Genomic Regions Influencing Preharvest Sprouting Tolerance in Two Doubled-Haploid Wheat Populations (Triticum aestivum L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Study Area and Experimental Design
2.2. Phenotypic Evaluation of PHS Tolerance
2.3. Phenotypic Evaluation and Statistical Analysis
2.4. Genotyping and Construction of Genetic Map
2.5. QTL Analysis
3. Results
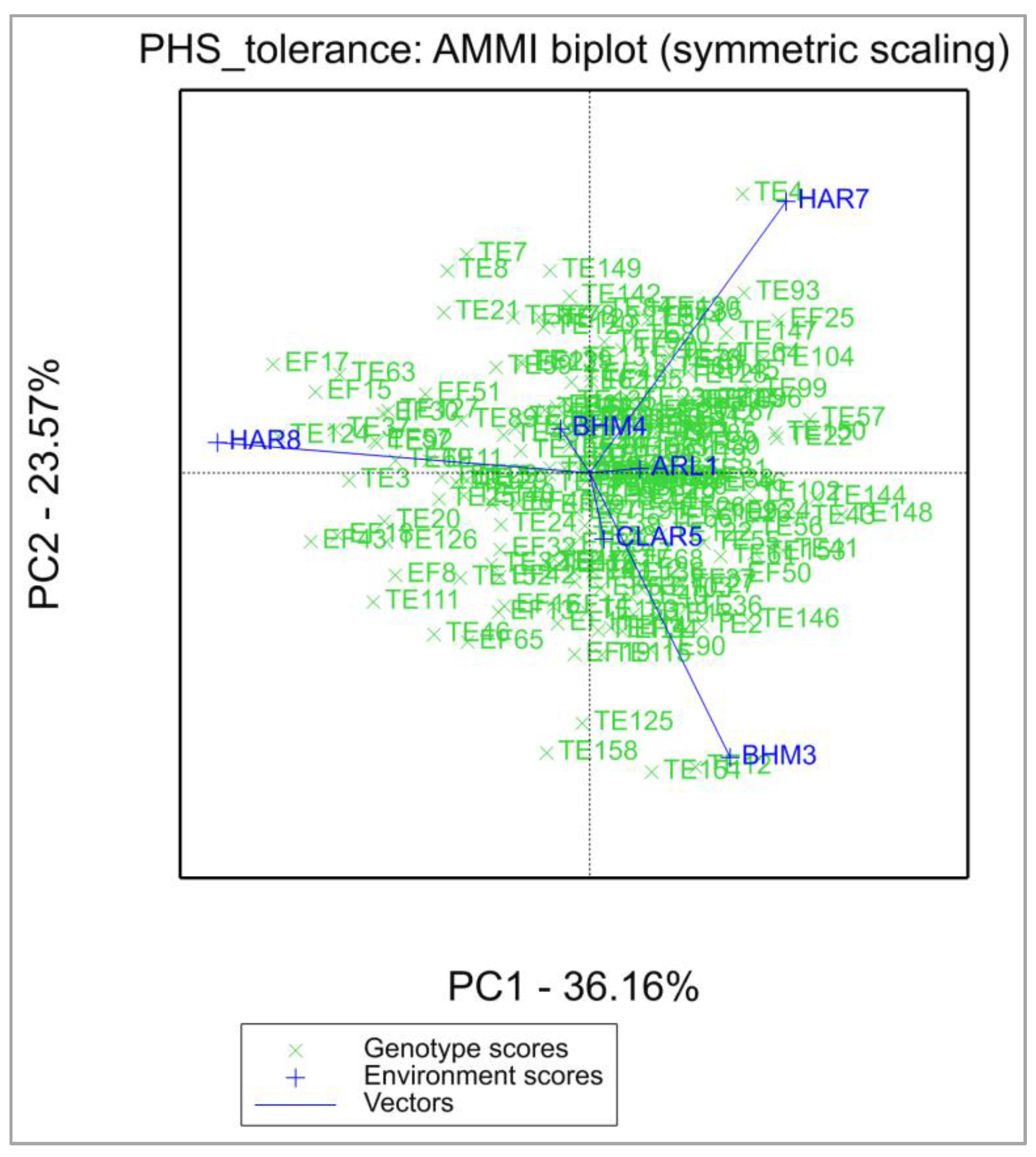
3.1. Phenotypic Performance of Genotypes and Parents across Multi-Environments
3.2. Selection of Best-Performing Genotypes
3.3. Genetic Linkage Map Construction
3.4. QTL Mapping Analysis
3.4.1. Additive QTLs Detected in the Tugela-Dn × Elands Mapping Population
3.4.2. Additive QTLs Detected in the Elands × Flamink Mapping Population
4. Discussion
4.1. Phenotypic Variations Attributed to Environmental Differences
4.2. QTL Mapping Analysis of PHS Tolerance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Groos, C.; Gay, G.; Perretant, M.R.; Gervais, L.; Bernard, M.; Dedryver, F.; Charmet, G. Study of the relationship between pre-harvest sprouting and grain colour by quantitative trait loci analysis in a white × red grain bread-wheat cross. Theor. Appl. Genet. 2002, 104, 39–47. [Google Scholar] [CrossRef]
- Rodriguez, M.V.; Barrero, J.M.; Corbineau, F.; Gubler, F.; Benech-Arnold, R.L. Dormancy in cereals (not too much, not so little): About the mechanisms behind this trait. Seed Sci. Res. 2015, 25, 99–119. [Google Scholar] [CrossRef] [Green Version]
- Mares, D.; Mrva, K.; Cheong, J.; Williams, K.; Watson, B.; Storlie, E.; Sutherland, M.; Zou, Y. A QTL located on chromosome 4A associated with dormancy in white- and red-grained wheats of diverse origin. Theor. Appl. Genet. 2005, 111, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Martinez, S.A.; Godoy, J.; Huang, M.; Zhang, Z.; Carter, A.H.; Garland, C.K.A.; Steber, C.M. Genome-wide association mapping for tolerance to preharvest sprouting and low falling numbers in wheat. Front. Plant Sci. 2018, 9, 141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, A.; Cao, J.; Jiang, H.; Chang, C.; Zhang, H.P.; Sheikh, S.W.; Shah, L.; Ma, C. Unraveling molecular and genetic studies of wheat (Triticum aestivum L.) resistance against factors causing pre-harvest sprouting. Agronomy 2019, 9, 117. [Google Scholar] [CrossRef] [Green Version]
- Gupta, P.K.; Balyan, H.S.; Sharma, S.; Kumar, R. Genetics of yield, abiotic stress tolerance and biofortification in wheat (Triticum aestivum L.): A review. Theor. Appl. Genet. 2020, 133, 1569–1602. [Google Scholar] [CrossRef]
- Andreoli, C.; Bassoi, M.C.; Brunetta, D. Genetic control of seed dormancy and pre-harvest sprouting in wheat. Sci. Agric. 2006, 63, 564–566. [Google Scholar] [CrossRef] [Green Version]
- Simsek, S.; Ohm, J.B.; Lu, H.; Rugg, M.; Berzonsky, W.; Alamri, M.S.; Mergoum, M. Effect of pre-harvest sprouting on physicochemical properties of starch in wheat. Foods 2014, 3, 194–207. [Google Scholar] [CrossRef] [Green Version]
- Ponce-García, N.; Ramírez-Wong, B.; Escalante-Aburto, A.; Torres-Chávez, P.I.; Serna-Saldivar, S.O. Grading factors of wheat kernels based on their physical properties. In Wheat Improvement, Management and Utilization; Wanyera, R., Owuoche, J., Eds.; IntechOpen: London, UK, 2017; pp. 275–291. [Google Scholar] [CrossRef]
- Gavazza, M.I.A.; Bassoi, M.C.; Carvalho, T.C.; de Bespalhok, F.J.C.; Panobianco, M. Methods for assessment of pre-harvest sprouting in wheat cultivars. Pesqui. Agropecuária Bras. 2012, 47, 928–933. [Google Scholar] [CrossRef] [Green Version]
