Boron and Zinc Diminish Grey Necrosis Incidence by the Promotion of Desirable Microorganisms on Hazelnut Orchards
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
2.2. Determination of Soil and Foliar Chemical Analyses
2.3. Structural Evaluation of Hazelnut Microbial Communities by Denaturing Gradient Gel of Electrophoresis (DGGE)
2.4. Isolation and Identification of Microorganisms from Kernels
2.4.1. Bacteria Consortium Formulation
2.4.2. Biocontrol Ability of Bacteria Consortium under Greenhouse Conditions
2.5. Microbial Occurrence and Biocontrol Ability Validation Using MALDI-TOF/MS
2.6. Statistical Analyses
3. Results
3.1. Chemical Parameters of Soil and Leaf Tissues
3.2. Microorganism Occurrence in Relation to B and Zn Fertilization
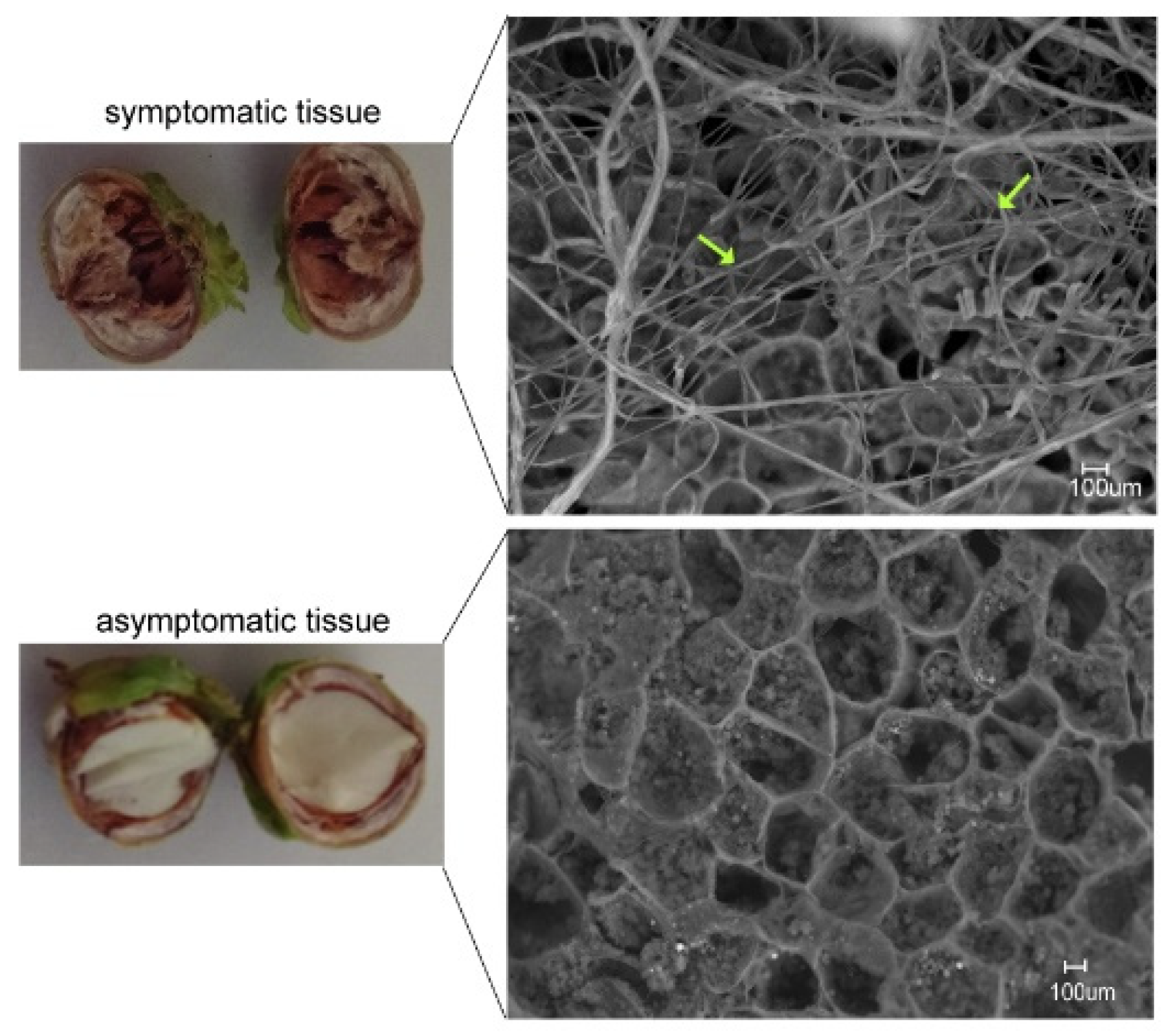
3.3. Microorganisms Involved in Gray Necrosis Symptoms
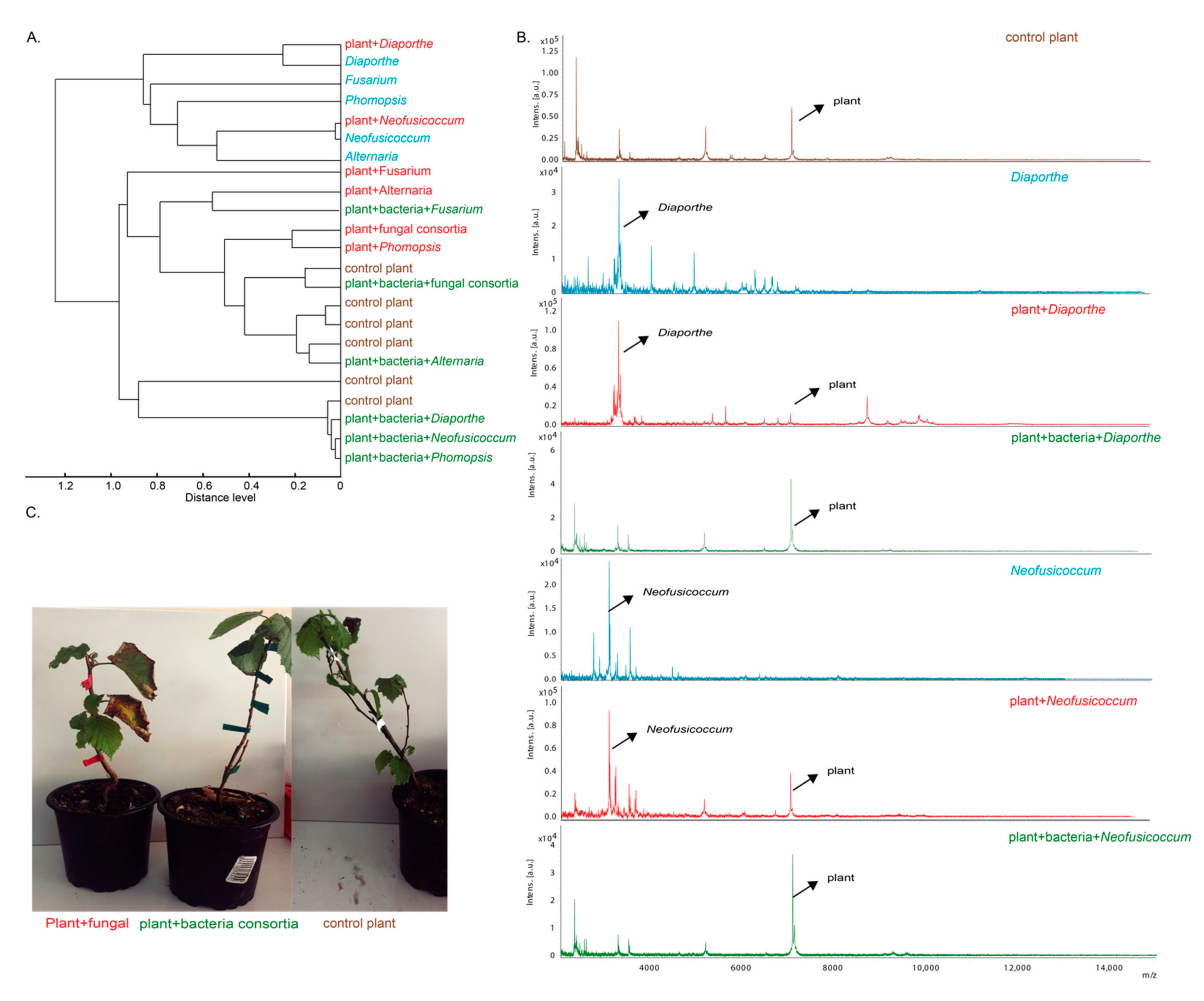
3.4. Biocontrol Capacity of Isolated Bacteria from BCNf Healthy Plants
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Özenç, N.; Bender Özenç, D. Nut traits and nutritional composition of hazelnut (Corylus avellana L.) as influenced by zinc fertilization. J. Sci. Food Agric. 2015, 95, 1956–1962. [Google Scholar] [CrossRef] [PubMed]
- Boccacci, P.; Botta, R. Investigating the origin of hazelnut (Corylus avellana L.) cultivars using chloroplast microsatellites. Genet. Resour. Crop Evol. 2009, 56, 851–859. [Google Scholar] [CrossRef]
- Paredes, C.; Staunton, S.; Duran, P.; Rodriguez, R.; Mora, M.D.L.L. Assessment of the combined effects of beef cattle manure and lemon peel waste on soil-plant biochemical properties and phosphorus uptake by ryegrass. Appl. Soil Ecol. 2021, 169, 104217. [Google Scholar] [CrossRef]
