf-removal: A new agronomic approach to increase dry matter, flower number and seed-yield of soybean in maize soybean relay intercropping system. Sci. Rep. 2019, 9, 13453. [Google Scholar] [CrossRef]
- Vieira, A.J.D.; Oliveira, D.A.; Soares, T.C.B.; Schuster, I.; Piovesan, N.D.; Martinez, C.A.; Barros, E.G.; Moreira, M.A. Use of the QTL approach to the study of soybean trait relationships in two populations of recombinant inbred lines at the F7 and F8 generations. Braz. J. Plant Physiol. 2006, 18, 281–290. [Google Scholar] [CrossRef]
- Sun, D.; Li, W.; Zhang, Z.; Chen, Q.; Ning, H.; Qiu, L.; Sun, G. Quantitative trait loci analysis for the developmental behavior of soybean (Glycine max L. Merr.). Theor. Appl. Genet. 2006, 112, 665–673. [Google Scholar] [CrossRef]
- Zhang, D.; Cheng, H.; Wang, H.; Zhang, H.; Liu, C.; Yu, D. Identification of genomic regions determining flower and pod numbers development in soybean (Glycine max L.). J. Genet. Genom. 2010, 37, 545–556. [Google Scholar] [CrossRef]
- Kuroda, Y.; Kaga, A.; Tomooka, N.; Yano, H.; Takada, Y.; Kato, S.; Vaughan, D. QTL affecting fitness of hybrids between wild and cultivated soybeans in experimental fields. Ecol. Evol. 2013, 3, 2150–2168. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Xin, D.; Liu, C.; Jiang, H.; Han, X.; Sun, Y.; Qi, Z.; Hu, G.; Chen, Q. Identification of QTLs for seed and pod traits in soybean and analysis for additive effects and epistatic effects of QTLs among multiple environments. Mol. Genet. Genom. 2013, 288, 651–667. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Li, M.; Hu, X.; Ma, Q.; Mu, Y.; Tan, Z.; Xia, Q.; Zhang, G.; Nian, H. Construction of high-density genetic map and QTL mapping of yield-related and two quality traits in soybean RILs population by RAD-sequencing. BMC Genom. 2017, 18, 466. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Zhao, X.; Hu, Y.; Liu, S.; Nan, H.; Li, X.; Fang, C.; Cao, D.; Shi, X.; Kong, L.; et al. Natural variation at the soybean J locus improves adaptation to the tropics and enhances yield. Nat. Genet. 2017, 49, 773–779. [Google Scholar] [CrossRef]
- Cregan, P.B.; Jarvik, T.; Bush, A.L. An integrated genetic linkage map of the soybean genome. Crop Sci. 1999, 39, 1464–1490. [Google Scholar] [CrossRef]
- Song, Q.J.; Marek, L.F.; Shoemaker, R.C.; Lark, K.G.; Concibido, V.C.; Delannay, X.; Specht, J.E.; Cregan, P.B. A new integrated genetic linkage map of the soybean. Theor. Appl. Genet. 2004, 109, 122–128. [Google Scholar] [CrossRef]
- Richards, E.; Reichardt, M.; Rogers, S. Preparation of genomic DNA from plant tissue. Curr. Protoc. Mol. Biol. 2001, 2, Unit2.3. [Google Scholar] [CrossRef]
- Manly, K.F.; Cudmore, J.; Robert, H.; Meer, J.M. Map Manager QTX, cross-platform software for genetic mapping. Mamm. Genome 2001, 12, 930–932. [Google Scholar] [CrossRef]
- Van Ooijen, J.W.; Voorrips, R.E. JoinMap®, Version 3.0; Software for the Calculation of Genetic Linkage Maps; Plant Research International: Wageningen, The Netherlands, 2001.
- Van Ooijen, J.W. MapQTL®, Version 5; Software for the Mapping of Quantitative Trait Loci in Experimental Populations; Kyazma BV: Wageningen, The Netherlands, 2004.