- Depauw, R.M.; Hucl, P.; Knox, R.E.; Singh, A.K.; Fox, S.L.; Humphreys, D.G. Developing standardized methods for breeding preharvest sprouting resistant wheat, challenges and successes in Canadian wheat. Euphytica 2012, 188, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Olaerts, H.; Courtin, C.M. Impact of preharvest sprouting on endogenous hydrolases and technological quality of wheat and bread: A review. Compr. Rev. Food Sci. Food Technol. 2018, 17, 698–713. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations (FAO). The Future of Food and Agriculture—Alternative Pathways to 2050; Summary Version; FAO: Rome, Italy, 2018; 60p. [Google Scholar]
- Ben Mariem, S.; Soba, D.; Zhou, B.; Loladze, I.; Morales, F.; Aranjuelo, I. Climate Change, Crop Yields, and Grain Quality of C3 Cereals: A Meta-Analysis of [CO2], Temperature, and Drought Effects. Plants 2021, 10, 1052. [Google Scholar] [CrossRef] [PubMed]
- Barnard, A.; Purchase, J.L.; Smith, M.F.; van Lill, D. Determination of the preharvest sprouting resistance of South African winter wheat (Triticum aestivum L.) cultivars. S. Afr. J. Plant Soil 1997, 14, 4–8. [Google Scholar] [CrossRef]
- Van Niekerk, H.A. Southern Africa Wheat Pool. In The World Wheat Book: The History of Wheat Breeding; Bonjean, A.P., Angus, W.J., Eds.; Lavoisier Publishing: Paris, France, 2001; pp. 923–926. [Google Scholar]
- Barnard, A.; Bona, L. Sprout damage and falling number in South African and Hungarian wheats. Cereal Res. Commun. 2004, 32, 259–264. [Google Scholar] [CrossRef]
- Barnard, A.; Smith, M.F. The effect of rainfall and temperature on the preharvest sprouting tolerance of winter wheat in the dryland production areas of the Free State Province. Field Crops Res. 2009, 112, 158–164. [Google Scholar] [CrossRef]
- Sydenham, S.L.; Barnard, A. Targeted haplotype comparisons between South African wheat cultivars appear predictive of pre-harvest sprouting tolerance. Front. Plant Sci. 2018, 9, 63. [Google Scholar] [CrossRef] [Green Version]
- Barnard, A.; Grain, S.A. Preharvest Sprouting Research—20 Years Later. 2018. Available online: https://www.grainsa.co.za/preharvest-sprouting-research-20-years-later (accessed on 6 January 2022).
- Barnard, A. Genetic diversity of South African winter wheat cultivars in relation to preharvest sprouting and falling number. Euphytica 2001, 119, 107–110. [Google Scholar] [CrossRef]
- Craven, M.; Barnard, A.; Labuschagne, M.T. The impact of cold temperatures during grain maturation on selected quality parameters of wheat. J. Sci. Food Agric. 2007, 87, 1783–1793. [Google Scholar] [CrossRef]
- Smit, H.A.; Tolmay, V.L.; Barnard, A.; Jordaan, J.P.; Koekemoer, F.P.; Otto, W.M. An overview of the context and scope of wheat (Triticum aestivum) research in South Africa from 1983 to 2008. S. Afr. J. Plant Soil 2010, 27, 81–96. [Google Scholar] [CrossRef]
- Barnard, A.; Van Deventer, C.S.; Maartens, H. Genetic variability of preharvest sprouting in the South African situation. Euphytica 2005, 143, 291–296. [Google Scholar] [CrossRef]
- ProAgri, SA. Wheat Production: The Use of Molecular Markers to Assist in Pre-Harvest Sprouting Research. 2016. Available online: https://www.proagri.co.za/en/wheat-production-use-molecular-markers-assist-pre-harvest-sprouting-research/ (accessed on 9 September 2021).
