Molecular Mapping of a New Brown Planthopper Resistance Gene Bph43 in Rice (Oryza sativa L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials, Mapping Populations, and NIL-Bph43-9311 Construction
2.2. BPH Insects and Evaluation of BPH Resistance
2.3. DNA Extraction and Gene Mapping
2.4. RNA-Seq and Transcriptome Analysis
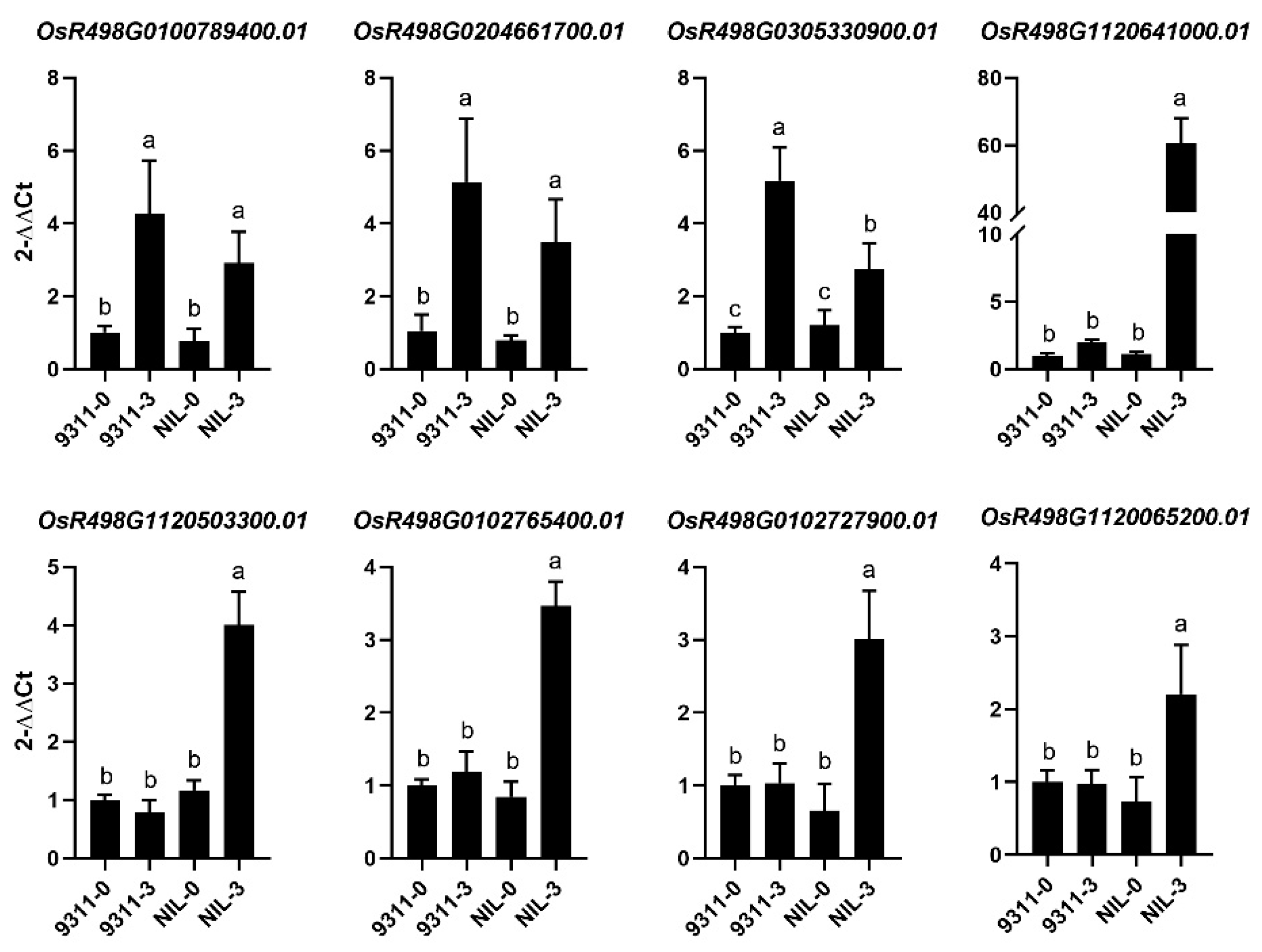
2.5. Quantitative Real-Time PCR (qRT-PCR) Analysis
2.6. Statistical Analysis
3. Results
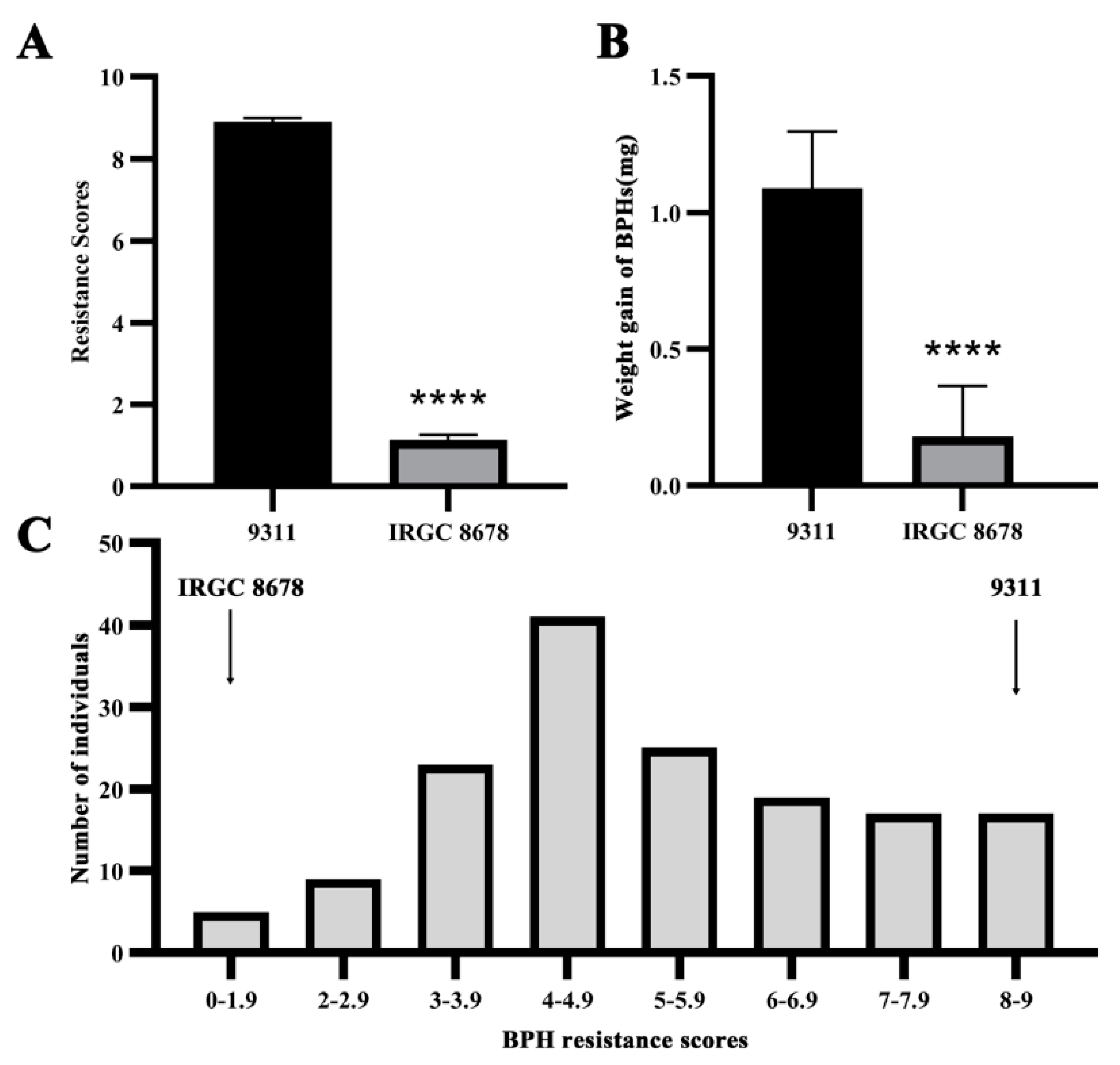
3.1. Genetic Analysis of BPH Resistance
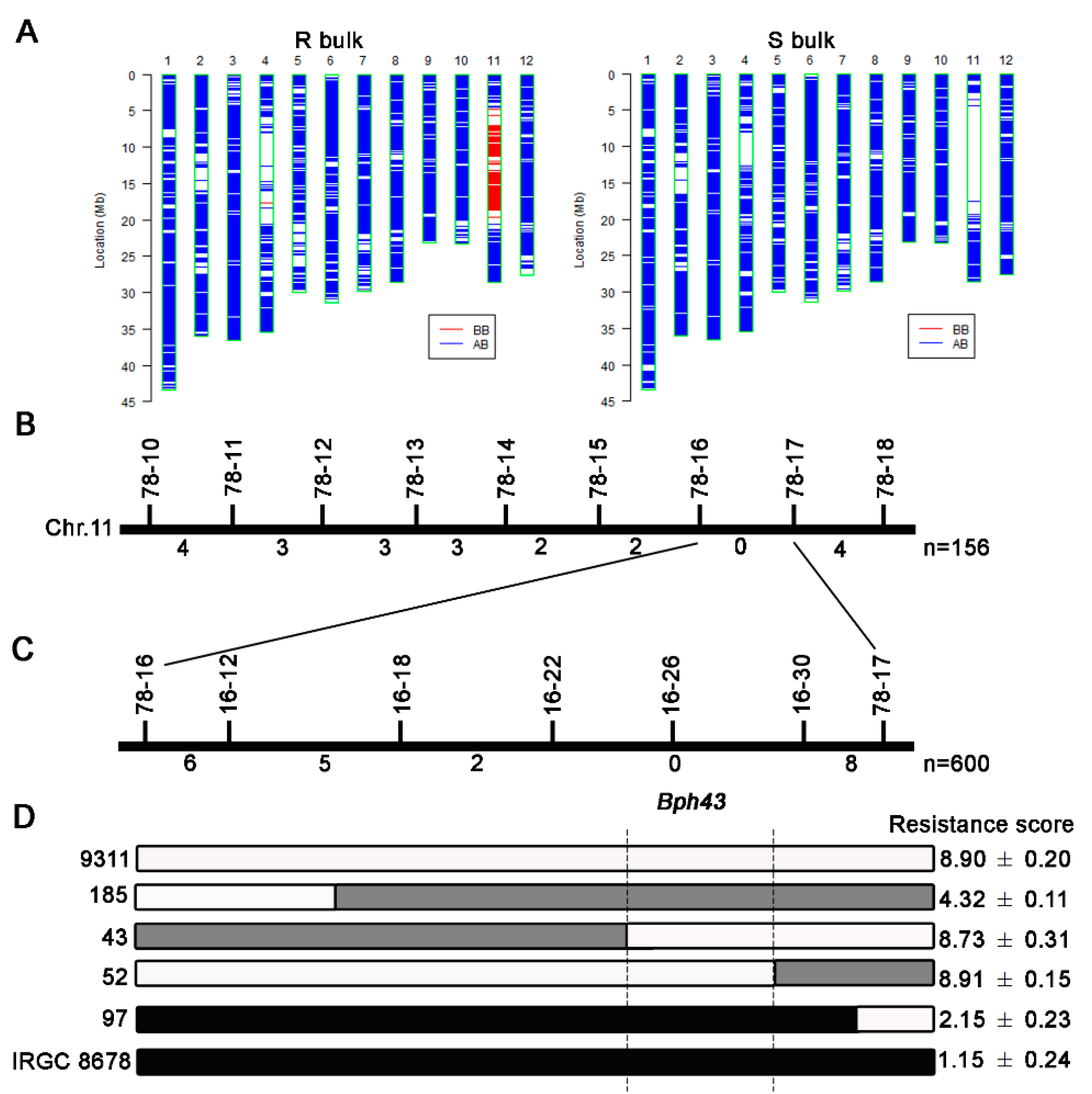
3.2. Molecular Mapping of Bph43
3.3. Verification of Bph43 in BC2F2 Populations
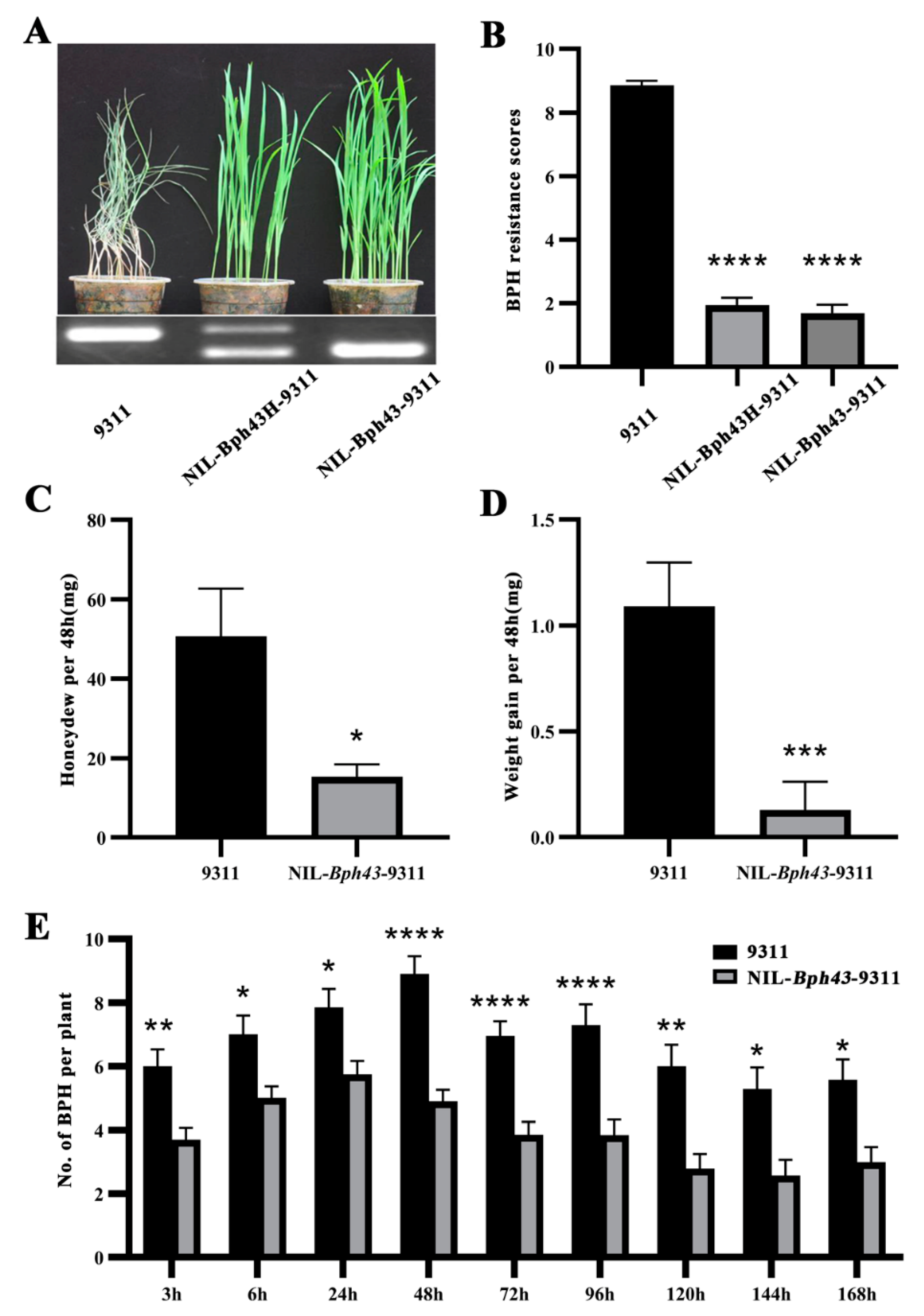
3.4. Development and Characterization of NIL-Bph43-9311
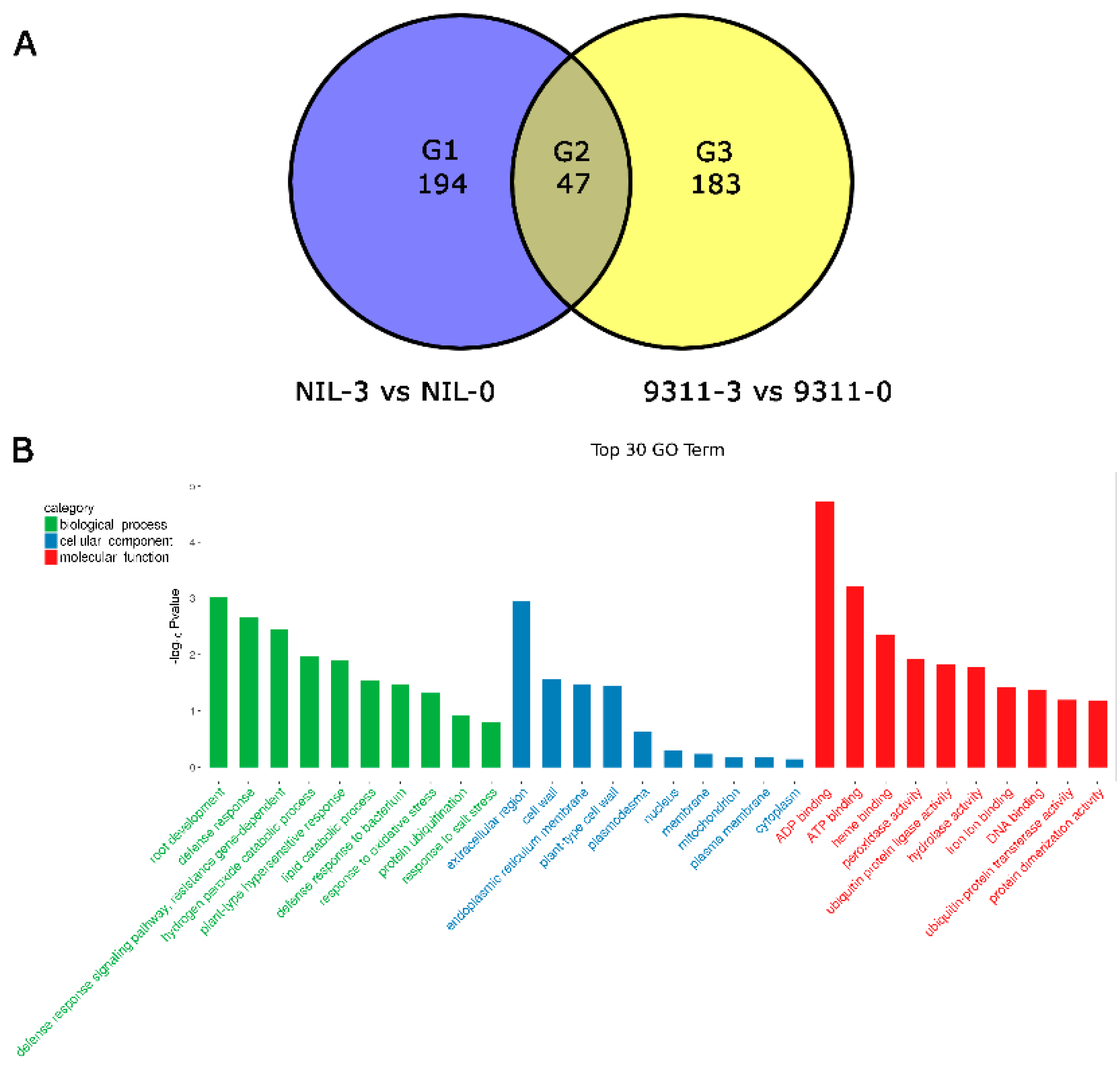
3.5. Comparative Transcriptome Analysis of NIL-Bph43-9311 and 9311 Underlying BPH Infestation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sasaki, T.; Burr, B. International Rice Genome Sequencing Project: The effort to completely sequence the rice genome. Curr. Opin. Plant Biol. 2000, 3, 138–142. [Google Scholar] [CrossRef]
- Douglas, A.E. Strategies for Enhanced Crop Resistance to Insect Pests. Annu. Rev. Plant Biol. 2018, 69, 637–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Sogawa, K.; Liu, G.J.; Shen, J.H. A review on the hyper-susceptibility of Chinese hybrid rice to insect pests. Chin. J. Rice Sci. 2003, 17, 23–30. [Google Scholar] [CrossRef]
- Brar, D.S.; Virk, P.S.; Jena, K.; Khush, G.S. Breeding for resistance to planthoppers in rice. In Planthoppers: New Threats to the Sustainability of Intensive Rice Production Systems in Asia; Heong, K.L., Hardy, B., Eds.; International Rice Research Institute: Los Baños, Philippines, 2009; pp. 401–427. [Google Scholar]