- Contreras, J.G.; Gutierrez, R.G.; Contreras, K.O.; Fuentealba, S.P. First Report of Diaporthe foeniculina Causing Black Tip and Necrotic Spot on Hazelnut Kernel in Chile. Plant Dis. 2020, 104, 975. [Google Scholar] [CrossRef]
- Ellena, M. Avellano europeo: Establecimiento y formación de la estructura productiva. In Instituto de Investigaciones Agropecuarias; Boletín INIA: Temuco, Chile, 2013; pp. 1–202. [Google Scholar]
- ODEPA Ficha Nacional. Oficina de Estudios y Políticas Agrarias. 2021, pp. 1–17. Available online: www.odepa.cl (accessed on 1 March 2022).
- Hanssen, I.M.; Lapidot, M.; Thomma, B.P.H.J. Enfermedades Virales Tomate. Mol. Plant-Microbe Interact. 2010, 23, 539–548. [Google Scholar] [CrossRef] [Green Version]
- Martin, R.R.; Tzanetakis, I.E. Pathogen-Tested Planting Material. Encycl. Agric. Food Syst. 2014, 304–312. [Google Scholar] [CrossRef]
- Durán, P.; Tortella, G.; Sadowsky, M.J.; Viscardi, S.; Barra, P.J.; Mora, M.d.l.L. Engineering Multigenerational Host-Modulated Microbiota Climate Change. Biology 2021, 10, 865. [Google Scholar] [CrossRef]
- Simler, A.B.; Williamson, M.A.; Schwartz, M.W.; Rizzo, D.M. Amplifying plant disease risk through assisted migration. Conserv. Lett. 2019, 12, e12605. [Google Scholar] [CrossRef]
- Fones, H.N.; Gurr, S.J. NOX ious gases and the unpredictability of emerging plant pathogens under climate change. BMC Biol. 2017, 15, 36. [Google Scholar] [CrossRef] [Green Version]