- Johansson, E. Effect of two genotypes and Swedish environment on falling number, amylase activities, and protein concentration and composition. Euphytica 2002, 126, 143–149. [Google Scholar] [CrossRef]
- Gao, X.; Hu, C.H.; Li, H.Z.; Yao, Y.J.; Meng, M.; Dong, J. Factors affecting pre-harvest sprouting resistance in wheat (Triticum aestivum L.): A review. J. Anim. Plant Sci. 2013, 23, 556–565. [Google Scholar]
- Nornberg, R.; de Souza, L.H.; da Silva, J.A.G.; Zimmer, C.M.; Cima, F.F.; Olivo, M.; de Oliveira, A.C. The challenge of finding high grain yield and pre-harvest sprouting tolerant genotypes in Brazilian wheat germplasm. Aust. J. Crop Sci. 2016, 10, 977–984. [Google Scholar] [CrossRef]
- Liu, S.; Sehgal, S.K.; Li, J.; Lin, M.; Trick, H.N.; Yu, J. Cloning and characterization of a critical regulator for preharvest sprouting in wheat. Genetics 2013, 195, 263–273. [Google Scholar] [CrossRef] [Green Version]
- Cabral, A.L.; Jordan, M.C.; McCartney, C.A.; You, F.M.; Humphreys, D.G.; MacLachlan, R.; Pozniak, C.J. Identification of candidate genes, regions and markers for pre-harvest sprouting resistance in wheat (Triticum aestivum L.). BMC Plant Biol. 2014, 14, 340. [Google Scholar] [CrossRef] [Green Version]
- Gao, F.; Wen, W.; Liu, J.; Rasheed, A.; Yin, G.; Xia, X. Genome-wide linkage mapping of QTL for yield components, plant height and yield-related physiological traits in the Chinese wheat cross Zhou 8425B/Chinese Spring. Front. Plant Sci. 2015, 6, 1099. [Google Scholar] [CrossRef] [Green Version]
- Lin, M.; Cai, S.; Wang, S.; Liu, S.; Zhang, G.; Bai, G. Genotyping-by-sequencing (GBS) identified SNP tightly linked to QTL for pre-harvest sprouting resistance. Theor. Appl. Genet. 2015, 128, 1385–1395. [Google Scholar] [CrossRef]
- Cao, L.; Hayashi, K.; Tokui, M.; Mori, M.; Miura, H.; Onishi, K. Detection of QTLs for traits associated with pre-harvest sprouting resistance in bread wheat (Triticum aestivum L.). Breed. Sci. 2016, 66, 260–270. [Google Scholar] [CrossRef] [Green Version]
- Su, Z.; Jin, S.; Lu, Y.; Zhang, G.; Chao, S.; Bai, G. Single nucleotide polymorphism tightly linked to a major QTL on chromosome 7A for both kernel length and kernel weight in wheat. Mol. Breed. 2016, 36, 15. [Google Scholar] [CrossRef]
- El-Feki, W.M.; Byrne, P.F.; Reid, S.D.; Haley, S.D. Mapping quantitative trait loci for agronomic traits in winter wheat under different soil moisture levels. Agronomy 2018, 8, 133. [Google Scholar] [CrossRef] [Green Version]
- Guan, P.; Lu, L.; Jia, L.; Kabir, M.R.; Zhang, J.; Lan, T. Global QTL analysis identifies genomic regions on chromosomes 4A and 4B harboring stable loci for yield-related traits across different environments in wheat (Triticum aestivum L.). Front. Plant Sci. 2018, 9, 529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeeshan, M.; Arshad, W.; Khan, M.I.; Ali, S.; Nawaz, A.; Batool, A. Breeding for pre-harvest sprouting resistance in bread wheat under rainfed conditions. Front. Agric. Sci. Eng. 2018, 5, 253–261. [Google Scholar] [CrossRef]
- Wang, X.; Liu, H.; Liu, G.; Mia, M.S.; Siddique, K.H.M.; Yan, G. Phenotypic and genotypic characterization of near-isogenic lines targeting a major 4BLQTL responsible for pre-harvest sprouting in wheat. BMC Plant Biol. 2019, 19, 348. [Google Scholar] [CrossRef] [Green Version]
- Gautam, T.; Kumar, K.; Agarwal, P.; Tyagi, S.; Jaiswal, V.; Gahlaut, V. Development of white-grain pre-harvest sprouting tolerant and pyramided protein-rich leaf rust resistant wheats using molecular breeding. (Version 1). Res. Sq. 2021. preprint. [Google Scholar] [CrossRef]
- He, J.; Zhang, D.; Chen, X.; Li, Y.; Hu, M.; Sun, S. Identification of QTLs and a candidate gene for reducing pre-harvest sprouting in Aegilops tauschii–Triticum aestivum chromosome segment substitution lines. Int. J. Mol. Sci. 2021, 22, 3729. [Google Scholar] [CrossRef]
- Sharma, S.K.; Dhaliwal, H.S.; Multani, D.S.; Bains, S.S. Inheritance of preharvest sprouting tolerance in Triticum aestivum and its transfer to an amber-grained cultivar. J. Hered. 1994, 85, 312–314. [Google Scholar] [CrossRef]
- Singh, K.A.; Knox, R.E.; Clarke, J.M.; Clarke, F.R.; Singh, A.; DePauw, R.M.; Cuthbert, R.D. Genetics of pre-harvest sprouting resistance in a cross of Canadian adapted durum wheat genotypes. Mol. Breed. 2014, 33, 919–929. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.Q.; Cloutier, S.; Lycar, L.; Radovanovic, N.; Humphreys, D.G.; Noll, J.S. Molecular detection of QTLs for agronomic and quality traits in a doubled haploid population derived from two Canadian wheats (Triticum aestivum L.). Theor. Appl. Genet. 2006, 113, 753–766. [Google Scholar] [CrossRef]
- Guzman, C.; Peña, R.J.; Singh, R.; Autrique, E.; Dreisigacker, S.; Crossa, J. Wheat quality improvement at CIMMYT and the use of genomic selection on it. Appl. Transl. Genom. 2016, 11, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Mokone, M. Understanding the Wheat Import Tariff. 2017. Available online: https://www.grainsa.co.za/understanding-the-wheat-import-tariff (accessed on 29 October 2021).
