Nitrate-Induced MtCLE34 Gene Lacks the Ability to Reduce Symbiotic Nodule Number and Carries Nonsense Mutation in a Few Accessions of Medicago truncatula
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Bacterial Strains, and Growth Conditions
2.2. RNA Extraction and cDNA Synthesis
2.3. Quantitative Reverse Transcription PCR (qRT-PCR) Analysis
2.4. Molecular Cloning
2.5. GFP Fluorescence Detection
2.6. Statistical Methods and Computer Software
3. Results
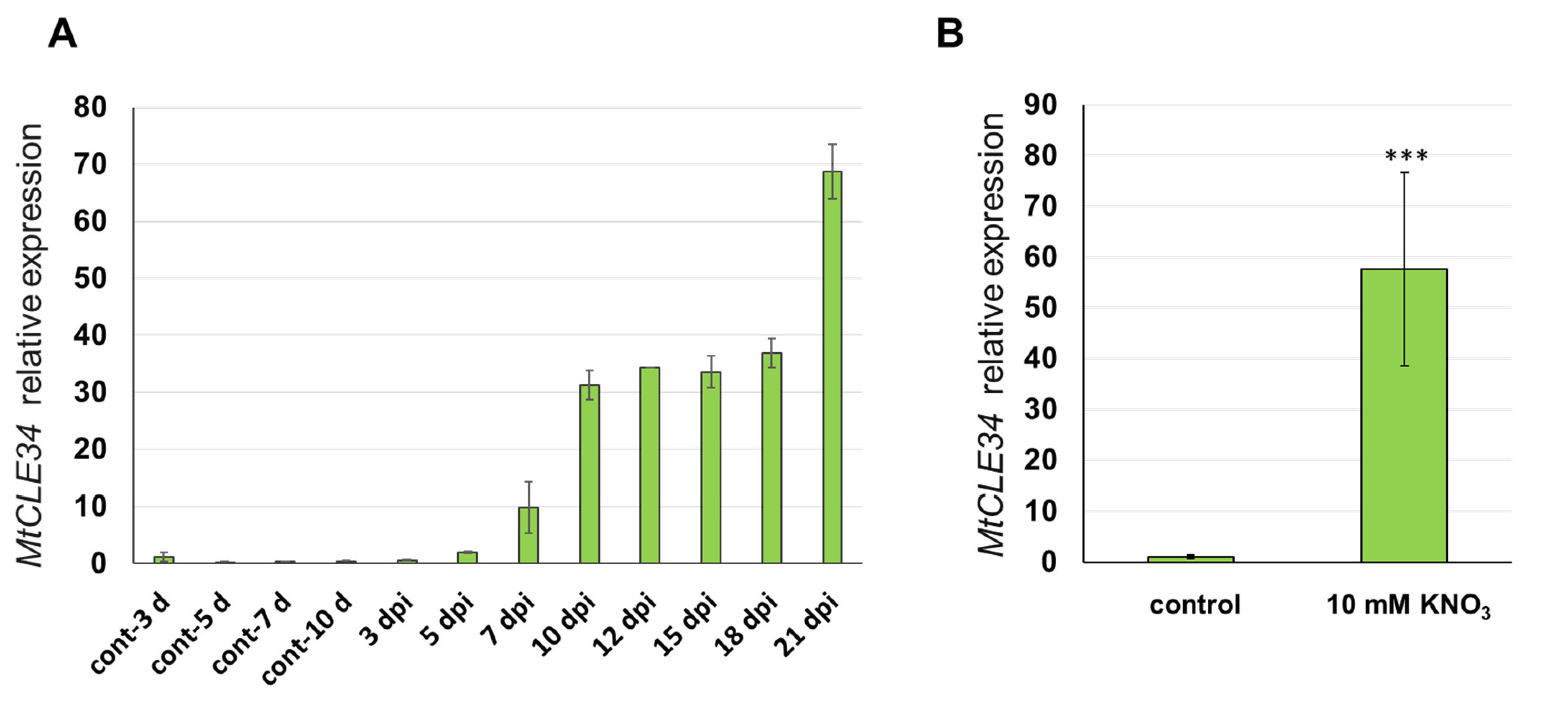
3.1. The Expression Analysis of the MtCLE34 Gene in Response to Nodulation and Nitrate Treatment
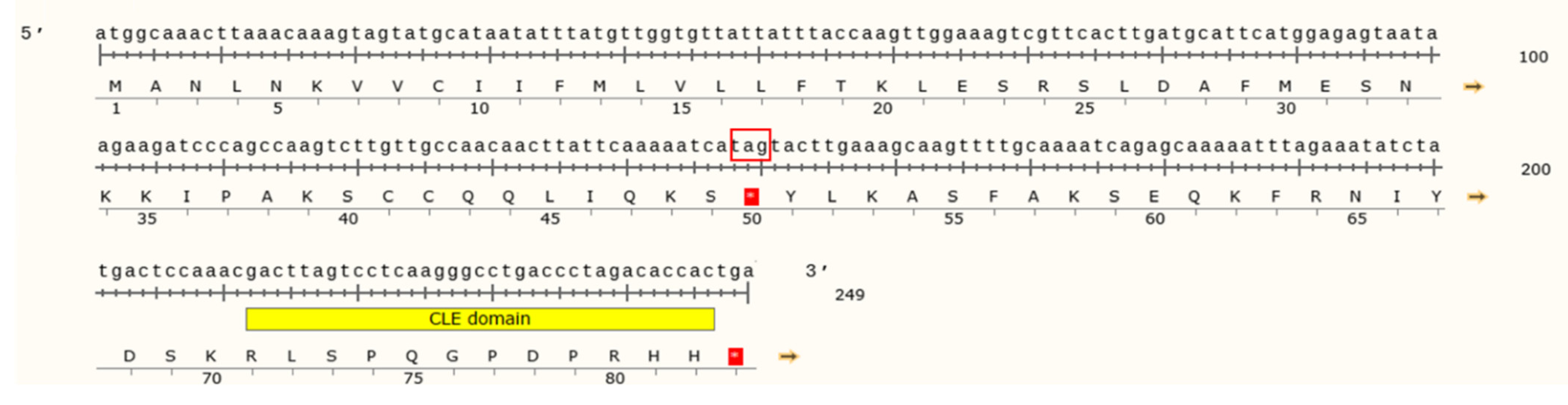
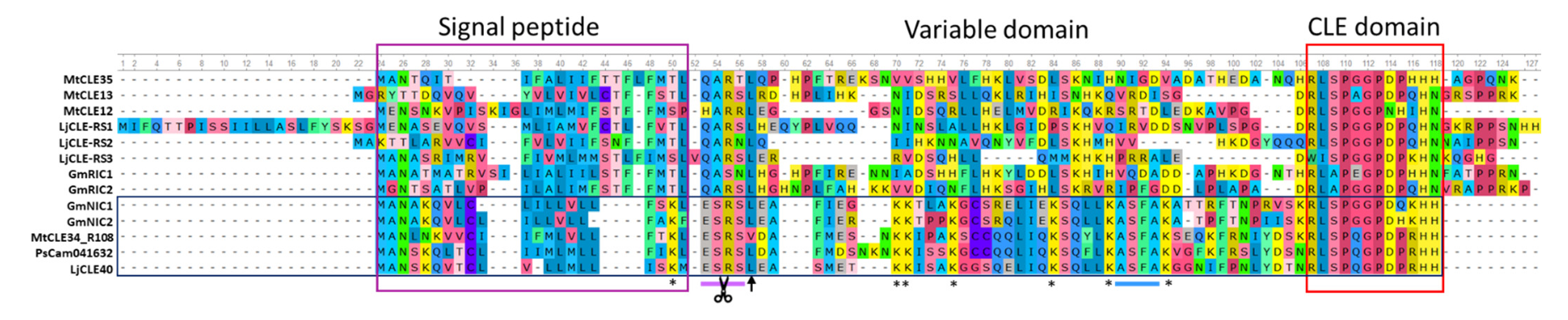
3.2. Characterization of the MtCLE34 Gene in the A17 and R108 Lines
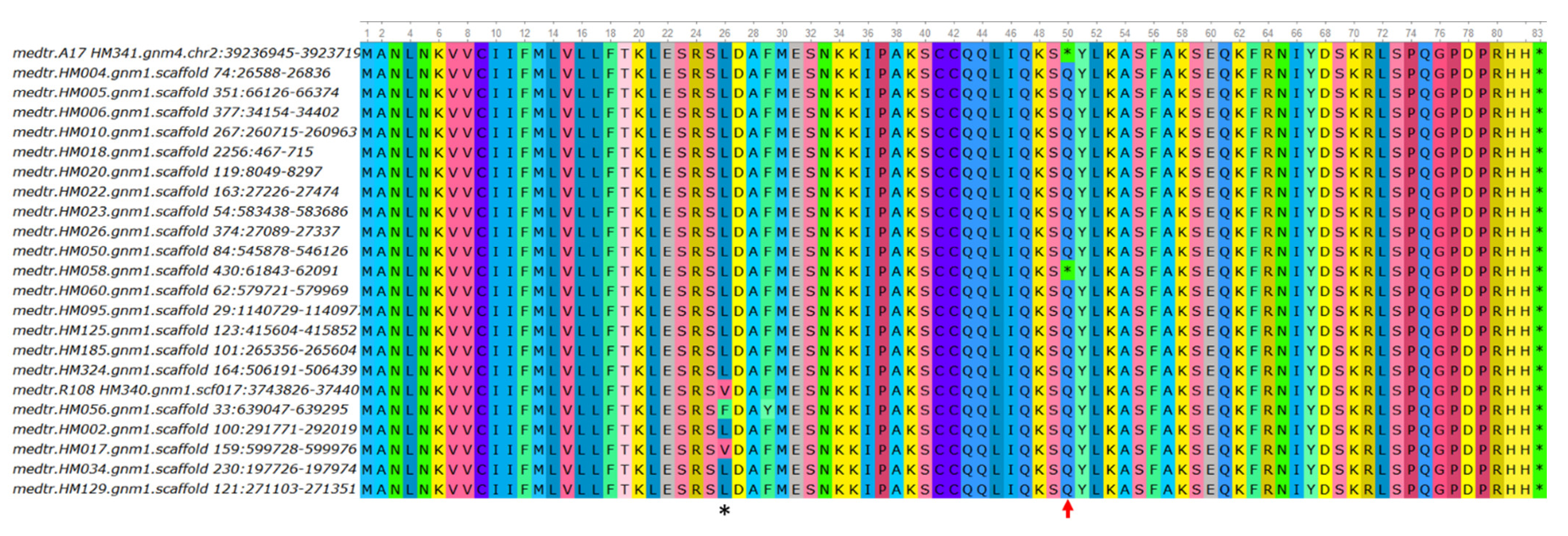
3.3. Natural Variation in the MtCLE34 Gene in M. truncatula Accessions from Medicago Hapmap Project
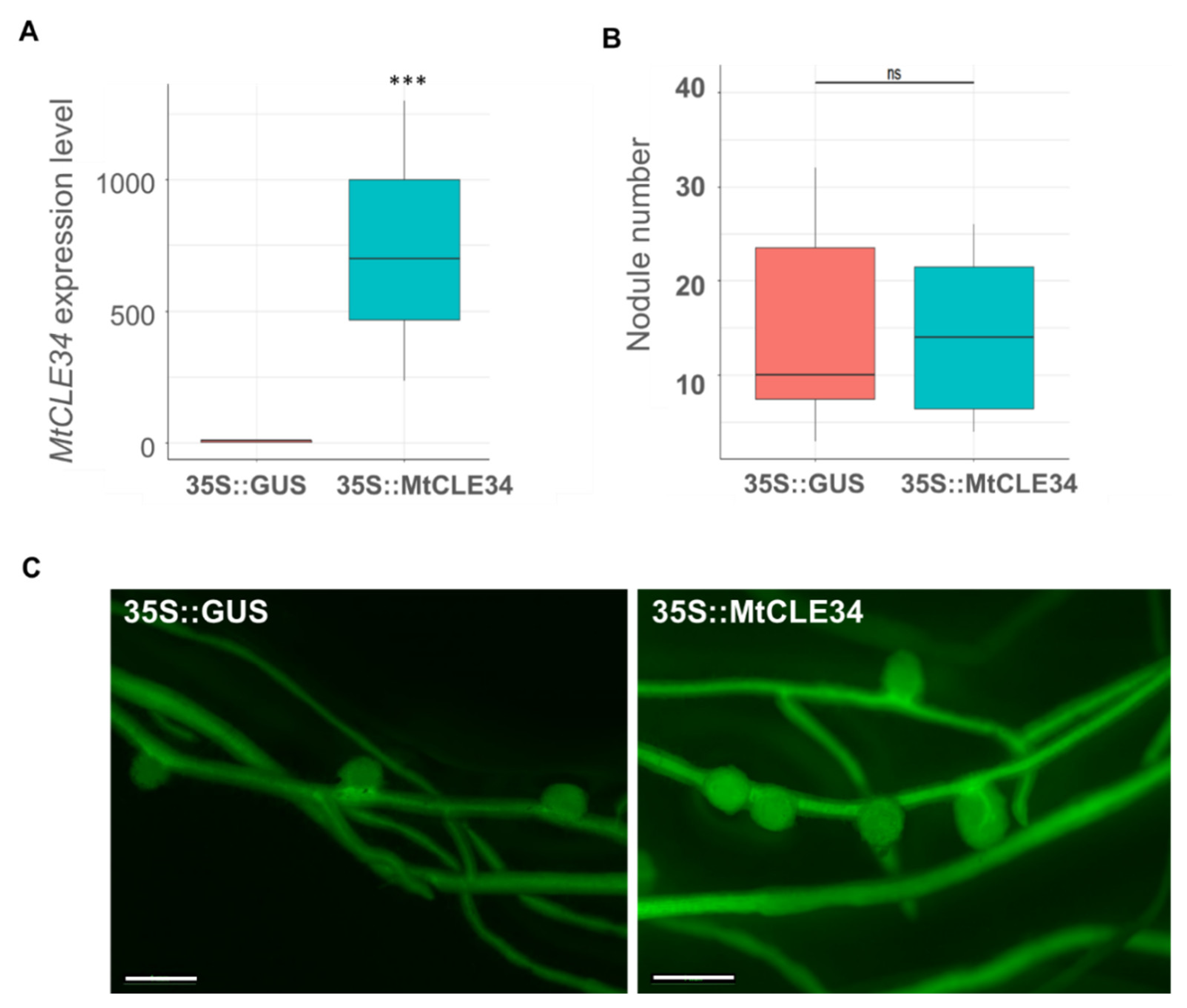
3.4. Overexpression of the MtCLE34 Gene from the R108 Line Does Not Inhibit Nodulation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Okamoto, S.; Tabata, R.; Matsubayashi, Y. Long-Distance Peptide Signaling Essential for Nutrient Homeostasis in Plants. Curr. Opin. Plant Biol. 2016, 34, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Oelkers, K.; Goffard, N.; Weiller, G.F.; Gresshoff, P.M.; Mathesius, U.; Frickey, T. Bioinformatic Analysis of the CLE Signaling Peptide Family. BMC Plant Biol. 