Abstract
Two trials were conducted under Mediterranean conditions to monitor several physiological indicators before harvest (leaf chlorophyll concentration, quantum yield of photosystem II electron transport, stem water potential, and stomatal conductance) and some agronomic performance parameters before and at harvest (vigor, fruit growth, fruit size, fruit weight, and yield), of ‘Vairo’ almond and ‘Big Top’ nectarine cultivars grafted onto eight Prunus rootstocks, six of which are common in both cultivars. For both ‘Vairo’ almond and ‘Big Top’ nectarine cultivars, factors including rootstock, date, and the interaction between rootstock and date, from fruit set to harvest were evaluated. Significantly affected were certain physiological and agronomical traits which were evaluated before harvest, with stem water potential being the parameter affected by interaction in both cultivars. In fact, the stem water potential presented low levels in Rootpac-20 and high levels in Rootpac-40 for both cultivars. With regard to the other physiological traits evaluated during the growing period, changes in stomatal conductance were observed in ‘Vairo’, but not in ‘Big Top’. Comparing rootstocks throughout the season, Rootpac-40 and IRTA-1 exhibited the highest stomatal conductance values, whereas the lowest was observed in Rootpac-R; Rootpac-20 and Ishtara also presented low values. Regarding agronomical traits at harvest, GF-677 and IRTA-1 produced high yields for ‘Vairo’ almond cultivar, whereas Rootpac-40 and Ishtara performed better with ‘Big Top’ nectarine cultivar.
1. Introduction
Almond (Prunus dulcis (Mill.) D.A. Webb. syn. P. amygdalus (L.) Batsch) and peach (Prunus persica (L.) Batsch) belong to the genus Prunus, subgenus Amygdalus, and family Rosaceae. Both species are diploid, with n = 8 for almond and 2n = 16 for peach [1,2]. Unlike peach—and other Prunus fruits whose commercial interest lies in their juicy flesh or mesocarp—almond is the only Prunus species grown for its edible seeds [3]. Today, the total worldwide production of almonds is 1,684,395 t of kernel, from a production area of 2,162,263 ha (in 2020). They are cultivated in more than 50 countries, with approximately 79% being produced in California, and with other major producers being Spain (7%), Australia (7%), and the Mediterranean Basin [4,5]. In terms of crop area, Spain was the second largest almond producer, in 2020, and had the largest area under cultivation (718,540 ha) [4,6]. Spain is also the world’s second largest peach producer, after China, with an average annual production (from 2018 to 2020) of 1,453,000 t, and the leading peach exporter, with exports of 785,000 t/year for the same period [6]. The large-scale introduction of new cultivars, rootstocks, and training systems, for both species, has made Spain the world-leader in the use of technological innovation in the production of these two crops [7,8].
As with other tree fruit crops, the planting and long-term management of new sustainable agro-systems for almond and peach production must consider the impact of potential changes in climatic and edaphic conditions when selecting new cultivars and rootstocks. Adapting plant material, and in particular rootstock, to meet future challenges will be essential for the economic and environmental sustainability of new orchards. In fact, combining appropriate plant material with the best training systems and crop production technology is, and will be, the main pillar of efficient and sustainable production [9].
Many plants now have integrated signaling and response mechanisms to help them tolerate constantly changing environments [10]. As a result, plant status can be assessed through a series of physiological parameters that, whether used alone or in combination, provide useful information on their physiological performance [11]. The literature lists many such parameters. Some of these, such as stem water potential, plant growth rate, stomatal conductance, and photosynthesis, are destructive and labor intense, while others, such as remote sensing, trunk sap flow, stem diameter shrinkage, and daily fruit growth rates, are more precise and are non-destructive, but require sophisticated equipment and high-level technical skills [11,12,13,14,15].
The sustainability of production, viewed from both the environmental and grower perspectives, will also be determined by the genetics of the chosen plant materials and the efficient use of other inputs [16]. Once appropriately defined, this efficiency will be based on the right combination of cultivar/rootstock and mainly determined by the training system and the fruit production technology [9,17,18]. Thus, bidimensional canopies, for more intensive systems, must be combined with available data about plant and soil status, climatic conditions, etc. This must be achieved through digitalization, where necessary, to achieve a more efficient application of technology in production (irrigation, fertilization, pruning, harvest time, etc.) and to help growers and technicians to take relevant decisions.
For Prunus, as with other tree fruit species, climate change is imposing major and severe constraints on productivity. This relates to blooming (chill and heat accumulation, flowering time, pollination, and flower fecundation), irrigation (reduction of water availability, increasing evapotranspiration and competition for water with other sectors) and other associated biotic and abiotic stresses. These last ones include increases in mean temperatures of up to 2.4 °C by the end of the century, unpredictable precipitation patterns, changes in the dynamics of pest and disease populations, and increases in atmospheric concentrations of CO2 [19,20,21,22]. Rising temperatures and extreme weather events are increasingly affecting the Mediterranean basin, which is an area that has been classified as extremely vulnerable to global warming [23], and such events are expected to become worse by the end of the century [24]. To minimize the impact of climate change, but also maintain the most efficient possible use of inputs in orchards, in the future, it will be necessary to accurately select the optimum combination of cultivars and rootstocks for each set of edaphoclimatic conditions. To achieve these objectives, it is essential to know, and understand, the physiological and productive performance of different cultivar × rootstock interactions under the prevailing agroclimatic conditions and to thereby enhance sustainable crop production.
In addition to examining the most appropriate choice of rootstock required to minimize the negative impact of climate change, many studies of Prunus spp. have demonstrated that it can help improve fruit quality and yield efficiency [8,25,26,27,28,29]. To date, there have been few Prunus rootstock studies related to physiological and agronomical performance before harvest (conducted throughout the growing season) [15,30]. Other studies have demonstrated the effect of rootstock, row orientation and cultivar on bud differentiation, leaf area index (LAI), yield efficiency and fruit quality in almond [31,32]. However, to the best of our knowledge, no previous studies have tried to interrelate all these parameters for two Prunus species.
The aim of this study was to characterize physiology and agronomy of two different Prunus species (P. dulcis and P. persica) grafted onto different Prunus rootstocks, some of them in common. Therefore, some physiological traits before harvest (leaf chlorophyll content, photosynthesis, stomatal conductance, and tree water status) and some agronomical assessments made both before and at harvest (fruit growth, tree vigor, production, fruit size and fruit weight) were evaluated in ‘Vairo’ almond and ‘Big Top’ nectarine cultivars.