- Nuttalla, J.G.; O’Learya, G.J.; Panozzoa, J.F.; Walkera, C.K.; Barlowb, K.M.; Fitzgerald, G.J. Models of grain quality in wheat—A review. Field Crops Res. 2017, 202, 136–145. [Google Scholar] [CrossRef] [Green Version]
- Tadele, Z. Raising crop productivity in Africa through intensification: A review. Agronomy 2017, 7, 22. [Google Scholar] [CrossRef]
- Kuzay, S.; Xu, Y.; Zhang, J.; Katz, A.; Pearce, S.; Su, Z. Identification of a candidate gene for a QTL for spikelet number per spike on wheat chromosome arm 7AL by high-resolution genetic mapping. Theor. Appl. Genet. 2019, 132, 2689–2705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lephuthing, M.; Tolmay, V.; Baloyi, T.; Hlongoane, T.; Oliphant, T.; Tsilo, T.J. Relationship of grain micronutrient concentrations and grain yield components in a doubled haploid bread wheat (Triticum Aestivum L.) population. Crop Pasture Sci. 2021, 73, 116–126. [Google Scholar] [CrossRef]
- Khumalo, T.P.; Barnard, A.; Dube, E.; Tsilo, T.J. Characterization of vegetative vigor of two doubled-haploid wheat populations. J. Crop Improv. 2021, 1–19. [Google Scholar] [CrossRef]
- ARC. Agricultural Research Council Guidelines for the Production of Small Grains in the Winter Rainfall Area; ARC Small Grain Institute: Bethlehem, South Africa, 1993. [Google Scholar]
- ARC. Agricultural Research Council Guidelines for the Production of Small Grains in the Summer Rainfall Area; ARC Small Grain Institute: Bethlehem, South Africa, 1999. [Google Scholar]
- VSN International. Genstat for Windows, 18th ed.; VSN International: Hemel, Hempstead, UK, 2015; Available online: www.genstat.co.uk (accessed on 12 June 2019).
- Federer, W.T. Augmented designs with one-way elimination of heterogeneity. Biometrics 1961, 17, 447–473. [Google Scholar] [CrossRef]
- Tsilo, T.J.; Kolmer, J.A.; Anderson, J.A. Molecular mapping and improvement of leaf rust resistance in wheat breeding lines. Phytopathology 2014, 104, 865–870. [Google Scholar] [CrossRef] [Green Version]
- RStudio Team. RStudio: Integrated Development for R; RStudio, Inc.: Boston, MA, USA, 2019; Available online: http://www.rstudio.com/ (accessed on 15 January 2019).
- van Ooijen, J.W. JoinMap 4, Software for the Calculation of Genetic Linkage Maps in Experimental Populations; Kyazma B.V.: Wageningen, The Netherlands, 2006. [Google Scholar]
- Stam, P. Construction of integrated genetic linkage maps by means of a new computer package: JoinMap. Plant J. 1993, 3, 739–744. [Google Scholar] [CrossRef]
- Kosambi, D.D. The estimation of map distances from recombination values. Ann. Eugen. 1943, 12, 172–175. [Google Scholar] [CrossRef]
- Wang, S.; Basten, C.J.; Zeng, Z.B. Windows QTL Cartographer 2.5; Department of Statistics, North Carolina State University: Raleigh, NC, USA, 2012. [Google Scholar]
- McIntosh, R.A.; Yamazaki, Y.; Devos, K.M.; Dubcovsky, J.; Rogers, W.J.; Appels, R. Catalogue of Gene Symbols for Wheat. In Tenth International Wheat Genetics Symposium, Paestum, Italy, 2003. Available online: https://wheat.pw.usda.gov/ggpages/wgc/98/intro.htm#Intro6 (accessed on 23 May 2015).
- Alipour, H.; Bai, G.; Zhang, G.; Bihamta, M.R.; Mohammadi, V.; Peyghambari, S.A. Imputation accuracy of wheat genotyping-by-sequencing (GBS) data using barley and wheat genome references. PLoS ONE 2019, 14, e0208614. [Google Scholar] [CrossRef]
- Liu, C.; Sukumaran, S.; Jarquin, D.; Crossa, J.; Dreisigacker, S.; Sansaloni, C.; Reynolds, M. Comparison of Array- and Sequencing-based Markers for Genome Wide Association Mapping and Genomic Prediction in Spring Wheat. Crop Sci. 2019, 60, 211–225. [Google Scholar] [CrossRef]
- Zou, C.; Karn, A.; Reisch, B.; Nguyen, A.; Sun, Y.; Bao, Y. Haplotyping the Vitis collinear core genome with rhAmpSeq improves marker transferability in a diverse genus. Nat. Commun. 2020, 11, 413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elshire, R.J.; Glaubitz, J.C.; Sun, Q.; Poland, J.A.; Kawamoto, K.; Buckler, E.S.; Mitchell, S.E. A robust, simple genotyping-by-sequencing (GBS) approach for high diversity species. PLoS ONE 2011, 6, e19379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heslot, N.; Rutkoski, J.; Poland, J.; Jannink, J.L.; Sorrells, M.E. Impact of marker ascertainment bias on genomic selection accuracy and estimates of genetic diversity. PLoS ONE 2013, 8, e74612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Zeng, Z.-B. A general mixture model approach for mapping quantitative trait loci from diverse cross designs involving multiple inbred lines. Genet. Res. 2000, 75, 345–355. [Google Scholar] [CrossRef] [Green Version]
- Qu, P.; Wang, J.; Wen, W.; Gao, F.; Liu, J.; Xia, X. Construction of consensus genetic map with applications in gene mapping of wheat (Triticum aestivum L.) using 90K SNP Array. Front. Plant Sci. 2021, 12, 1777. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.M.; Barker, G.L.; Wilkinson, P.; Burridge, A.; Winfield, M.; Coghill, J. Discovery and development of exome-based, co-dominant single nucleotide polymorphism markers in hexaploid wheat (Triticum aestivum L.). Plant Biotechnol. 2013, 11, 279–295. [Google Scholar] [CrossRef] [Green Version]
- Mackay, T.F.C. The genetic architecture of quantitative traits: Lessons from Drosophila. Curr. Opin. Genet. Dev. 2004, 14, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Miles, C.M.; Wayne, M. Quantitative Trait Locus (QTL) Analysis—What Statistical Method Would You Use to Analyze Complex Traits? Nat. Educ. 2008. Available online: https://www.nature.com/scitable/topicpage/quantitative-trait-locus-qtl-analysis-53904 (accessed on 11 January 2019).