- Duran, P.; Barra, P.J.; Mora, M.D.L.; Morina, F.; Viscardi, S.; Meriño, C. First report of fungal complex causing grey necrosis of hazelnut in Chile. New Dis. Rep. 2020, 42, 7. [Google Scholar] [CrossRef]
- Machado, P.P.; Steiner, F.; Zuffo, A.M.; Machado, R.A. Could the Supply of Boron and Zinc Improve Resistance of Potato to Early Blight? Potato Res. 2018, 61, 169–182. [Google Scholar] [CrossRef]
- Awan, Z.A.; Shoaib, A.; Khan, K.A. Crosstalk of zn in combination with other fertilizers underpins interactive effects and induces resistance in tomato plant against early blight disease. Plant Pathol. J. 2019, 35, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Pérez, C.D.P.; Pozza, E.A.; Pozza, A.A.A.; Elmer, W.H.; Pereira, A.B.; Guimarães, D.D.S.G.; Monteiro, A.C.A.; De Rezende, M.L.V. Boron, zinc and manganese suppress rust on coffee plants grown in a nutrient solution. Eur. J. Plant Pathol. 2020, 156, 727–738. [Google Scholar] [CrossRef]
- Sutulienė, R.; Ragelienė, L.; Duchovskis, P.; Miliauskienė, J. The Effects of Nano-copper, -molybdenum, -boron, and -silica on Pea (Pisum sativum L.) Growth, Antioxidant Properties, and Mineral Uptake. J. Soil Sci. Plant Nutr. 2021, 22, 801–814. [Google Scholar] [CrossRef]
- Hussain, M.; Mehboob, N.; Naveed, M.; Shehzadi, K.; Yasir, T.A. Optimizing Boron Seed Coating Level and Boron-Tolerant Bacteria for Improving Yield and Biofortification of Chickpea. J. Soil Sci. Plant Nutr. 2020, 20, 2471–2478. [Google Scholar] [CrossRef]