- Abuín, J.M.; Pichel, J.C.; Pena, T.F.; Amigo, J. BigBWA: Approaching the Burrows-Wheeler aligner to Big Data technologies. Bioinformatics 2015, 31, 4003–4005. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. 1000 genome project data processing subgroup. The sequence alignment/map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.T.; Demarest, B.L.; Bisgrove, B.W.; Gorsi, B.; Su, Y.C.; Yost, H.J. MMAPPR: Mutation mapping analysis pipeline for pooled RNA-seq. Genome Res. 2013, 23, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.; Jiang, C.; Yang, J.; Wang, L.; Wu, X.; Wei, W. Rapid identification of candidate genes for seed weight using the SLAF-Seq method in Brassica napus. PLoS ONE 2016, 11, e147580. [Google Scholar]
- Fehr, W.R.; Caviness, C.E.; Bumood, D.T.; Pennington, J.S. Stage of development descriptions for soybeans, Glycine max L. Merrill. Crop Sci. 1971, 11, 929–931. [Google Scholar] [CrossRef]
- Han, Y.; Li, D.; Zhu, D.; Li, H.; Li, X.; Teng, W.; Li, W. QTL analysis of soybean seed weight across multi-genetic backgrounds and environments. Theor. Appl. Genet. 2012, 125, 671–683. [Google Scholar] [CrossRef]
- Adzhubei, I.A.; Schmidt, S.; Peshkin, L.; Ramensky, V.E.; Gerasimova, A.; Bork, P.; Kondrashov, A.S.; Sunyaev, S.R. A method and server for predicting damaging missense mutations. Nat. Methods 2010, 7, 248–249. [Google Scholar] [CrossRef]
- Liu, Z.Z.; Yao, D.; Zhang, J.; Li, Z.L.; Ma, J.; Liu, S.Y.; Qu, J.; Guan, S.Y.; Wang, D.D.; Pan, L.D.; et al. Identification of genes associated with the increased number of four-seed pods in soybean (Glycine max L.) using transcriptome analysis. Genet. Mol. Res. 2015, 14, 18895–18912. [Google Scholar] [CrossRef]
- Zhou, Z.; Jiang, Y.; Wang, Z.; Gou, Z.; Lyu, J.; Li, W.; Yu, Y.; Shu, L.; Zhao, Y.; Ma, Y.; et al. Resequencing 302 wild and cultivated accessions identifies genes related to domestication and improvement in soybean. Nat. Biotechnol. 2015, 33, 408–414. [Google Scholar] [CrossRef]
- Suzuki, T.; Narciso, J.O.; Zeng, W.; van de Meene, A.; Yasutomi, M.; Takemura, S.; Lampugnani, E.R.; Doblin, M.S.; Bacic, A.; Ishiguro, S. KNS4/UPEX1: A Type II arabinogalactan beta-(1,3)-Galactosyltransferase required for pollen exine development. Plant Physiol. 2017, 173, 183–205. [Google Scholar] [CrossRef]
- Wang, D.; Skibbe, D.S.; Walbot, V. Maize Male sterile 8 (Ms8), a putative beta-1,3-galactosyltransferase, modulates cell division, expansion, and differentiation during early maize anther development. Plant Reprod. 2013, 26, 329–338. [Google Scholar] [CrossRef]
- Board, J.E.; Tan, Q. Assimilatory capacity effects on soybean yield components and pod number. Crop Sci. 1995, 35, 846–851. [Google Scholar] [CrossRef]
- Zhang, J.L.; Wang, Y.Y.; Sun, L.Q.; Wei, T.T.; Gu, X.H.; Gao, F.; Li, X.D. Effects of paclobutrazol on the yield, quality, and related enzyme activities of different quality type peanut cultivars. Ying Yong Sheng Tai Xue Bao 2013, 24, 2850–2856. [Google Scholar] [PubMed]
- Wang, S.; Liu, S.; Wang, J.; Yokosho, K.; Zhou, B.; Yu, Y.C.; Liu, Z.; Frommer, W.B.; Ma, J.F.; Chen, L.Q.; et al. Simultaneous changes in seed size, oil content, and protein content driven by selection of SWEET homologues during soybean domestication. Natl. Sci. Rev. 2020, 7, 1776–1786. [Google Scholar] [CrossRef]