2008, 8, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohyama, K.; Shinohara, H.; Ogawa-Ohnishi, M.; Matsubayashi, Y. A Glycopeptide Regulating Stem Cell Fate in Arabidopsis Thaliana. Nat. Chem. Biol. 2009, 5, 578–580. [Google Scholar] [CrossRef] [PubMed]
- Imin, N.; Patel, N.; Corcilius, L.; Payne, R.J.; Djordjevic, M.A. CLE Peptide Tri-Arabinosylation and Peptide Domain Sequence Composition Are Essential for SUNN-Dependent Autoregulation of Nodulation in Medicago Truncatula. New Phytol. 2018, 218, 73–80. [Google Scholar] [CrossRef] [Green Version]
- Hastwell, A.H.; Gresshoff, P.M.; Ferguson, B.J. The Structure and Activity of Nodulation-Suppressing CLE Peptide Hormones of Legumes. Funct. Plant Biol. 2015, 42, 229–238. [Google Scholar] [CrossRef] [Green Version]
- Okamoto, S.; Ohnishi, E.; Sato, S.; Takahashi, H.; Nakazono, M.; Tabata, S.; Kawaguchi, M. Nod Factor/Nitrate-Induced CLE Genes That Drive HAR1-Mediated Systemic Regulation of Nodulation. Plant Cell Physiol. 2009, 50, 67–77. [Google Scholar] [CrossRef] [Green Version]
- Nishida, H.; Handa, Y.; Tanaka, S.; Suzaki, T.; Kawaguchi, M. Expression of the CLE-RS3 Gene Suppresses Root Nodulation in Lotus Japonicus. J. Plant Res. 2016, 129, 909–919. [Google Scholar] [CrossRef] [Green Version]
- Mortier, V.; Den Herder, G.; Whitford, R.; Van de Velde, W.; Rombauts, S.; D’haeseleer, K.; Holsters, M.; Goormachtig, S. CLE Peptides Control Medicago Truncatula Nodulation Locally and Systemically. Plant Physiol. 2010, 153, 222–237. [Google Scholar] [CrossRef] [Green Version]