2. Materials and Methods
2.1. Plant Material, Site Description and Experimental Design
2.1.1. Almond
This study was carried out over one growing season (2017). It was conducted at an IRTA experimental orchard, located at Les Borges Blanques, NE Spain (41°30′31.89″ N; 0°51 10.70″ E), and used ‘Vairo’ as the scion cultivar [33,34]. ‘Vairo’ was selected due to its high vigor, late-flowering and self-pollinating characteristics. Eight rootstocks of different genetic origins were evaluated (Table 1).

Table 1.
List of the rootstocks evaluated for ‘Vairo’ almond and ‘Big Top’ nectarine cultivars, their genetics, and their origins.
The experimental orchard was planted in March, 2010, using dormant bud plants. It had a loam clay soil, with a good water-holding capacity, was well drained and fertile, and had an organic matter content of around 2%. The trees were trained to an open vase system, with a tree spacing of 5 m × 4.5 m. The trees were drip-irrigated. The climate was cold semiarid Mediterranean (Bsk, according to the Köppen-Geiger climate classification system), with mean annual rainfall of 350 mm and a mean summer daily temperature of 32 °C. The plots were subject to IPM management, according to general industry standards. The experiment was set up in a randomized complete block design, with 6 single-tree replications.
2.1.2. Peach
This study was carried out over one growing season (2017). It was conducted at the IRTA experimental orchard, located at Gimenells, NE Spain (41°39′18.77′′ N and 0°23′31.41′′ E). The mid-season nectarine ‘Big Top’, which is a yellow fleshed cultivar, released by Zaiger Genetics Inc., was selected as it is the most widely planted and popular nectarine grown in Europe and a key reference cultivar [28]. The attributes of the ‘Big Top’ nectarine cultivar include its intense, early, red color, sweet taste, slow softening and excellent postharvest storage potential [35,36,37]. Eight rootstocks of different genetic origins were evaluated (Table 1).
Dormant bud trees were planted in the winter of 2008, on Aquic Xerofluent soil. They were then trained to an open vase system, known as a Catalan vase, which is relatively small and easy-to-train, and spaced at 5 m × 2.6 m. The trees were then drip-irrigated. The climate was cold semiarid Mediterranean (Bsk, according to the Köppen-Geiger climate classification system), with annual precipitation of 350 mm, and a mean summer daily temperature of 32 °C. The plots were subject to IPM management, according to general industry standards. The experiment was set up in a randomized complete block design, with four blocks, with the base plot consisting of three trees per scion-rootstock combination. The central tree in each base plot was used for the study.
2.2. Agronomical Assessments before Harvest
2.2.1. Almond
To evaluate fruit growth (FG), the fruit and kernel dimensions (length (L), diameter (D), and width (W)) [38] were monitored once every 2–3 weeks until harvest, using a Digital Vernier Caliper (Absolute Digimatic Caliper Mitutoyo). Five fruits per tree/replication and rootstock were randomly harvested, from different branches around the tree, from mid-May to the end of August. The basic ellipsoid volume formula: V = 4/3 πLWD was used to calculate the fruit volume.
2.2.2. Peach
To evaluate fruit growth (FG), two fruiting shoots on each central tree/replication and rootstock, each containing 3 fruits, were selected and tagged [37]. The fruit diameter (FD) was then measured on a weekly basis using a Digital Vernier Caliper from the end of April to the end of June.
2.3. Physiological Assessments before Harvest
The physiological measurements used in this study were taken between May and September for ‘Vairo’ almond cultivar and between May and July for ‘Big Top’ nectarine cultivar.
2.3.1. Leaf Chlorophyll Concentration
Leaf chlorophyll concentration, or leaf greenness (SPAD value), was measured using a SPAD-502 meter hand-held device (Konica Minolta SPAD-502 Plus) [15]. Measurements were taken once every two weeks, from each tree in each replicate and from each central tree replicate, for both almond and peach trees, from 2–3 randomly selected leaves, which were at the same height and stage of development.
2.3.2. Quantum Yield of Photosynthesis
The effective quantum yield of photosystem II electron transport (ΦPSII) was checked using a leaf fluorometer (FluorPen FP100 Photon Systems Instruments) [15]. Measurements of leaf chlorophyll concentration were taken once every two weeks, from three randomly selected leaves which were at the same height and stage of development.
2.3.3. Stem Water Potential
The stem water potential (SWP) was used to monitor the tree water status, expressed in MPa. The SWP was measured using a digital pressure chamber (SF-Pres, Type “Scholander”) [12,15]. Once every two weeks, two additional mature leaves per tree were randomly selected, bagged in black plastic covered by aluminum foil for two hours to equilibrate the water potential between their leaves, stems, and branches, and then measured. The SWP of the bagged leaves was measured at between 12:30 h and 14:30 h.
2.3.4. Stomatal Conductance
Stomatal conductance (SC) is an indicator of the foliar transpiration rate and of a plant’s water status; it is measured in mmol m−2 s−1 [39]. It was measured using a leaf porometer (Decagon SC-1 Leaf Porometer). Measurements were made once every two weeks, from two leaves per tree, and taken at between 12:30 h and 14:30 h.
2.4. Agronomical Assessments at Harvest
2.4.1. Almond
The harvest date was established when >75% of the almond fruits growing on most of the rootstocks had completely dried hulls. At harvest, the trees were shaken mechanically, using commercial equipment. In-shell nuts were then collected, using a reversed-umbrella, and shelled and dehulled with a self-moving production huller. Once the in-shell nuts had been dehulled, their fresh weight was measured, and the gross yield per tree was calculated [27].
A 1 kg in-shell nut sample was collected from each replicate and naturally dried for about three–four weeks (until reaching 6% kernel moisture). The dry weight was determined and then one sample of 100 in-shell nuts per 1 kg was collected to determine shell and kernel dry weights, and the shelling percentage (kernel weight/in-shell weight * 100). The kernels were separated by sieving them into four different categories, based on their respective calipers (<12 mm; 12 mm ≤ 14 mm; 14 mm ≤ 16 mm; and ≥16 mm). Kernel yield was calculated by multiplying the in-shell nut yield (kg tree−1) by the shelling percentage (kernel weight/in-shell weight [27].
Tree vigor (cm2) was measured at the end of the season. It was evaluated based on the trunk cross-sectional area (TCSA) at 20 cm from the graft union. Kernel efficiency, expressed as g cm−2, was then calculated [27].
Because of the great differences in tree vigor and size, corresponding to the different rootstocks, and the fact that the same planting distance was used for all the rootstocks, we had to adjust the tree spacing and consequently planting density based on the vigor (TCSA) of each rootstock. We calculated the theoretical kernel yield per hectare by multiplying kernel yield per hectare by a theoretical coefficient which expresses the relative vigor (%) when comparing each rootstock with the reference rootstock GF-677 [27].