- Fujita, D.; Kohli, A.; Horgan, F.G. Rice Resistance to Planthoppers and Leafhoppers. Crit. Rev. Plant Sci. 2013, 32, 162–191. [Google Scholar] [CrossRef]
- Watanabe, T.; Kitagawa, H. Photosynthesis and Translocation of Assimilates in Rice Plants Following Phloem Feeding by the Planthopper Nilaparvata lugens (Homoptera: Delphacidae). J. Econ. Entomol. 2000, 93, 1192–1198. [Google Scholar] [CrossRef]
- Qiu, Y.; Guo, J.; Jing, S.; Zhu, L.; He, G. Development and characterization of japonica rice lines carrying the brown planthopper-resistance genes BPH12 and BPH6. Theor. Appl. Genet. 2012, 124, 485–494. [Google Scholar] [CrossRef]
- Hu, J.; Xiao, C.; He, Y. Recent progress on the genetics and molecular breeding of brown planthopper resistance in rice. Rice 2016, 9, 30. [Google Scholar] [CrossRef] [Green Version]
- Min, S.; Lee, S.W.; Choi, B.R.; Lee, S.H.; Kwon, D.H. Insecticide resistance monitoring and correlation analysis to select appropriate insecticides against Nilaparvata lugens (Stål), a migratory pest in Korea. J. Asia-Pac. Entomol. 2014, 17, 711–716. [Google Scholar] [CrossRef]
- Tanaka, K.; Endo, S.; Kazano, H. Toxicity of insecticides to predators of rice planthoppers:Spiders, the mirid bug and the dryinid wasp. Appl. Entomol. Zool. 2000, 35, 177–187. [Google Scholar] [CrossRef]
- Matsumura, M.; Takeuchi, H.; Satoh, M.; Sanada-Morimura, S.; Thanh, D.V. Current status of insecticide resistance in rice planthoppers in Asia. In Planthoppers: New Threats to the Sustainability of Intensive Rice Production Systems in Asia; Heong, K.L., Hardy, B., Eds.; International Rice Research Institute: Los Baños, Philippines, 2009; pp. 233–244. [Google Scholar]
- Du, B.; Chen, R.; Guo, J.; He, G. Current understanding of the genomic, genetic, and molecular control of insect resistance in rice. Mol. Breed. 2020, 40, 24. [Google Scholar] [CrossRef] [Green Version]