- Lebedeva, M.; Azarakhsh, M.; Yashenkova, Y.; Lutova, L. Nitrate-Induced CLE Peptide Systemically Inhibits Nodulation in Medicago Truncatula. Plants 2020, 9, 1456. [Google Scholar] [CrossRef]
- Mens, C.; Hastwell, A.H.; Su, H.; Gresshoff, P.M.; Mathesius, U.; Ferguson, B.J. Characterisation of Medicago Truncatula CLE34 and CLE35 in Nitrate and Rhizobia Regulation of Nodulation. New Phytol. 2021, 229, 2525–2534. [Google Scholar] [CrossRef]
- Moreau, C.; Gautrat, P.; Frugier, F. Nitrate-Induced CLE35 Signaling Peptides Inhibit Nodulation through the SUNN Receptor and MiR2111 Repression. Plant Physiol. 2021, 185, 1216–1228. [Google Scholar] [CrossRef]
- Reid, D.E.; Ferguson, B.J.; Gresshoff, P.M. Inoculation-and Nitrate-Induced CLE Peptides of Soybean Control NARK-Dependent Nodule Formation. Mol. Plant Microbe Interact. 2011, 24, 606–618. [Google Scholar] [CrossRef] [Green Version]
- Samorodova, A.P.; Tvorogova, V.E.; Tkachenko, A.A.; Potsenkovskaya, E.A.; Lebedeva, М.А.; Tikhonovich, I.A.; Lutova, L.А. Agrobacterial Tumors Interfere with Nodulation and Demonstrate the Expression of Nodulation-Induced CLE Genes in Pea. J. Plant Physiol. 2018, 221, 94–100. [Google Scholar] [CrossRef]