- Zhang, H.; Goettel, W.; Song, Q.; Jiang, H.; Hu, Z.; Wang, M.L.; An, Y.C. Selection of GmSWEET39 for oil and protein improvement in soybean. PLoS Genet. 2020, 16, e1009114. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Qiu, J.; Gao, Q. QTL-BSA: A bulked segregant analysis and visualization pipeline for QTL-seq. Interdiscip Sci. 2019, 11, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.K. Plant glycosyltransferases: Their potential as novel biocatalysts. Chemistry 2005, 11, 5486–5494. [Google Scholar] [CrossRef] [PubMed]
- Keegstra, K.; Raikhel, N. Plant glycosyltransferases. Curr. Opin. Plant Biol. 2001, 4, 219–224. [Google Scholar] [CrossRef]
- Taylor, L.P.; Miller, K.D. The use of a photoactivatable kaempferol analogue to probe the role of flavonol 3-O-galactosyltransferase in pollen germination. Adv. Exp. Med. Biol. 2002, 505, 41–50. [Google Scholar]
- Joly, C.; Léonard, R.; Maftah, A.; Riou-Khamlichi, C. alpha4-Fucosyltransferaseis regulated during flower development: Increases in activity are targeted to pollen maturation and pollen tube elongation. J. Exp. Bot. 2002, 53, 1429–1436. [Google Scholar]
- Sim, J.S.; Kesawat, M.S.; Kumar, M.; Kim, S.Y.; Mani, V.; Subramanian, P.; Park, S.; Lee, C.M.; Kim, S.R.; Hahn, B.S. Lack of the alpha1,3-Fucosyltransferase Gene (Osfuct) Affects Anther Development and Pollen Viability in Rice. Int. J. Mol. Sci. 2018, 19, 1225. [Google Scholar] [CrossRef]
- Ishikawa, N.; Takabayashi, A.; Ishida, S.; Hano, Y.; Endo, T.; Sato, F. NDF6: A thylakoid protein specific to terrestrial plants is essential for activity of chloroplastic NAD(P)H dehydrogenase in Arabidopsis. Plant Cell Physiol. 2008, 49, 1066–1073. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Doerks, T.; Copley, R.; Bork, P. DDT—A novel domain in different transcription and chromosome remodeling factors. Trends Biochem. Sci. 2001, 26, 145–146. [Google Scholar] [CrossRef]
- Hennet, T. The galactosyltransferase family. Cell Mol. Life Sci. 2002, 59, 1081–1095. [Google Scholar] [CrossRef] [PubMed]
- Yi, K.; Guo, C.; Chen, D.; Zhao, B.; Yang, B.; Ren, H. Cloning and functional characterization of a formin-like protein (AtFH8) from Arabidopsis. Plant Physiol. 2005, 138, 1071–1082. [Google Scholar] [CrossRef]
- Zhang, L.; Smertenko, T.; Fahy, D.; Koteyeva, N.; Moroz, N.; Kuchařová, A.; Novák, D.; Manoilov, E.; Smertenko, P.; Galva, C.; et al. Analysis of formin functions during cytokinesis using specific inhibitor SMIFH2. Plant Physiol. 2021, 186, 945–963. [Google Scholar] [CrossRef]
- Gachomo, E.W.; Jimenez-Lopez, J.C.; Baptiste, L.J.; Kotchoni, S.O. GIGANTUS1 (GTS1), a member of Transducin/WD40 protein superfamily, controls seed germination, growth and biomass accumulation through ribosome-biogenesis protein interactions in Arabidopsis thaliana. BMC Plant Biol. 2014, 14, 37. [Google Scholar] [CrossRef]
| PNPP | Seed Number per Plant | Yield per Plant | |
|---|---|---|---|
| TFPN | 0.903 | 0.807 ** | 0.833 ** |
| PNPP | - | 0.868 ** | 0.903 ** |
| Traits | QTL | Chromosome | Flanking Marker | Genomic Region (Mb) | LOD | PVE (%) | Additive Effect | Re-Identification |
|---|---|---|---|---|---|---|---|---|
| TFPN | qFPN2 | 2 | Sat_069—PSI0295 | 43.2–48.3 | 2.28 | 14.4 | 23.04 | |
| qFPN3 | 3 | Sat_266—GMABAB | 34.0–37.9 | 2.67 | 13.9 | 26.10 | ||
| qFPN4 | 4 | Satt190—Satt195 | 16.7–44.4 | 2.10 | 9.2 | 16.81 | ||
| qFPN7 | 7 | Satt201—Satt245 | 2.0–9.4 | 4.28 | 18.1 | 33.09 | ||
| qFPN10-1 | 10 | Sat_321—Satt653 | 2.4–4.6 | 2.28 | 11.3 | 23.34 | ||
| qFPN10-2 | 10 | Satt653—Satt259 | 4.6–4.9 | 2.02 | 8.9 | 20.06 | ||