2.4.2. Peach
‘Big Top’ trees were harvested in two different picks that were made 4–7 days apart. The criteria established for the first pick were: fruit size ≥60 mm and fruit color ≥80% of fruit surface. After each of the two picks, the whole yield of each control tree was graded for fruit size and weight using a commercial electronic fruit grader (MAF RODA Iberica, Alzira, Spain). Total yield per tree, average fruit weight and the total number of fruits per fruit size (<60 mm; 60−65 mm; 65−70 mm; 70−75 mm; and >75 mm) were then calculated for each pick.
At the end of the season, in order to measure the vigor, the tree circumference was measured at 20 cm above the graft union, and the trunk cross-sectional area (TCSA, cm2) was then calculated. After that, the yield efficiency (kg cm−2) was calculated [28].
Because of the great differences in tree vigor and size corresponding to the different rootstocks and the fact that the same planting distance was used for all rootstocks, we had to adjust the tree spacing and consequently planting density based on the vigor (TCSA) of each rootstock. We calculated the theoretical fruit yield per hectare by multiplying yield per hectare by a theoretical coefficient which expresses the relative vigor (%) when comparing each rootstock with the reference rootstock GF-677 [27,28].
2.5. Statistical Analysis
Data evaluated before the harvest, for both ‘Vairo’ and ‘Big Top’ cultivars, were analyzed using a linear mixed model with a repeated-measures design. The data were then square root transformed to normalize their distribution. This model included the replicate as a random effect, and rootstock, date, and their interaction as fixed factors. If the interaction was significative, one-way analysis of variances (ANOVA) was used to evaluate the effect of the rootstock at each date and also the harvest data parameters. Tukey’s HSD test with a 95% confidence interval was used as a post hoc test for means separation. A two-way hierarchical cluster was performed with Ward’s criterion [40,41] for ‘Vairo’ and ‘Big Top’ cultivars to classify the same rootstocks; this was evaluated based on all the coincident assessments performed on the two cultivars. All the data were standardized before their analysis. The date was expressed as a Julian day. The data were analyzed using the JMP® statistical software (Version 16; SAS Institute Inc., SAS Campus Drive, Cary, NC, USA).
3. Results
3.1. Agronomical and Physiological Assessments before Harvest
For ‘Vairo’ almond cultivar, Table 2 shows that rootstock and date, but not their interaction, affected the two agronomical traits evaluated (FV and KV) before harvest. On all the assessment dates, the values of the two parameters were highest for GF-677 and Rootpac-40 trees and lowest for Rootpac-20 and Rootpac-R trees (Table 3). In contrast, for ‘Big Top’ nectarine cultivar, date was the only variable that influenced FD as a normal factor in fruit growth (Table 2).

Table 2.
Statistical significance of rootstock, date, and the interaction between rootstock and date, based on agronomical and physiological assessments made before harvest, corresponding to eight-year-old ‘Vairo’ almond trees and ten-year-old ‘Big Top’ nectarine trees.

Table 3.
Agronomical and physiological assessments made before harvest, corresponding to eight-year-old ‘Vairo’ almond trees and ten-year-old ‘Big Top’ nectarine trees grafted onto eight different Prunus rootstocks.
For ‘Vairo’ cultivar, all the physiological parameters evaluated were influenced by the rootstock and date interaction, with the exception of the quantum yield of photosynthesis (ΦPSII) (Table 2). In this case, Rootpac-20 was the only rootstock that induced differences in ΦPSII, exhibiting low values compared to the rest of the rootstocks evaluated (Table 3). Leaf greenness (SPAD) values constantly increased through the period in which all the rootstocks were evaluated (Figure 1a), although there were some differences between them. On the last measurement date (Julian day 246, which was 12 days before harvest), the SPAD values were over 38. The GF-677 and IRTA-1 rootstocks tended to produce the highest SPAD values on ‘Vairo’, whereas Ishtara and Rootpac-20 produced the lowest (Figure 1a). Changes in stomatal conductance (SC) were also observed during the growing period (Figure 1b). Comparing rootstocks over the whole season, Rootpac-40 and IRTA-1 had the highest values, whereas Rootpac-R produced the lowest, although Rootpac-20 and Ishtara also presented low values (Figure 1b). A general decrease in stem water potential (SWP) values was observed over the period of evaluation (Figure 1c). ‘Vairo’ trees grafted onto Rootpac-R and Rootpac-20 presented the lowest values from the beginning of the trial and until Julian day 206, with the former continuing to decrease until it reached values of below −1.6 MPa. However, ‘Vairo’ trees grafted onto Rootpac-40 showed the opposite tendency (Figure 1c).
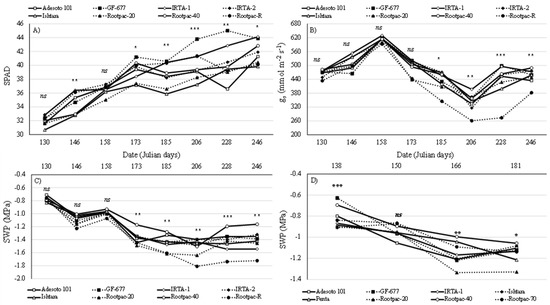
Figure 1.
Seasonal patterns of leaf chlorophyll content (SPAD) (A), stomatal conductance (B) and tree water status (C) for ‘Vairo’ almond cultivar and the seasonal patterns of tree water status (D) for ‘Big Top’ nectarine cultivar grafted onto eight different Prunus rootstocks. Ns = not significant; * p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001.
A different trend was observed for ‘Big Top’ trees. Their rootstock only affected SC, while the date affected both SWP and SC; however, the rootstock and date interaction only affected the SWP (Table 2). Slightly significant differences were observed among rootstocks for SC (Table 3). On average, they all exhibited similar values, except for Penta rootstock, which produced values of below 400 mmol m−2s−1. SWP values decreased constantly for all the rootstocks (Figure 1d), in a similar way to that observed with ‘Vairo’ cultivar. ‘Big Top’ trees grown on Rootpac-20 seemed to be the ones most affected, with values falling to −1.32 MPa, whereas Rootpac-40 was the least affected.
3.2. Agronomical Assessments at Harvest
For ‘Vairo’ almond cultivar, the trees grown on IRTA-2 were the most vigorous, according to their TCSA values (Table 4). The observed values did not however differ significantly from those relating to GF-677. In contrast, IRTA-1, followed by Rootpac-20, Ishtara and Rootpac-R, induced lower vigor in ‘Vairo’ cultivar. The GF-677, IRTA-1, and Rootpac-40 trees produced the highest yield and kernel efficiency values. They also presented the highest shelling percentage, whereas the highest kernel weights were for GF-677, IRTA-1, IRTA-2 and Adesoto 101 (Table 4). Rootpac-20, together with Rootpac-R and Ishtara, induced low vigor, produced small kernels and had low yield values and, therefore, also low kernel efficiency values.