- Cheng, X.; Wu, Y.; Guo, J.; Du, B.; Chen, R.; Zhu, L.; He, G. A rice lectin receptor-like kinase that is involved in innate immune responses also contributes to seed germination. Plant J. 2013, 76, 687–698. [Google Scholar] [CrossRef]
- Ji, H.; Kim, S.-R.; Kim, Y.-H.; Suh, J.-P.; Park, H.-M.; Sreenivasulu, N.; Misra, G.; Kim, S.-M.; Hechanova, S.L.; Kim, H.; et al. Map-based Cloning and Characterization of the BPH18 Gene from Wild Rice Conferring Resistance to Brown Planthopper (BPH) Insect Pest. Sci. Rep. 2016, 6, 34376. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Gao, F.; Wu, X.; Lu, X.; Zeng, L.; Lv, J.; Su, X.; Luo, H.; Ren, G. Bph32, a novel gene encoding an unknown SCR domain-containing protein, confers resistance against the brown planthopper in rice. Sci. Rep. 2016, 6, 37645. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Zhang, W.; Liu, B.; Hu, J.; Wei, Z.; Shi, Z.; He, R.; Zhu, L.; Chen, R.; Han, B.; et al. Identification and characterization of Bph14, a gene conferring resistance to brown planthopper in rice. Proc. Natl. Acad. Sci. USA 2009, 106, 22163–22168. [Google Scholar] [CrossRef] [Green Version]
- Tamura, Y.; Hattori, M.; Yoshioka, H.; Yoshioka, M.; Takahashi, A.; Wu, J.; Sentoku, N.; Yasui, H. Map-based Cloning and Characterization of a Brown Planthopper Resistance Gene BPH26 from Oryza sativa L. ssp. indica Cultivar ADR52. Sci. Rep. 2014, 4, 5872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Cao, L.; Zhang, Y.; Cao, C.; Liu, F.; Huang, F.; Qiu, Y.; Li, R.; Luo, X. Map-based cloning and characterization of BPH29, a B3 domain-containing recessive gene conferring brown planthopper resistance in rice. J. Exp. Bot. 2015, 66, 6035–6045. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Huang, J.; Wang, Z.; Jing, S.; Wang, Y.; Ouyang, Y.; Cai, B.; Xin, X.