- Kulwal, P.L.; Mir, R.R.; Kumar, S.; Gupta, P.K. QTL analysis and molecular breeding for seed dormancy and pre-harvest sprouting tolerance in bread wheat. J. Plant Biol. 2010, 37, 59–74. [Google Scholar]
- Marzougui, S.; Sugimoto, K.; Yamanouchi, U.; Shimono, M.; Hoshino, T.; Hori, K. Mapping and characterization of seed dormancy QTLs using chromosome segment substitution lines in rice. Theor. Appl. Genet. 2012, 124, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Barrero, J.M.; Cavanagh, C.; Verbyla, K.L.; Tibbits, J.F.; Verbyla, A.P.; Huang, B.E. Transcriptomic analysis of wheat near-isogenic lines identifies PM19-A1 and A2 as candidates for a major dormancy QTL. Genome Biol. 2015, 16, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mares, D.J.; Mrva, K. Wheat grain preharvest sprouting and late maturity alpha-amylase. Planta 2014, 240, 1167–1178. [Google Scholar] [CrossRef] [PubMed]
- Mares, D.J. Genetic studies of sprouting tolerance in red and white wheats. In Pre-Harvest Sprouting in Cereals 1992; Walker-Simmons, M.K., Ried, J.L., Eds.; American Association of Cereal Chemists: St. Paul, MN, USA, 1993; pp. 21–29. [Google Scholar]
- Biddulph, T.B.; Plummer, J.A.; Setter, T.L.; Mares, D.J. Influence of high temperature and terminal moisture stress on dormancy in wheat (Triticum aestivum L.). Field Crops Res. 2007, 103, 139–153. [Google Scholar] [CrossRef]
- DAFF. Production Guideline for Wheat. 2016. Available online: https://www.daff.gov.za/wheat (accessed on 20 September 2017).
- Wang, B.; Liu, H.; Liu, Z.; Dong, X.; Guo, J.; Li, W. Identification of minor effect QTLs for plant architecture related traits using super high density genotyping and large recombinant inbred population in maize (Zea mays). BMC Plant Biol. 2018, 18, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collard, B.C.Y.; Jahufer, M.Z.Z.; Brouwer, J.B.; Pang, E.C.K. An introduction to markers, quantitative trait loci (QTL) mapping and marker-assisted selection for crop improvement: The basic concepts. Euphytica 2005, 142, 169–196. [Google Scholar] [CrossRef]
- Almeida, G.D.; Makumbi, D.; Magorokosho, C.; Nair, S.; Borém, A.; Ribaut, J.M. QTL mapping in three tropical maize populations reveals a set of constitutive and adaptive genomic regions for drought tolerance. Theor. Appl. Genet. 2012, 126, 583–600. [Google Scholar] [CrossRef] [Green Version]
- Stange, M.; Utz, H.F.; Schrag, T.A.; Melchinger, A.E.; Würschum, T. High-density genotyping: An overkill for QTL mapping? Lessons learned from a case study in maize and simulations. Theor. Appl. Genet. 2013, 126, 2563–2574. [Google Scholar] [CrossRef]
- Lin, M.; Zhang, D.; Liu, S.; Zhang, G.; Yu, J.; Fritz, A.K.; Bai, G. Genome-wide association analysis on pre-harvest sprouting resistance and grain color in U.S. winter wheat. BMC Genom. 2016, 17, 794. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Matus-Cádiz, M.; Båga, M.; Hucl, P.; Chibbar, R.N. Genetic Mapping of Pre-Harvest Sprouting Resistance Loci in Bread Wheat (Triticum aestivum L.). 2006. Available online: https://harvest.usask.ca/bitstream/handle/10388/9460/R.%20Singh%20et%20al.%2C%202006.pdf?sequence=1&isAllowed=y (accessed on 15 June 2021).