Table 4.
Agronomical data at harvest for ‘Vairo’ almond cultivar grafted onto eight different Prunus rootstocks at the IRTA’s Lleida Experimental Station (Borges Blanques, Lleida) in the eighth crop year (2017).
Kernel size distribution was only affected by rootstock on those kernels that were smaller than 12 mm (Figure 2). Rootpac-20, followed by Rootpac-R, Ishtara and IRTA-1, exhibited the highest percentage of fruits with a kernel size of less than 12 mm.
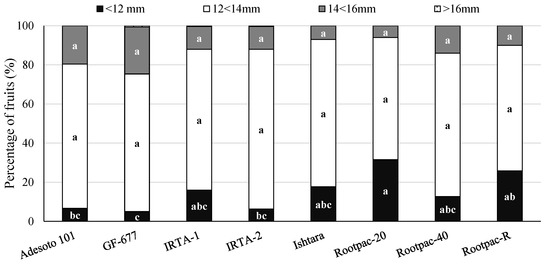
Figure 2.
Mean kernel distribution of fruits from ‘Vairo’ almond cultivar grafted onto eight different Prunus rootstocks at the IRTA’s Lleida Experimental Station (Borges Blanques, Lleida) in the eighth crop year (2017). For each distribution, different letters denote significant differences among rootstocks (Tukey’s honestly significant difference test, p ≤ 0.05).
‘Big Top’ trees grown on Rootpac-70 were the most vigorous, but also the least productive and efficient (Table 5). In contrast, Ishtara, followed by Rootpac-20, Rootpac-40 and Adesoto 101, were the most productive and efficient trees. No significant differences were found between rootstocks in terms of fruit weight (Table 5) or the distribution of each fruit size (Figure 3). The most important fruit size for ‘Big Top’ was 65–70 mm; all ‘Big Top’ rootstocks produced 30–40% of their total fruits within this size range (Figure 4).

Table 5.
Agronomical data at harvest for ‘Big Top’ nectarine cultivar grafted onto eight different Prunus rootstocks at the IRTA’s Lleida Experimental Station (Gimenells, Lleida) in the tenth crop year (2017).
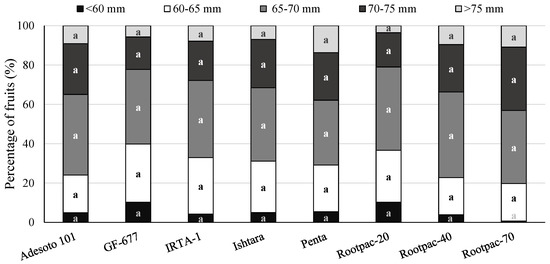
Figure 3.
Mean distribution of fruit size for ‘Big Top’ nectarine cultivar grafted onto eight Prunus rootstocks at the IRTA’s Lleida Experimental Station (Gimenells, Lleida) in the tenth crop year (2017). For each distribution, different letters denote significant differences among rootstocks (Tukey’s honestly significant difference test, p ≤ 0.05).
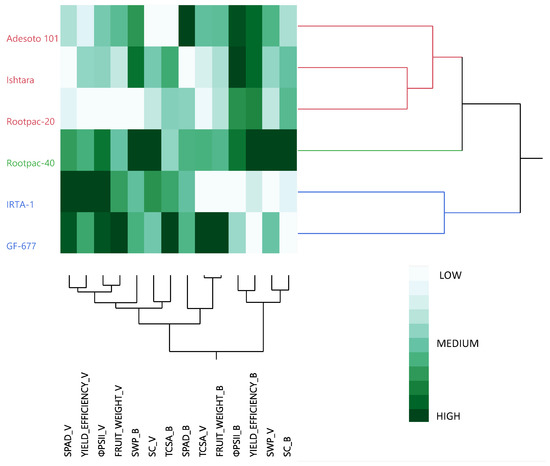
Figure 4.
Clustering the six common rootstocks based on the most relevant traits evaluated. Data about their physiological parameters were collected from the last date of the evaluations applied to both cultivars. The same nomenclature has been used for both cultivars: fruit weight and yield efficiency for ‘Vairo’ almond cultivar represent kernel weight and kernel yield efficiency, as shown in Table 4. The letter V after each of the traits evaluated refers to ‘Vairo’ and the B refers to ‘Big Top’.
3.3. Overall Rootstock Performance and Interaction with Almond and Peach
Taking the values for the last day of the evaluation for all the physiological assessments together and combining them with those for the most relevant agronomical assessments at harvest (vigor, fruit weight, and yield efficiency), for both ‘Vairo’ and ‘Big Top’ cultivars, the six common rootstocks were grouped within three clusters (Figure 4). The first cluster, which included Adesoto 101, Ishtara, and Rootpac-20, was characterized by mainly low-to-medium SPAD, ΦPSII, fruit weight, vigor, and SC values, and medium-level SWP values for the ‘Vairo’ almond cultivar, and medium-to-high ΦPSII values, and medium-level fruit weight and SC values for ‘Big Top’ nectarine cultivar. Rootpac-40 clustered alone, since it had the highest SWP and SC values on both cultivars, and the highest yield efficiency for ‘Big Top’ nectarine cultivar. For the rest of the traits considered in this dendrogram, Rootpac-40 exhibited medium-to-high values on both cultivars. The last group was comprised by IRTA-1 and GF-677. Both rootstocks had high SPAD, ΦPSII and yield efficiency values, and medium-level SC values for ‘Vairo’ almond cultivar, and medium-level SWP and SPAD values, and low yield efficiency and SC values for ‘Big Top’ nectarine cultivar.
4. Discussion
There is an increasing interest in selecting and breeding Prunus rootstocks that are better adapted to different cultivation areas. This adaptation process must take into account the scenario of climate change, in which efforts must be directed towards obtaining greater water-use efficiency (WUE), a better photosynthetic balance; a higher osmotic adjustment; and better nutrient efficiency, in order to improve water productivity, yield efficiency and fruit quality and to better adapt fruit production to future climate change [12,16,20].
In this study, we evaluated the adaptive response of several different Prunus root-stocks grafted with ‘Vairo’ almond and ‘Big Top’ nectarine cultivars and grown under Mediterranean conditions, in the 2017 growing season. In general, the almond cultivar was more affected by changes in rootstock than the peach cultivar, based on a series of parameters that were mainly related to compatibility and the vigor provided by the rootstock. Improving almond crop efficiency involves seeking high production, with a large number of fruits; this is the opposite of improving peach crop efficiency, in which the number of fruits is determined and forced. Then, an appropriate crop load management is achieved by thinning to obtain optimum pack-out [8]. For this reason, any physiological influence from the rootstock could have a dramatic impact on kernel weight and yield in the almond cultivar, but much less in the peach cultivar.