-F.; Liu, X.; Zhang, C.; et al. Allelic diversity in an NLR gene BPH9 enables rice to combat planthopper variation. Proc. Natl. Acad. Sci. USA 2016, 113, 12850–12855. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wu, H.; Chen, H.; Liu, Y.; He, J.; Kang, H.; Sun, Z.; Pan, G.; Wang, Q.; Hu, J.; et al. A gene cluster encoding lectin receptor kinases confers broad-spectrum and durable insect resistance in rice. Nat. Biotechnol. 2015, 33, 301–305. [Google Scholar] [CrossRef]
- Jing, S.; Zhao, Y.; Du, B.; Chen, R.; Zhu, L.; He, G. Genomics of interaction between the brown planthopper and rice. Curr. Opin. Insect Sci. 2017, 19, 82–87. [Google Scholar] [CrossRef]
- Guo, J.; Xu, C.; Wu, D.; Zhao, Y.; Qiu, Y.; Wang, X.; Ouyang, Y.; Cai, B.; Liu, X.; Jing, S.; et al. Bph6 encodes an exocyst-localized protein and confers broad resistance to planthoppers in rice. Nat. Genet. 2018, 50, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Wang, H.; Nie, L.; Tan, D.; Zhou, C.; Zhang, Q.; Li, Y.; Du, B.; Guo, J.; Huang, J.; et al. Bph30 confers resistance to brown planthopper by fortifying sclerenchyma in rice leaf sheaths. Mol. Plant 2021, 14, 1714–1732. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Zhang, Q.; Chen, Y.; Huang, J.; Guo, Q.; Li, Y.; Wang, W.; Qiu, Y.; Guan, W.; Zhang, J.; et al. Balancing selection and wild gene pool contribute to resistance in global rice germplasm against planthopper. J. Integr. Plant Biol. 2021, 63, 1695–1711. [Google Scholar] [CrossRef] [PubMed]
- Jena, K.K.; Kim, S.-M. Current Status of Brown Planthopper (BPH) Resistance and Genetics. Rice 2010, 3, 161–171. [Google Scholar] [CrossRef] [Green Version]
- Khush, G.S.; Coffman, W.R. Genetic Evaluation and Utilization (GEU) program. Theor. Appl. Genet. 1977, 51, 97–110. [Google Scholar] [CrossRef]