- Fofana, B.; Humphreys, G.; Rasul, G.; Cloutier, S.; Somers, D. Assessment of molecular diversity at QTLs for preharvest sprouting resistance in wheat using microsatellite markers. Genome 2008, 51, 375–386. [Google Scholar] [CrossRef]
- Zhou, S.H.; Lin, F.U.; Wu, Q.H.; Chen, J.J.; Chen, Y.X.; Xie, J.Z. QTL mapping revealed TaVp-1A conferred pre-harvest sprouting resistance in wheat population Yanda 1817×Beinong 6. J. Integr. Agric. 2017, 16, 435–444. [Google Scholar] [CrossRef]
- Mori, M.; Uchino, N.; Chono, M.; Kato, K.; Miura, H. Mapping QTLs for grain dormancy on wheat chromosome 3A and the group 4 chromosomes, and their combined effect. Theor. Appl. Genet. 2005, 110, 1315–1323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munkvold, J.D.; Tanaka, J.; Benscher, D.; Sorrells, M.E. Mapping quantitative trait loci for preharvest sprouting resistance in white wheat. Theor. Appl. Genet. 2009, 119, 1223–1235. [Google Scholar] [CrossRef]
- Somyong, S.; Ishikawa, G.; Munkvold, J.D.; Tanaka, J.; Benscher, D.; Cho, Y.G.; Sorrells, M.E. Fine mapping of a preharvest sprouting QTL interval on chromosome 2B in white wheat. Theor. Appl. Genet. 2014, 127, 1843–1855. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tang, H.; Cheng, M.P.; Dankwa, K.O.; Chen, Z.X.; Li, Z.Y. Genome-wide association study for pre-harvest sprouting resistance in a large germplasm collection of Chinese wheat landraces. Front. Plant Sci. 2017, 8, 401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, S.; Abe, F.; Kawahigashi, H.; Nakazono, K.; Tagiri, A.; Matsumoto, T. A wheat homolog of MOTHER OF FT AND TFL1 acts in the regulation of germination. Plant Cell 2011, 23, 3215–3229. [Google Scholar] [CrossRef] [Green Version]
- Rutter, M.; Moffitt, T.E.; Caspi, A. Gene-environment interplay and psychopathology: Multiple varieties but real effects. J. Child Psychol. Psychiatry 2006, 47, 226–261. [Google Scholar] [CrossRef]
- Hospital, F. Challenges for effective marker-assisted selection in plants. Genetica 2009, 136, 303–310. [Google Scholar] [CrossRef]
- Ogbonnaya, F.C.; Rasheed, A.; Okechukwu, E.C.; Jighly, A.; Makdis, F.; Wuletaw, T. Genome-wide association study for agronomic and physiological traits in spring wheat evaluated in a range of heat prone environments. Theor. Appl. Genet. 2017, 130, 1819–1835. [Google Scholar] [CrossRef]
- Jannink, J.-L.; Bink, M.C.A.M.; Jansen, R.C. Using complex plant pedigrees to map valuable genes. Trends Plant Sci. 2001, 6, 337–342. [Google Scholar] [CrossRef]
- Shen, L.; Courtois, B.; McNally, K.L.; Robin, S.; Li, Z. Evaluation of near-isogenic lines of rice introgressed with QTLs for root depth through marker-aided selection. Theor. Appl. Genet. 2001, 103, 75–83. [Google Scholar] [CrossRef]

| † Env | Period | ‡ Geographic Position | Average Daily Temperature (°C) | Average Daily Humidity (%) | Average Daily Rainfall (mm) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Longit. | Latit. | Altit. ǂ (m.a.s.l.) | Min | Max | Min | Max | |||
| ARL1 | October 2016 | 26.7732 | 28.0046 | 1435 | 12.67 | 27.00 | 28.33 | 48.67 | 1.66 |
| November 2016 | 17.67 | 31.33 | 33.00 | 62.67 | 0.02 | ||||
| December 2016 | 18.67 | 31.67 | 30.00 | 52.00 | 0.03 | ||||
| January 2017 | 17.33 | 31.33 | 26.00 | 46.00 | 0.00 | ||||
| BHM3 | October 2016 | 28.2973 | −28.1628 | 1721 | 11.00 | 26.00 | 33.50 | 92.50 | 1.38 |
| November 2016 | 14.05 | 27.26 | 35.25 | 94.40 | 4.01 | ||||
| December 2016 | 13.63 | 27.94 | 37.29 | 93.77 | 3.11 | ||||
| January 2017 | 13.32 | 26.20 | 41.65 | 94.53 | 4.56 | ||||
| BHM4 | October 2017 | 28.2973 | −28.1628 | 1721 | 7.06 | 24.61 | 27.28 | 90.13 | 1.39 |
| November 2017 | 9.04 | 26.76 | 25.27 | 90.81 | 3.14 | ||||
| December 2017 | 12.23 | 26.66 | 35.84 | 93.01 | 3.69 | ||||