To be more precise, for ‘Vairo’ almond cultivar, the agronomical assessments undertaken throughout the growing season (before harvest) were significantly affected by both rootstock and date, but not by the interaction rootstock × date. To the best of our knowledge, there is no previously published data available on Prunus rootstocks grafted with ‘Vairo’ almond cultivar, or even on other almond cultivars, grown under similar or different climatic conditions, influencing fruit growth during the season. As is evident, the differences found in kernel size and fruit weight at harvest started to emerge from the beginning of the growing season, when the rootstock had a clear influence on fruit growth. This could have been due to differences in rootstock efficiency in the use of inputs, such as water and nutrients, which were also demonstrated by differences in their respective physiological parameters. This could also have been due, in both cases, to the different genetic rootstock backgrounds (plum, peach, almond × peach, interspecific Prunus hybrids), as illustrated in Table 1.
Throughout the experiment, Ishtara and Rootpac-20 produced the lowest SPAD values in ‘Vairo’ leaves. The results for ‘Vairo’ were within the range reported by Yahmed et al. [15] for other almond cultivars, such as ‘Belona’, ‘Soleta’, ‘Guara’, ‘Lauranne’, and ‘Tuono’. On the other hand, despite no differences being found between rootstocks for ‘Big Top’, Ishtara and Rootpac-20, they also tended to exhibit lower SPAD throughout the growing season than the rest of the rootstocks evaluated. In fact, Reig et al. [28] reported low SPAD values, below 30, for both rootstocks when Fe-chelate was not applied after fruit set through harvest date in the 2019 season. These results therefore suggest that Rootpac-20 and Ishtara could be equally sensitive to calcareous soil conditions for both Prunus species: almond and peach, and that under conditions in which they receive insufficient Fe-chelate doses, they may more readily present symptoms of chlorosis. It is also worth mentioning that in the SPAD evaluation, the IRTA-1 rootstock showed a similar trend to that of GF-677, with both producing their highest SPAD values on the last day of evaluation. In this case, their genetic backgrounds were similar: peach × almond (Table 1). This trend was not, however, observed when the ‘Big Top’ cultivar was evaluated. In summary, rootstocks from crosses of Prunus cerasifera may be more susceptible to chlorosis in calcareous soils, as has been widely reported by other authors [42,43].
Changes in ΦPSII were observed in rootstocks grafted with ‘Vairo’ almond cultivar. The lowest value for ΦPSII associated with Rootpac-20 was in line with the SPAD values and results reported by Yahmed et al. [15] for various almond cultivars. For ‘Soleta’ almond grafted onto Rootpac-20 and GF-677, Casanova-Gascón et al. [30] reported values of over 0.8, indicating an absence of environmental stress in those trees. In our study, conducted throughout the growing season vegetative cycle, all the rootstocks, for both ‘Vairo’ and ‘Big Top’ cultivars, showed values close to 0.8, but always below this level, indicating that the climatic conditions were up at the limit for stressing trees.
In this study, SWP was used to evaluate the plant water status. This parameter was significantly affected by the rootstock × date interaction on both Prunus species. Throughout the growing season, the rootstock distribution differed between the dates of evaluation and the Prunus species. The SWP decreased throughout the growing season in both Prunus species evaluated, regardless of their rootstocks and despite all the trees being watered. This decrease was also related to the increase in temperature and high evaporative demands [44] registered during the growing season. ‘Vairo’ trees grown on Rootpac-R showed the worst water status after the second evaluation date, whereas those grown on Rootpac-40 exhibited the best water status throughout the growing season, producing their highest value on the last evaluation date; this result agreed and was within the range values reported by Yahmed et al. [15] under Tunisian conditions. One important difference between the two rootstocks was their background, and particularly the absence of P. persica in the case of Rootpac-R (Table 1). The low SWP values for Rootpac-R suggest that this rootstock was acting as if it had a lower hydraulic conductivity or root biomass than the others and that this caused a fall in stem water potential [12].
On the other hand, in our study, Rootpac-40 induced medium-to-low vigor in ‘Vairo’ almond cultivar. Under our climatic conditions, these results were not, therefore, in agreement with previous studies [15]. They reported that dwarf rootstocks exhibited lower SWP values than the most vigorous ones, and that this could have been related to the low hydraulic conductivity of the root system of the dwarfing rootstock or to that at the graft union. It is worth mentioning that Rootpac-20, which showed similarly low SWP values to Rootpac-R at the beginning of the evaluation, subsequently exhibited an increase in its values, which reached similar levels to those of the rest of the rootstocks evaluated by the last day of evaluation. These results could suggest that despite both rootstocks displaying low vigor, Rootpac-20 recovered earlier due to rain and its more superficial root system [7]. In fact, because of its combination of multiple fine superficial roots and a few (3 to 5) strong roots, which give it good anchorage, it is able to stand upright without the need for any support structure.
For ‘Big Top’ trees, Rootpac-40 rootstock showed the same tree water status tendency as observed with ‘Vairo’ trees, but Rootpac-20 had the most unfavorable status. As reported for ‘Vairo’, and in agreement with Yahmed et al. [15], Rootpac-40 rootstock seemed to be very efficient in terms of water-use efficiency, regardless of the soil conditions or the scion that they were grafted with.
Stomatal conductance (SC), which was used as an indicator of foliar transpiration and plant water status, is related to the opening and closing of plant stomata. Stomatal closure limits photosynthesis and therefore has an important influence on plant function, growth, and yield [14]. As a result, stressed trees exhibited lower SC values when maintaining adequate water levels. As mentioned above, the trees in this study were not drought stressed, but the rootstock effect observed throughout the growing season was clearly reflected in ‘Vairo’ trees. The Rootpac-R rootstock exhibited the worst SC values throughout the growing season, whereas the rest of the rootstocks showed higher values after the fourth evaluation date, with these being highest for the IRTA-1 and Rootpac-40 rootstocks. The decrease in values for all the rootstocks from the third evaluation date until the last one (in the summer period), combined with the decrease in SWP, observed in the same period, could perhaps be explained by plants adapting by closing their stomata to regulate the flux of water in response to the increase in temperature. However, not all rootstocks adapt equally, as mentioned above. For ‘Big Top’, rootstock only slightly affected the SC values, with the Prunus domestica rootstock, Penta, exhibiting the worst performance.