- Ferguson, B.J.; Li, D.; Hastwell, A.H.; Reid, D.E.; Li, Y.; Jackson, S.A.; Gresshoff, P.M. The Soybean (Glycine max) Nodulation-Suppressive CLE Peptide, GmRIC1, Functions Interspecifically in Common White Bean (Phaseolus vulgaris), but Not in a Supernodulating Line Mutated in the Receptor PvNARK. Plant Biotechnol. J. 2014, 12, 1085–1097. [Google Scholar] [CrossRef]
- Okamoto, S.; Shinohara, H.; Mori, T.; Matsubayashi, Y.; Kawaguchi, M. Root-Derived CLE Glycopeptides Control Nodulation by Direct Binding to HAR1 Receptor Kinase. Nat. Commun. 2013, 4, 2191. [Google Scholar] [CrossRef]
- Soyano, T.; Hirakawa, H.; Sato, S.; Hayashi, M.; Kawaguchi, M. NODULE INCEPTION Creates a Long-Distance Negative Feedback Loop Involved in Homeostatic Regulation of Nodule Organ Production. Proc. Natl. Acad. Sci. USA 2014, 111, 14607–14612. [Google Scholar] [CrossRef] [Green Version]
- Laffont, C.; Ivanovici, A.; Gautrat, P.; Brault, M.; Djordjevic, M.A.; Frugier, F. The NIN Transcription Factor Coordinates CEP and CLE Signaling Peptides That Regulate Nodulation Antagonistically. Nat. Commun. 2020, 11, 3167. [Google Scholar] [CrossRef]
- Nishida, H.; Tanaka, S.; Handa, Y.; Ito, M.; Sakamoto, Y.; Matsunaga, S.; Betsuyaku, S.; Miura, K.; Soyano, T.; Kawaguchi, M.; et al. A NIN-LIKE PROTEIN Mediates Nitrate-Induced Control of Root Nodule Symbiosis in Lotus Japonicus. Nat. Commun. 2018, 9, 499. [Google Scholar] [CrossRef]