- Alam, S.N.; Cohen, M.B. Detection and analysis of QTLs for resistance to the brown planthopper, Nilaparvata lugens, in a doubled-haploid rice population. Theor. Appl. Genet. 1998, 97, 1370–1379. [Google Scholar] [CrossRef]
- Ketipearachchi, Y.; Kaneda, C.; Nakamura, C. Adaptation of the brown planthopper (BPH), Nilaparvata lugens (Stal) (Homoptera: Delphacidae), to BPH resistant rice cultivars carrying bph8 or Bph9. Appl. Entomol. Zool. 1998, 33, 497–505. [Google Scholar] [CrossRef]
- Heinrichs, E.A.; Medrano, F.G.; Rapusas, H.R. Genetic Evaluation for Insect Resistance in Rice; International Rice Research Institute: Los Baños, Philippines, 1985. [Google Scholar]
- Huang, Z.; He, G.; Shu, L.; Li, X.; Zhang, Q. Identification and mapping of two brown planthopper resistance genes in rice. Theor. Appl. Genet. 2001, 102, 929–934. [Google Scholar] [CrossRef]
- Porebski, S.; Bailey, L.G.; Baum, B.R. Modification of a CTAB DNA extraction protocol for plants containing high polysaccharide and polyphenol components. Plant Mol. Biol. Report. 1997, 15, 8–15. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 2010, 26, 589–595. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; Genome Project Data Processing Subgroup. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, Y.; Guo, J.; Jing, S.; Zhu, L.; He, G. High-resolution mapping of the brown planthopper resistance gene Bph6 in rice and characterizing its resistance in the 9311 and Nipponbare near isogenic backgrounds. Theor. Appl. Genet. 2010, 121, 1601–1611. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 2014, 31, 166–169. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [Green Version]
- Kanehisa, M.; Araki, M.; Goto, S.; Hattori, M.; Hirakawa, M.; Itoh, M.; Katayama, T.; Kawashima, S.; Okuda, S.; Tokimatsu, T.; et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2008, 36, D480–D484. [Google Scholar] [CrossRef]