| CLAR5 | October 2016 | 28.5838 | −28.5038 | 1849 | 8.17 | 25.47 | 18.71 | 84.18 | 1.22 |
| November 2016 | 11.37 | 25.38 | 34.19 | 92.64 | 3.45 | ||||
| December 2016 | 12.36 | 27.24 | 33.54 | 92.50 | 3.73 | ||||
| January 2017 | 12.30 | 25.00 | 41.07 | 93.13 | 3.90 | ||||
| HAR7 | October 2016 | 29.11596 | −28.3128 | 1720 | 9.17 | 25.85 | 23.08 | 87.88 | 1.42 |
| November 2016 | 12.09 | 25.42 | 42.99 | 91.43 | 4.43 | ||||
| December 2016 | 13.18 | 27.45 | 42.06 | 89.57 | 4.60 | ||||
| January 2017 | 12.66 | 26.58 | 47.29 | 89.68 | 5.54 | ||||
| HAR8 | October 2017 | 29.11596 | −28.3128 | 1720 | 7.65 | 24.89 | 30.41 | 84.01 | 1.6 |
| November 2017 | 9.76 | 27.05 | 28.93 | 80.81 | 2.73 | ||||
| December 2017 | 11.93 | 26.05 | 42.76 | 89.45 | 6.74 | ||||
| Source of Variation | Degrees of Freedom | Mean Square | F (p-Value) |
|---|---|---|---|
| Parents | 2 | 84.873 | <0.001 |
| Environment | 5 | 5.434 | <0.001 |
| Replications | 4 | 1.861 | |
| Parents × Environment | 9 | 1.580 | 0.017 |
| Residual | 56 | 0.627 | |
| Genotypes | 193 | 2.429 | <0.001 |
| Environment | 5 | 74.182 | <0.001 |
| Replications | |||
| Genotype × Environment | 799 | 1.114 | 0.017 |
| H2 | 0.5414 | ||
| H2 (%) | 54.14 |
| † Genotype/Parent | ǂ Environment | Average PHS Tolerance Score | |||||
|---|---|---|---|---|---|---|---|
| ARL1 | BHM3 | BHM4 | CLAR5 | HAR7 | HAR8 | ||
| PHS tolerance scores of the top ten best-performing DH lines | |||||||
| EF 44 | * | 2 | 1 | 2 | 2 | 1 | 1 |
| EF 15 | * | 2 | 2 | 1 | 1 | 4 | 2 |
| EF 17 | * | 2 | 1 | 1 | 2 | 5 | 2 |
| EF 47 | * | 4 | 1 | 1 | 1 | 1 | 2 |
| TE 21 | 1 | 2 | 2 | 1 | 3 | 3 | 2 |
| TE 37 | 2 | 2 | 2 | 2 | 1 | 4 | 2 |
| TE 62 | 2 | 2 | 4 | 2 | 2 | 1 | 2 |
| TE 73 | 2 | 3 | 2 | 1 | 3 | 1 | 2 |
| TE 122 | 2 | 4 | 1 | 1 | 2 | 1 | 2 |
| TE 127 | 1 | 3 | 2 | 2 | 2 | 4 | 2 |
| PHS tolerance scores of the top five worst-performing DH lines | |||||||
| TE 48 | 4 | 6 | 4 | 2 | 5 | 5 | 4 |
| TE 145 | 5 | 4 | 5 | 4 | 5 | 2 | 4 |
| TE 67 | 5 | 6 | 4 | 4 | 6 | 3 | 5 |
| TE 149 | 5 | 5 | 6 | 5 | 6 | 5 | 5 |
| TE 155 | 4 | 6 | 3 | 4 | 6 | 5 | 5 |
| PHS tolerance scores of the three parental cultivars | |||||||
| Tugela-Dn | 4 | 6 | 5 | 5 | 5 | 5 | 5 |
| Elands | 2 | 3 | 2 | 1 | 2 | 2 | 2 |
| Flamink | * | 5 | 3 | 3 | 4 | 2 | 3.4 |
| Tugela-Dn × Elands Linkage Map | Elands × Flamink Linkage Map | |||||||
|---|---|---|---|---|---|---|---|---|
| LG † | Chrom ǂ | No. of Markers | Map Length (cM) | Marker Density (cM) | Chrom ǂ | No. of Markers | Map Length (cM) | Marker Density (cM) |
| 1 | 1A | 19 | 49.33 | 2.60 | 1A | 67 | 19.96 | 0.30 |
| 2 | 1B | 30 | 90.05 | 3.00 | 1B | 81 | 19.76 | 0.24 |
| 3 | 1D | 25 | 76.50 | 3.06 | 1D | 51 | 21.69 | 0.43 |
| 4 | 2A | 17 | 96.05 | 5.65 | 2A | 83 | 17.76 | 0.21 |
| 5 | 2B | 40 | 75.98 | 1.90 | 2B | 57 | 13.63 | 0.24 |
| 6 | 2D | 11 | 73.56 | 6.69 | 2D | 56 | 12.02 | 0.21 |
| 7 | 3A | 8 | 49.80 | 6.22 | 3A | 79 | 20.89 | 0.26 |
| 8 | 3B | 29 | 58.84 | 2.03 | 3B | 64 | 14.45 | 0.23 |
| 9 | 3D.LG1 | 8 | 28.95 | 3.62 | 3D | 59 | 11.84 | 0.20 |
| 10 | 3D.LG2 | 7 | 60.95 | 8.71 | 4A | 25 | 9.38 | 0.38 |
| 11 | 4A | 8 | 48.35 | 6.04 | 4B | 11 | 13.29 | 1.21 |
| 12 | 4B | 23 | 82.90 | 3.60 | 4D | 53 | 14.07 | 0.27 |
| 13 | 4D | 15 | 74.35 | 4.96 | 5A | 72 | 15.64 | 0.22 |
| 14 | 5A | 14 | 53.35 | 3.81 | 5B | 47 | 13.89 | 0.30 |
| 15 | 5B | 31 | 76.91 | 2.48 | 5D | 67 | 14.47 | 0.22 |
| 16 | 5D | 15 | 34.15 | 2.28 | 6A | 31 | 11.78 | 0.38 |
| 17 | 6A | 20 | 57.27 | 2.86 | 6B | 32 | 15.27 | 0.48 |
| 18 | 6B.LG1 | 31 | 17.90 | 0.58 | 6D | 59 | 11.61 | 0.20 |
| 19 | 6B.LG2 | 27 | 75.70 | 2.80 | 7A | 42 | 12.18 | 0.29 |
| 20 | 6D | 7 | 55.74 | 7.96 | 7B | 40 | 12.97 | 0.32 |
| 21 | 7A | 34 | 126.62 | 3.72 | 7D | 68 | 15.07 | 0.22 |