- Stanton-Geddes, J.; Paape, T.; Epstein, B.; Briskine, R.; Yoder, J.; Mudge, J.; Bharti, A.K.; Farmer, A.D.; Zhou, P.; Denny, R.; et al. Candidate Genes and Genetic Architecture of Symbiotic and Agronomic Traits Revealed by Whole-Genome, Sequence-Based Association Genetics in Medicago Truncatula. PLoS ONE 2013, 8, e65688. [Google Scholar] [CrossRef] [Green Version]
- Fahraeus, G. The Infection of Clover Root Hairs by Nodule Bacteria Studied by a Simple Glass Slide Technique. J. Gen. Microbiol. 1957, 16, 374–381. [Google Scholar] [CrossRef] [Green Version]
- Azarakhsh, M.; Kirienko, A.; Zhukov, V.; Lebedeva, M.; Dolgikh, E.; Lutova, L. KNOTTED1-LIKE HOMEOBOX 3: A New Regulator of Symbiotic Nodule Development. J. Exp. Bot. 2015, 66, 7181–7195. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Schindelin, J.; Rueden, C.T.; Hiner, M.C.; Eliceiri, K.W. The ImageJ Ecosystem: An Open Platform for Biomedical Image Analysis. Mol. Reprod. Dev. 2015, 82, 518–529. [Google Scholar] [CrossRef] [Green Version]
- Danecek, P.; Bonfield, J.K.; Liddle, J.; Marshall, J.; Ohan, V.; Pollard, M.O.; Whitwham, A.; Keane, T.; McCarthy, S.A.; Davies, R.M.; et al. Twelve Years of SAMtools and BCFtools. Gigascience 2021, 10, giab008. [Google Scholar] [CrossRef]
- Bradbury, P.J.; Zhang, Z.; Kroon, D.E.; Casstevens, T.M.; Ramdoss, Y.; Buckler, E.S. TASSEL: Software for Association Mapping of Complex Traits in Diverse Samples. Bioinformatics 2007, 23, 2633–2635. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (ITOL) v5: An Online Tool for Phylogenetic Tree Display and Annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef]