- The Gene Ontology Consortium. The Gene Ontology Resource: 20 years and still GOing strong. Nucleic Acids Res. 2018, 47, D330–D338. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Wu, Y.; Wu, D.; Rao, W.; Guo, J.; Ma, Y.; Wang, Z.; Shangguan, X.; Wang, H.; Xu, C.; et al. The Coiled-Coil and Nucleotide Binding Domains of BROWN PLANTHOPPER RESISTANCE14 Function in Signaling and Resistance against Planthopper in Rice. Plant Cell 2017, 29, 3157–3185. [Google Scholar] [CrossRef] [Green Version]
- Lv, W.; Du, B.; Shangguan, X.; Zhao, Y.; Pan, Y.; Zhu, L.; He, Y.; He, G. BAC and RNA sequencing reveal the brown planthopper resistance gene BPH15 in a recombination cold spot that mediates a unique defense mechanism. BMC Genom. 2014, 15, 674. [Google Scholar] [CrossRef] [Green Version]
- Kawahara, Y.; de la Bastide, M.; Hamilton, J.P.; Kanamori, H.; McCombie, W.R.; Ouyang, S.; Schwartz, D.C.; Tanaka, T.; Wu, J.; Zhou, S.; et al. Improvement of the Oryza sativa Nipponbare reference genome using next generation sequence and optical map data. Rice 2013, 6, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishii, T.; Brar, D.S.; Multani, D.S.; Khush, G.S. Molecular tagging of genes for brown planthopper resistance and earliness introgressed from Oryza australiensis into cultivated rice, O. sativa. Genome 1994, 37, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Jena, K.K.; Jeung, J.U.; Lee, J.H.; Choi, H.C.; Brar, D.S. High-resolution mapping of a new brown planthopper (BPH) resistance gene, Bph18(t), and marker-assisted selection for BPH resistance in rice (Oryza sativa L.). Theor. Appl. Genet. 2006, 112, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.H.; Wang, C.M.; Su, C.C.; Liu, Y.Q.; Zhai, H.Q.; Wan, J.M. Mapping and Marker-assisted Selection of a Brown Planthopper Resistance Gene bph2 in Rice (Oryza sativa L.). Acta Genet. Sin. 2006, 33, 717–723. [Google Scholar] [CrossRef]