Fruit growth is the consequence of many different physiological processes that occur simultaneously at the plant level; these are related to both plant status and environmental conditions. When fruit growth is optimal, all the physiological processes within the tree are efficient. However, when trees experience drought stress, fruit growth is highly affected by SWP. In fact, their SWP decreases, and fruit growth rates tend to be reduced as it becomes more and more difficult for the fruit to obtain water from the vascular tissue [11]. The trees in this study were not drought stressed. For this reason, despite the rootstock effect being present throughout the growing season, it was clearly reflected in tree water status for both Prunus species, but not in fruit and kernel growth, which were expressed as fruit volume and kernel volume for ‘Vairo’ almond cultivar, and as fruit diameter for ‘Big Top’ nectarine cultivar. These results also confirmed that some rootstocks had lower hydraulic conductivities than others.
At the orchard level, the fruit represents the actual economic target of production, and the yield and yield efficiency are the maximum expressions of orchard profitability for apple [45,46], peach [8], pear [47] and almond orchards [27]. Hernandez-Santana et al. [14] have suggested that certain agronomic traits, such as vigor and yield parameters, and even some quality attributes, are a consequence of differences in either the root system architecture or the hydraulic properties of a given rootstock. These differences could influence the transpiration rate through their effects on stem water potential and the control of stomatal conductance. In agreement with this line of thought, GF-677, IRTA-1 and Rootpac-40 induced the highest theoretical kernel yields. Best fruit weights were obtained with GF-677, IRTA-1, IRTA-2 and Adesoto 101, and greater yield efficiencies resulted from GF-677, IRTA-1 and Rootpac-40 in ‘Vairo’ trees. The lack of compatibility observed in this trial and also reported by Lordan et al. [27] between some rootstocks with P. cerasifera as a parent (Rootpac-20 and Rootpac-R) and ‘Vairo’ almond cultivar could also help to explain the lower kernel yield and fruit weight values (Table 4). These results contrasted from high values obtained with Rootpac-40 (P. Amygdalus × P. persica) or Adesoto 101 (P. insititia). In contrast, for other rootstocks, which also had P. cerasifera as a parent (such as the vigorous rootstock IRTA-2), this lack of incompatibility could have been disguised; this may have been the case with high vigor cultivars, such as ‘Vairo’ [27]. However, when ‘Vairo’ was grafted onto GF-677 rootstock, which was one of the most vigorous rootstocks and produced intermediate SWP and SC values, it produced high yields and showed a good level of kernel efficiency.
For ‘Big Top’ nectarine cultivar, Rootpac-20 and Rootpac-40 had similar performances in terms of vigor and fruit yield. However, Rootpac-40 was more efficiently based on yield efficiency values (Table 5) and fruit size (Figure 4) than Rootpac-20, as were Ishtara and Adesoto 101. The results from this last rootstock agreed with those for previous long-term studies of Prunus rootstocks grafted with ‘Big Top’ [28,40,48], despite the fact that more and different rootstocks were evaluated. These results also suggested that rootstocks with P. cerasifera (Rootpac-20) as a parent were not ideal in terms of compatibility for grafting with ‘Big Top’ and other nectarine cultivars. Furthermore, different trials conducted with Rootpac-40 and GF-677 rootstocks grafted with ‘Noracila’ and ‘Luciana‘ nectarine cultivars, reported by Iglesias and Echeverría [8], clearly demonstrated the benefits of intensification by combining size-controlling rootstock (Rootpac-40) and training systems (central leader) in terms of early yields, cost reduction, fruit quality improvement and greater grower profitability. In any case, for each specific cultivar × rootstock combination, the selection of the right spacing is the fundamental consideration in order to reach optimum production levels, as has been demonstrated for both almond [7] and peach [8]. The differences between almond and peach trees concerning crop load management, the need for thinning and high labor costs for peach management have a clear influence on the training system/rootstock that should be used.
Finally, clustering the six common rootstocks on both the Prunus cultivars: ‘Vairo’ and ‘Big Top’, for the same, and most relevant, traits allowed us to confirm the different performances of each one according to the cultivar with which it was grafted, and enabled us to classify the rootstocks into three different large groups. These differences could be largely attributed to how the genetic background of the rootstock interacted with the genetic background of the cultivar.
5. Conclusions
This is the first report evaluating the physiological and agronomical performance of different Prunus rootstocks grafted with scions from different Prunus species: almond and peach. It is difficult to contrast our results with those of other studies as most of the parameters evaluated have yet to be tested with ‘Vairo’ and ‘Big Top’, and particularly those which were evaluated before harvest and during the growing season. Based on our results, and under our climatic conditions, the dwarfing rootstock IRTA-1 had the best performance for ‘Vairo’ almond cultivar; this was followed by the GF-677 rootstock. Both rootstocks presented high values on all traits evaluated. In contrast, the dwarfing rootstock Rootpac-20 did not perform like any of the other rootstocks evaluated when it was grafted with ‘Vairo’ almond cultivar; in fact, we observed a significant lack of compatibility, causing low values on most of the traits evaluated. It did, however, perform well when grafted with ‘Big Top’ nectarine cultivar. Adesoto 101 and Ishtara also exhibited good performance for ‘Big Top’ nectarine cultivar. Rootpac-40 was the only rootstock which performed well on both species. Finally, contrasting the agronomical performance of Prunus rootstocks grafted with P. dulcis and P. persica could help Prunus rootstock breeders, and therefore also almond and peach growers, to obtain more information prior to the release, or purchase, of what are currently the most appropriate rootstocks. It is also important to point out that the interest of a specific degree of vigor induced by the rootstock is dependent on the species. Thus, in almond with an important degree of mechanization of pruning or harvesting, the final volume of the tree is not a limiting factor. On the contrary, in peach where labor represents the main cost of production, having reduced bidimensional with good accessibility canopies from size-controlling rootstocks is the key factor for grower profitability.
Author Contributions
X.M. and I.I. developed the study. Conceptualization, X.M. and I.I.; method-ology, X.M., I.I., L.Z., L.T. and G.M.; software, G.R.; validation, X.M., I.I. and G.R.; formal analysis, X.M., I.I. and G.R.; investigation, X.M., I.I., L.Z., L.T. and G.M.; resources, X.M. and I.I.; data curation, X.M., I.I. and L.Z.; writing—original draft preparation, G.R., X.M. and I.I.; writing—review and editing, G.R., X.M. and I.I.; visualization, G.R., X.M. and I.I.; supervision, X.M. and I.I.; project administration, G.R., X.M. and I.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bassi, D.; Monet, R. Botany and Taxonomy. In The Peach: Botany, Production and Uses; Layne, D.R., Bassi, D., Eds.; CABI Publishing: Oxfordshire, UK, 2008; pp. 1–36. [Google Scholar]
- Socias i Company, R.; Ansón, J.M.; Espiau, M.T. Taxonomy, Botany and Physiology. In Almonds: Botany, Production and Uses; Socias i Company, R., Gradziel, T.M., Eds.; CABI Publishing: Oxfordshire, UK, 2017; pp. 1–41. [Google Scholar]
- Gradziel, T.M.; Martínez-Gómez, P. Almond breeding. In Plant Breeding Reviews; Jacnick, J., Ed.; Wiley & Blackwel: New York, NY, USA, 2013; Volume 37, pp. 207–258. [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations). The Importance of Ukraine and the Russian Federation for Global Agricultural Markets. Available online: https://www.fao.org (accessed on 15 December 2021).