- Okonechnikov, K.; Golosova, O.; Fursov, M. UGENE team Unipro UGENE: A Unified Bioinformatics Toolkit. Bioinformatics 2012, 28, 1166–1167. [Google Scholar] [CrossRef] [Green Version]
- Almagro Armenteros, J.J.; Tsirigos, K.D.; Sønderby, C.K.; Petersen, T.N.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 5.0 Improves Signal Peptide Predictions Using Deep Neural Networks. Nat. Biotechnol. 2019, 37, 420–423. [Google Scholar] [CrossRef]
- Hastwell, A.H.; de Bang, T.C.; Gresshoff, P.M.; Ferguson, B.J. CLE Peptide-Encoding Gene Families in Medicago Truncatula and Lotus Japonicus, Compared with Those of Soybean, Common Bean and Arabidopsis. Sci. Rep. 2017, 7, 9384. [Google Scholar] [CrossRef] [PubMed]
- Boschiero, C.; Dai, X.; Lundquist, P.K.; Roy, S.; Christian de Bang, T.; Zhang, S.; Zhuang, Z.; Torres-Jerez, I.; Udvardi, M.K.; Scheible, W.-R.; et al. MtSSPdb: The Medicago Truncatula Small Secreted Peptide Database. Plant Physiol. 2020, 183, 399–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pecrix, Y.; Staton, S.E.; Sallet, E.; Lelandais-Brière, C.; Moreau, S.; Carrère, S.; Blein, T.; Jardinaud, M.-F.; Latrasse, D.; Zouine, M.; et al. Whole-Genome Landscape of Medicago Truncatula Symbiotic Genes. Nat. Plants 2018, 4, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.W.; Lee, Y.W.; Lee, S.C.; Hwang, C.H. Nitrate Inhibits Soybean Nodulation by Regulating Expression of CLE Genes. Plant Sci. 2014, 229, 1–9. [Google Scholar] [CrossRef] [PubMed]