- Rahman, M.L.; Jiang, W.; Chu, S.H.; Qiao, Y.; Ham, T.H.; Woo, M.O.; Lee, J.; Khanam, M.S.; Chin, J.H.; Jeung, J.U.; et al. High-resolution mapping of two rice brown planthopper resistance genes, Bph20(t) and Bph21(t), originating from Oryza minuta. Theor. Appl. Genet. 2009, 119, 1237–1246. [Google Scholar] [CrossRef]
- Myint, K.K.M.; Fujita, D.; Matsumura, M.; Sonoda, T.; Yoshimura, A.; Yasui, H. Mapping and pyramiding of two major genes for resistance to the brown planthopper (Nilaparvata lugens [Stål]) in the rice cultivar ADR52. Theor. Appl. Genet. 2012, 124, 495–504. [Google Scholar] [CrossRef] [Green Version]
- Jairin, J.; Phengrat, K.; Teangdeerith, S.; Vanavichit, A.; Toojinda, T. Mapping of a broad-spectrum brown planthopper resistance gene, Bph3, on rice chromosome 6. Mol. Breed. 2007, 19, 35–44. [Google Scholar] [CrossRef]
- Jairin, J.; Sansen, K.; Wongboon, W.; Kothcharerk, J. Detection of a brown planthopper resistance gene bph4 at the same chromosomal position of Bph3 using two different genetic backgrounds of rice. Breed. Sci. 2010, 60, 71–75. [Google Scholar] [CrossRef] [Green Version]
- Yara, A.; Phi, C.N.; Matsumura, M.; Yoshimura, A.; Yasui, H. Development of near-isogenic lines for BPH25(t) and BPH26(t), which confer resistance to the brown planthopper, Nilaparvata lugens (Stål.) in indica rice ‘ADR52’. Breed. Sci. 2010, 60, 639–647. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Liu, Y.; He, J.; Liu, Y.; Jiang, L.; Liu, L.; Wang, C.; Cheng, X.; Wan, J. Fine mapping of brown planthopper (Nilaparvata lugens Stål) resistance gene Bph28(t) in rice (Oryza sativa L.). Mol. Breed. 2014, 33, 909–918. [Google Scholar] [CrossRef]