- International Nut Council. Almond Working Group. Statistical Year Book. 2020–2021. Available online: https://www.nutfruit.org (accessed on 15 December 2021).
- MAPA. Short-Term Outlook for Eu Agricultural Markets in 2019 and 2020. Available online: https://www.mapa.gob.es (accessed on 15 December 2021).
- Iglesias, I.; Foles, P.; Oliveira, C. El cultivo del almendro en España y Portugal: Situación, innovación tecnológica, costes, rentabilidad y perspectivas. Rev. Frutic. 2021, 81, 6–49. [Google Scholar]
- Iglesias, I.; Echeverría, G. Current situation, trends and challenges for efficient and sustainable peach production. Sci. Hortic. 2022, 296, 110899. [Google Scholar] [CrossRef]
- Iglesias, I. Situación actual e innovación tecnológica en fruticultura: Una apuesta por la eficiencia y la sostenibilidad. Rev. De Frutic. 2022, 85, 6–45. [Google Scholar]
- Zhang, Y.; Barthe, G.; Grosser, J.W.; Wang, N. Transcriptome analysis of root response to Citrus blight bas on the newly as-sembled swingle citrumelo draft genome. BMC Genom. 2016, 17, 485. [Google Scholar] [CrossRef] [Green Version]
- Boini, A.; Manfrini, L.; Bortolotti, G.; Corelli-Grappadelli, L.; Morandi, B. Monitoring fruit daily growth indicates the onset of mild drought stress in apple. Sci. Hortic. 2019, 256, 108520. [Google Scholar] [CrossRef]
- Bellvert, J.; Nieto, H.; Pelechá, A.; Jofre-Cekalovic, C.; Zazurca, L.; Miarnau, X. Remote sensing energy balance model for the assessment of crop evapotranspiration and water status in an almond rootstock collection. Front. Plant Sci. 2021, 12, 608967. [Google Scholar] [CrossRef]
- Gonzalez-Dugo, V.; Lopez-Lopez, M.; Espadafor, M.; Orgaz, F.; Testi, L.; Zarco-Tejada, P.J.; Lorite, I.J.; Fereres, E. Transpiration from canopy temperature: Implications for the assessment of crop yield in almond orchards. European. J. Agron. 2019, 105, 78–85. [Google Scholar] [CrossRef]
- Hernandez-Santana, V.; Fernández, J.E.; Rodriguez-Dominguez, C.M.; Romero, R.; Diaz-Espejo, A. The dynamics of radial sap flux density reflects changes in stomatal conductance in response to soil and air water deficit. Agric. Forest Meteorol. 2016, 218, 92–101. [Google Scholar] [CrossRef] [Green Version]
- Yahmed, J.B.; Ghrab, M.; Mimoun, M.B. Eco-physiological evaluation of different scion-rootstock combinations of almond grown in Mediterranean conditions. Fruits 2016, 71, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Iglesias, I. La intensificación sostenible como respuesta al Pacto Verde de la Unión Europea: Retos y ejemplos en la producción frutícola y en el consumo alimentario. Rev. Frutic. 2020, 79, 45–57. [Google Scholar]
- Maldonado, M.; Torguet, L.; Girabet, R.; Zazurca, L.; Martinez, G.; Miarnau, X. Nuevos modelos productivos para la intensi-ficación del almendro. Vida Rural 2019, 22, 50–55. [Google Scholar]
- Miarnau, X.; Torguet, L.; Batlle, I.; Alegre, S. El cultivo del almendro en alta densidad. Rev. Frutic. 2016, 49, 68–87. [Google Scholar]
- Díez-Palet, I.; Funes, I.; Savé, R.; Biel, C.; de Herralde, F.; Miarnau, X.; Vargas, F.; Àvila, G.; Carbó, J.; Aranda, X. Blooming under Mediterranean climate: Estimating cultivar-specific chill and heat requirements of almond and apple trees using a statistical approach. Agronomy 2019, 9, 760. [Google Scholar] [CrossRef] [Green Version]
- Gogorcena, Y.; Sánchez, G.; Moreno-Vázquez, S.; Pérez, S.; Ksouri, N. Genomic-Based Breeding for Climate-Smart Peach Varieties. In Genomic Designing of Climate-Smart Fruit Crops; Kole, C., Ed.; Springer Nature: Cham, Germany, 2020; pp. 271–332. [Google Scholar]
- Penso, G.A.; Citadin, I.; Scariotto, S.; Magalhae dos Santos, C.E.; Junior, A.W.; Bruckner, C.H.; Rodrigo, J. Development of peach flower buds under low Winter chilling conditions. Agronomy 2020, 10, 428. [Google Scholar] [CrossRef] [Green Version]
- Prudencio, A.S.; Sánchez-Pérez, R.; Martínez-García, P.J.; Dicenta, F.; Gradziel, T.M.; Martínez-Gómez, P. Genomic Designing for New Climate-Resilient Almond Varieties. In Genomic Designing of Climate-Smart Fruit Crops; Kole, C., Ed.; Springer Nature: Cham, Germany, 2020; pp. 1–22. [Google Scholar]
- Paniagua, L.L.; García-Martín, A.; Moral, F.J.; Rebollo, F.J. Aridity in the Iberian Peninsula (1960–2017): Distribution, tenden-cies, and changes. Theor. Appl. Climatol. 2019, 138, 811–830. [Google Scholar] [CrossRef]
- Ozturk, T.; Zeynep, P.C.; Turkes, M.; Levent Kurnaz, M. Projections of climate change in the Mediterranean Basin by using downscaled global climate model outputs. Int. J. Climatol. 2015, 35, 4276–4292. [Google Scholar] [CrossRef]
- Font i Forcada, C.; Reig, G.; Mestre, L.; Mignard, P.; Betrán, J.A.; Moreno, M.A. Scion × rootstock response on production, mineral composition, and fruit quality under heavy-calcareous soil and hot climate. Agronomy 2020, 10, 1159. [Google Scholar] [CrossRef]
- Jiménez, S.; Dridi, J.; Gutiérrez, D.; Moret, D.; Irigoyen, J.J.; Moreno, M.A.; Gogorcena, Y. Physiological, biochemical and molecular responses in four Prunus rootstocks submitted to drought stress. Tree Physiol. 2013, 33, 1061–1075. [Google Scholar] [CrossRef]