| Accession | Depth of Coverage (Allele Depth: TAG/CAG) SNP chr2:39237092 |
|---|---|
| HM341/HM101 (=A17) | 26 (26/0) |
| HM256 | 3 (3/0) |
| HM038 | 1 (1/0) |
| HM058 | 6 (4/2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lebedeva, M.; Dvornikova, K.; Lutova, L. Nitrate-Induced MtCLE34 Gene Lacks the Ability to Reduce Symbiotic Nodule Number and Carries Nonsense Mutation in a Few Accessions of Medicago truncatula. Agronomy 2022, 12, 842. https://doi.org/10.3390/agronomy12040842
Lebedeva M, Dvornikova K, Lutova L. Nitrate-Induced MtCLE34 Gene Lacks the Ability to Reduce Symbiotic Nodule Number and Carries Nonsense Mutation in a Few Accessions of Medicago truncatula. Agronomy. 2022; 12(4):842. https://doi.org/10.3390/agronomy12040842
Chicago/Turabian StyleLebedeva, Maria, Kristina Dvornikova, and Lyudmila Lutova. 2022. "Nitrate-Induced MtCLE34 Gene Lacks the Ability to Reduce Symbiotic Nodule Number and Carries Nonsense Mutation in a Few Accessions of Medicago truncatula" Agronomy 12, no. 4: 842. https://doi.org/10.3390/agronomy12040842
APA StyleLebedeva, M., Dvornikova, K., & Lutova, L. (2022). Nitrate-Induced MtCLE34 Gene Lacks the Ability to Reduce Symbiotic Nodule Number and Carries Nonsense Mutation in a Few Accessions of Medicago truncatula. Agronomy, 12(4), 842. https://doi.org/10.3390/agronomy12040842