| 22 | 7B | 39 | 110.63 | 2.84 | ||||
| 23 | 7D | 25 | 42.67 | 1.71 | ||||
| A sub-genome | 120 | 480.77 | 4.01 | A sub-genome | 399 | 107.59 | 0.27 | |
| B sub-genome | 250 | 588.92 | 2.36 | B sub-genome | 332 | 103.25 | 0.31 | |
| D sub-genome | 113 | 446.88 | 3.96 | D sub-genome | 413 | 100.75 | 0.24 | |
| Total Genome | 483 | 1516.57 | 3.87 | Total Genome | 1144 | 311.59 | 0.27 | |
| QTLs Mapped in the Tugela-Dn × Elands DH Mapping Population | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trait | Nearby Marker | Position a | QTL b | Detected Environments c | QTL Effects d | |||||||||||
| ARL1 | BHM3 | BHM4 | CLAR5 | HAR7 | HAR8 | AVE | LOD | Add | PVE (%) | LOD | Add | PVE (%) | ||||
| PHS | 4910940|F|0–22:A > G; 4395011|F|0–8:T > C | 5B (28–29) | QPhs.sgi-5B.3+ | ¥ | ¥ | √ | ¥ | ¥ | √ | ¥ | 3.11 | 0.33 | 10.08 | 3.01 | −0.45 | 11.56 |
| 5582828|F|0–6:C > T; 3024652|F|0–22:C > T | 7B (60–66) | QPhs.sgi-7B.4+ | ¥ | ¥ | ¥ | √ | √ | ¥ | ¥ | 3.10 | −0.52 | 20.30 | 2.85 | −0.49 | 11.89 | |
| 3021324|F|0–20:T > C; 6041508|F|0–33:G > T | 7B (100–101) | QPhs.sgi-7B.2+ | ¥ | √ | √ | ¥ | ¥ | ¥ | ¥ | 2.76 | −0.48 | 10.66 | 2.73 | −0.36 | 11.00 | |
| 7329308 | 7B (11) | QPhs.sgi-7B.1 | ¥ | √ | ¥ | ¥ | ¥ | ¥ | ¥ | 3.21 | 0.51 | 10.97 | ¥ | ¥ | ¥ | |
| 3025468|F|0–18:T > G; 12343039|F|0–26:G > T | 3B (7) | QPhs.sgi-3B+ | ¥ | ¥ | ¥ | √ | ¥ | ¥ | √ | 3.87 | −0.40 | 11.49 | 4.09 | −0.23 | 11.92 | |
| 4394765|F|0–8:C > G; 1684411|F|0–9:G > T | 7A (30–64) | QPhs.sgi-7A+ | ¥ | ¥ | ¥ | ¥ | ¥ | √ | √ | 3.80 | 0.69 | 28.75 | 5.85 | 0.29 | 18.41 | |
| 5050436|F|0–32:T > C | 1A (20) | QPhs.sgi-1A | ¥ | ¥ | √ | ¥ | ¥ | ¥ | ¥ | 4.63 | −0.42 | 14.19 | ¥ | ¥ | ¥ | |
| 1082843|F|0–43:T > C | 1B (22) | QPhs.sgi-1B | ¥ | ¥ | ¥ | ¥ | √ | ¥ | ¥ | 2.56 | −0.53 | 16.97 | ¥ | ¥ | ¥ | |
| 5582507|F|0–13:C > G | 2A (90) | QPhs.sgi-2A | √ | ¥ | ¥ | ¥ | ¥ | ¥ | ¥ | 3.66 | −0.33 | 11.06 | ¥ | ¥ | ¥ | |
| 5324489; 5324039 | 2B (45–53) | QPhs.sgi-2B.2 | ¥ | √ | ¥ | ¥ | ¥ | ¥ | ¥ | 2.92 | −0.49 | 8.15 | ¥ | ¥ | ¥ | |
| 3029334|F|0–25:C > G | 3DLG2 (4) | QPhs.sgi-3DLG2 | ¥ | ¥ | ¥ | ¥ | ¥ | ¥ | √ | 2.52 | 0.27 | 16.03 | ¥ | ¥ | ¥ | |
| 4395594|F|0–22:T > C | 5B (38) | QPhs.sgi-5B.1 | √ | ¥ | ¥ | ¥ | ¥ | ¥ | ¥ | 4.12 | 0.36 | 12.96 | ¥ | ¥ | ¥ | |
| 3064906|F|0–10:T > A | 5B (44) | QPhs.sgi-5B.2 | √ | ¥ | ¥ | ¥ | ¥ | ¥ | ¥ | 3.78 | 0.47 | 22.63 | ¥ | ¥ | ¥ | |
| 1268172|F|0–33:C > G | 6B.LG2 (4) | QPhs.sgi-6BLG2 | ¥ | √ | ¥ | ¥ | ¥ | ¥ | ¥ | 3.46 | −0.53 | 12.78 | ¥ | ¥ | ¥ | |
| QTLs Mapped in the Elands × Flamink DH Mapping Population | ||||||||||||||||
| PHS | 3029487 | 2D (0) | QPhs.sgi-2D | ¥ | ¥ | ¥ | ¥ | √ | ¥ | ¥ | 3.51 | −1.29 | 21.84 | ¥ | ¥ | ¥ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khumalo, T.P.; Hlongoane, T.; Barnard, A.; Tsilo, T.J. Genomic Regions Influencing Preharvest Sprouting Tolerance in Two Doubled-Haploid Wheat Populations (Triticum aestivum L.). Agronomy 2022, 12, 832. https://doi.org/10.3390/agronomy12040832
Khumalo TP, Hlongoane T, Barnard A, Tsilo TJ. Genomic Regions Influencing Preharvest Sprouting Tolerance in Two Doubled-Haploid Wheat Populations (Triticum aestivum L.). Agronomy. 2022; 12(4):832. https://doi.org/10.3390/agronomy12040832
Chicago/Turabian StyleKhumalo, Thobeka Philile, Tsepiso Hlongoane, Annelie Barnard, and Toi John Tsilo. 2022. "Genomic Regions Influencing Preharvest Sprouting Tolerance in Two Doubled-Haploid Wheat Populations (Triticum aestivum L.)" Agronomy 12, no. 4: 832. https://doi.org/10.3390/agronomy12040832
APA StyleKhumalo, T. P., Hlongoane, T., Barnard, A., & Tsilo, T. J. (2022). Genomic Regions Influencing Preharvest Sprouting Tolerance in Two Doubled-Haploid Wheat Populations (Triticum aestivum L.). Agronomy, 12(4), 832. https://doi.org/10.3390/agronomy12040832