- Lordan, J.; Zazurca, L.; Maldonado, M.; Torguet, L.; Alegre, S.; Miarnau, X. Horticultural performance of ‘Marinada’ and ‘Vairo’ almond cultivars grown on a genetically diverse set of rootstocks. Sci. Hortic. 2019, 256, 108558. [Google Scholar] [CrossRef]
- Reig, G.; Garanto, X.; Mas, N.; Iglesias, I. Long-term agronomical performance and iron chlorosis susceptibility of several Prunus rootstocks grown under loamy and calcareous soil conditions. Sci. Hortic. 2020, 262, 10935. [Google Scholar] [CrossRef]
- Yahmed, J.B.; Ghrab, M.; Moreno, M.A.; Pinochet, J.; Mimoun, M.B. Performance of ‘Subirana’ flat peach cultivar budded on different Prunus rootstocks in a warm production area in North Africa. Sci. Hortic. 2016, 206, 24–32. [Google Scholar] [CrossRef] [Green Version]
- Casanova-Gascòn, J.; Figueras-Panillo, M.; Iglesias-Castellarnau, J.; Martin-Ramos, P. Comparison of SHD and ppen-center training systems in almond tree orchards cv. “Soleta”. Agronomy 2019, 9, 874. [Google Scholar] [CrossRef] [Green Version]
- Maldera, F.; Vivaldi, G.A.; Iglesias-Castellarnau, I.; Camposeo, S. Row orientation and canopy position affect bud differentia-tion, LAI and some agronomical traits of a super high-density almond orchard. Agronomy 2021, 11, 251. [Google Scholar] [CrossRef]
- Maldera, F.; Vivaldi, G.A.; Iglesias-Castellarnau, I.; Camposeo, S. Two almond cultivars trained in a super-high density orchard show different growth, yield efficiencies and damages by mechanical harvesting. Agronomy 2021, 11, 1406. [Google Scholar] [CrossRef]
- Vargas, F.J.; Romero, M.; Clave, J.; Verges, J.; Santos, J.; Batlle, I. Vairo’, ‘Marinada’, ‘Constanti’, and ‘Tarraco’ almonds. HortScience 2008, 43, 535–537. [Google Scholar] [CrossRef] [Green Version]
- Vargas, F.J.; Romero, M.A.; Clave, J.; Batlle, I.; Miarnau, X.; Alegre, S. Important traits in IRTA’s new almond cultivars. Acta Hortic. 2011, 912, 359–365. [Google Scholar] [CrossRef]
- Iglesias, I.; Echeverría, G. Differential effect of cultivar and harvest date on nectarine colour, quality and consumer acceptance. Sci. Hortic. 2009, 120, 41–50. [Google Scholar] [CrossRef]
- Iglesias, I.; Echeverría, G. Overview of peach industry in the European Union with special reference to Spain. Acta Hortic. 2021, 1304, 163–176. [Google Scholar] [CrossRef]
- Reig, G.; Alegre, S.; Cantín, C.M.; Gatius, F.; Puy, J.; Iglesias, I. Tree ripening and postharvest firmness loss of eleven commercial nectarine cultivars under Mediterranean conditions. Sci. Hortic. 2017, 219, 335–343. [Google Scholar] [CrossRef]
- Sakar, E.H.; Yamani, M.E.; Rharrabti, Y. Geometrical traits in almond fruit as affected by genotypic and environmental variations in Northern Morocco. Erwerbs-Obstbau 2019, 61, 103–112. [Google Scholar] [CrossRef]
- Toro, G.; Flexas, J.; Escalona, J.M. Contrasting leaf porometer and infra-red gas analyser methodologies: An oldl paradigm about the stomatal conductance measurement. Theor. Exp. Plant Physiol. 2019, 31, 483–492. [Google Scholar] [CrossRef]
- Kuiper, F.K.; Fisher, L. A Monte Carlo comparison of six clustering procedures. Biometrics 1975, 31, 777–783. [Google Scholar] [CrossRef]
- Ward, J.H., Jr. Hierarchical groupings to optimize an objective function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Mestre, L.; Reig, G.; Betrán, J.A.; Pinochet, J.; Moreno, M.A. Influence of peach–almond hybrids and plum-based rootstocks on mineral nutrition and yield characteristics of ‘Big Top’ nectarine in replant and heavy-calcareous soil conditions. Sci. Hortic. 2015, 215, 475–481. [Google Scholar] [CrossRef] [Green Version]
- Reig, G.; Font i Forcada, C.; Mestre, L.; Betrán, J.A.; Moreno, M.A. Potential of new Prunus cerasifera based rootstocks for adapting under heavy and calcareous soil conditions. Sci. Hortic. 2018, 234, 193–200. [Google Scholar] [CrossRef]
- Conejero, W.; Mellisho, C.D.; Ortuño, M.F.; Galindo, A.; Pérez-Sarmiento, F.; Torrecillas, A. Establishing maximum daily trunk shrinkage and midday stem water potential reference equations for irrigation scheduling of early maturing peach trees. Irrig. Sci. 2011, 29, 299–309. [Google Scholar] [CrossRef]
- Reig, G.; Lordan, J.; Fazio, G.; Grusak, M.A.; Hoying, S.; Cheng, L.; Francescatto, P.; Robinson, T. Horticultural performance and elemental nutrient concentrations on ‘Fuji’ grafted on apple rootstocks under New York climatic conditions. Sci. Hortic. 2018, 227, 22–37. [Google Scholar] [CrossRef]
- Reig, G.; Lordan, J.; Miranda, M.; Hoying, S.A.; Fargione, M.; Reginato, G.; Donahue, D.J.; Francescatto, P.; Fazio, G.; Robinson, T. Long-term performance of ‘Gala’, Fuji’ and ‘Honeycrisp’ apple trees grafted on Geneva® rootstocks and trained to four production systems under New York State climatic conditions. Sci. Hortic. 2019, 244, 277–293. [Google Scholar] [CrossRef]
- Musacchi, S.; Neri, D.; Iglesias, I. Training systems and sustainable orchard management for European pear (Pyrus communis L.) in the Mediterranean area: A Review. Agronomy 2021, 11, 1765. [Google Scholar] [CrossRef]
- Reig, G.; Mestre, L.; Betrán, J.A.; Pinochet, J.; Moreno, M.A. Agronomic and physicochemical fruit properties of ‘Big Top’ nectarine budded on peach and plum based rootstocks in Mediterranean conditions. Sci. Hortic. 2016, 210, 85–92. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).