- Kaneda, C.; Ito, K.; Ikeda, R. Screening of Rice Cultivars for Resistance to the Brown Planthopper, Nilaparvata lugens Stal., by Three Biotypes. Jpn. J. Breed. 1981, 31, 141–151. [Google Scholar] [CrossRef] [Green Version]
- Cohen, M.B.; Alam, S.N.; Medina, E.B.; Bernal, C.C. Brown planthopper, Nilaparvata lugens, resistance in rice cultivar IR64: Mechanism and role in successful N. lugens management in Central Luzon, Philippines. Entomol. Exp. Et Appl. 1997, 85, 221–229. [Google Scholar] [CrossRef]
- Tan, J.; Wu, Y.; Guo, J.; Li, H.; Zhu, L.; Chen, R.; He, G.; Du, B. A combined microRNA and transcriptome analyses illuminates the resistance response of rice against brown planthopper. BMC Genom. 2020, 21, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; An, X.; Yang, K.; Miao, S.; Qin, Y.; Hu, Y.; Du, B.; Zhu, L.; He, G.; Chen, R. Molecular Mapping of a New Brown Planthopper Resistance Gene Bph43 in Rice (Oryza sativa L.). Agronomy 2022, 12, 808. https://doi.org/10.3390/agronomy12040808
Kim J, An X, Yang K, Miao S, Qin Y, Hu Y, Du B, Zhu L, He G, Chen R. Molecular Mapping of a New Brown Planthopper Resistance Gene Bph43 in Rice (Oryza sativa L.). Agronomy. 2022; 12(4):808. https://doi.org/10.3390/agronomy12040808
Chicago/Turabian StyleKim, JangChol, Xin An, Ke Yang, Si Miao, Yushi Qin, Yinxia Hu, Bo Du, Lili Zhu, Guangcun He, and Rongzhi Chen. 2022. "Molecular Mapping of a New Brown Planthopper Resistance Gene Bph43 in Rice (Oryza sativa L.)" Agronomy 12, no. 4: 808. https://doi.org/10.3390/agronomy12040808
APA StyleKim, J., An, X., Yang, K., Miao, S., Qin, Y., Hu, Y., Du, B., Zhu, L., He, G., & Chen, R. (2022). Molecular Mapping of a New Brown Planthopper Resistance Gene Bph43 in Rice (Oryza sativa L.). Agronomy, 12(4), 808. https://doi.org/10.3390/agronomy12040808