| PNPP | qPN2 | 2 | Sat_069—PSI0295 | 43.2–48.3 | 2.38 | 15.9 | 17. 55 | [6,12] |
| qPN4 | 4 | Satt190—Satt195 | 16.7–44.4 | 2.19 | 9.6 | 13.89 | [6,9,14] | |
| qPN5 | 5 | Sat_407 | 34.0 | 2.00 | 8.8 | 14.18 | [13] | |
| qPN7 | 7 | Satt201—Satt245 | 2.0–9.4 | 3.19 | 13.7 | 21.21 | [6,12] | |
| qPN10 | 10 | Sat_321—Satt653 | 2.4–4.6 | 2.15 | 10.3 | 17.17 | [6,12] |
| Gene Name | SNP Position | CDS | Allele for MFP | Allele for LFP | NSSNP | Homolog in Ath. | Protein Family |
|---|---|---|---|---|---|---|---|
| Glyma.04G166600 | 41834809 | exon2 | C | T | A78V | AT2G03220.1 | Fucosyltransferase 1 |
| Glyma.04G167400 | 42009788 | exon1 | G | T | R32I | AT1G18730.1 | Photosynthetic NAD(P)H dehydrogenase subcomplex B4 |
| Glyma.04G176500 | 43949063 | exon6 | A | C | K259T | AT1G18950.1 | DDT domain superfamily |
| Glyma.04G176600 | 43971465 | exon1 | C | A | W11L | AT5G62620.1 | Galactosyltransferase family protein |
| Glyma.04G176800 | 44006761 | exon2 | A | G | N154D | AT3G47850.1 | - |
| Glyma.04G177800 | 44202428 | exon2 | C | G | K600N | AT1G70140.1 | Formin 8 |
| Glyma.04G178800 | 44358968 | exon1 | AACG | A | N146del.3 | AT5G50120.1 | Transducin/WD40 repeat-like Superfamily protein |
| Glyma.04G178900 | 44372615 | exon1 | C | CT | T5ins.1 | - | - |
| Gene Name | * Variation Position | Variation | G. soja | Landrace | Improved Cultivar |
|---|---|---|---|---|---|
| C | 62.90% | 63.85% | 85.45% | ||
| Glyma.04G166600 | 233 | T | 27.42% | 30.77% | 11.82% |
| Y | 9.68% | 5.38% | 2.73% | ||
| G | 53.23% | 50% | 65.45% | ||
| Glyma.04G167400 | 95 | T | 33.87% | 43.08% | 28.18% |
| K | 12.90% | 6.92% | 6.36% | ||
| A | 100% | 90.77% | 90.91% | ||
| Glyma.04G176500 | 776 | C | 0 | 6.92% | 6.36% |
| M | 0 | 2.31% | 2.73% | ||
| C | 98.39% | 54.62% | 44.55% | ||
| Glyma.04G176600 | 32 | A | 0 | 40.77% | 47.27% |
| M | 1.61% | 4.61% | 8.18% | ||
| A | 83.87% | 53.85% | 42.73% | ||
| Glyma.04G176800 | 460 | G | 6.45% | 38.46% | 47.27% |
| R | 9.68% | 7.69% | 10% | ||
| C | 50% | 84.61% | 60.91% | ||
| Glyma.04G177800 | 1800 | G | 38.71% | 11.54% | 30.91% |
| S | 11.29% | 3.85% | 8.18% | ||
| AACG | 75.81% | 63.08% | 54.54% | ||
| Glyma.04G178800 | 437 | A | 19.35% | 27.69% | 41.82% |
| H | 4.84% | 9.23% | 3.64% | ||
| C | 98.39% | 89.23% | 67.27% | ||
| Glyma.04G178900 | 16 | CT | 0 | 11.54% | 32.73% |
| H | 1.61% | 0.77% | 6.36% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Sun, X.; Pan, X.; Zhang, H.; Wang, Y.; Ren, H.; Wang, F. Identification and Fine Mapping of a Quantitative Trait Locus Controlling the Total Flower and Pod Numbers in Soybean. Agronomy 2022, 12, 790. https://doi.org/10.3390/agronomy12040790
Sun X, Sun X, Pan X, Zhang H, Wang Y, Ren H, Wang F. Identification and Fine Mapping of a Quantitative Trait Locus Controlling the Total Flower and Pod Numbers in Soybean. Agronomy. 2022; 12(4):790. https://doi.org/10.3390/agronomy12040790
Chicago/Turabian StyleSun, Xia, Xiaohuan Sun, Xiangwen Pan, Hengyou Zhang, Yanping Wang, Haixiang Ren, and Feifei Wang. 2022. "Identification and Fine Mapping of a Quantitative Trait Locus Controlling the Total Flower and Pod Numbers in Soybean" Agronomy 12, no. 4: 790. https://doi.org/10.3390/agronomy12040790
APA StyleSun, X., Sun, X., Pan, X., Zhang, H., Wang, Y., Ren, H., & Wang, F. (2022). Identification and Fine Mapping of a Quantitative Trait Locus Controlling the Total Flower and Pod Numbers in Soybean. Agronomy, 12(4), 790. https://doi.org/10.3390/agronomy12040790