- Saadati, S.; Moallemi, N.; Mortazavi, S.M.H.; Seyyednejad, S.M. Effects of zinc and boron foliar application on soluble carbohydrate and oil contents of three olive cultivars during fruit ripening. Sci. Hortic. 2013, 164, 30–34. [Google Scholar] [CrossRef]
- Keshavarz, K.; Vahdati, K.; Samar, M.; Azadegan, B.; Brown, P.H. Foliar application of zinc and boron improves walnut vegetative and reproductive growth. Horttechnology 2011, 21, 181–186. [Google Scholar] [CrossRef] [Green Version]
- Spann, T.M.; Schumann, A.W. The Role of Plant Nutrients in Disease Development with Emphasis on Citrus and Huanglongbing. Proc. Fla. State Horicult. Sci. 2009, 122, 169–171. [Google Scholar]
- Meriño-Gergichevich, C.; Luengo-Escobar, A.; Alarcón, D.; Reyes-Díaz, M.; Ondrasek, G.; Morina, F.; Ogass, K. Combined Spraying of Boron and Zinc During Fruit Set and Premature Stage Improves Yield and Fruit Quality of European Hazelnut cv. Tonda di Giffoni. Front. Plant Sci. 2021, 12, 984. [Google Scholar] [CrossRef]
- Maziah, M.; Zuraida, A.R.; Halimi, M.S.; Zulkifli, H.S.; Sreeramanan, S. Influence of boron on the growth and biochemical changes in plant growth promoting rhizobacteria (PGPR) inoculated banana plantlets. World J. Microbiol. Biotechnol. 2010, 26, 933–944. [Google Scholar] [CrossRef]
- Franczuk, J.; Rosa, R.; Zaniewicz-Bajkowska, A.; Słonecka, D. Effects of boron application and treatment with effective microorganisms on the growth, yield and some quality attributes of broccoli. J. Elem. 2019, 24, 1335–1348. [Google Scholar] [CrossRef]
- Hussain Shah, A.; Naz, I.; Ahmad, H.; Nasreen Khokhar, S.; Khan, K. Impact of Zinc Solubilizing Bacteria on Zinc Contents of Wheat. J. Agric. Environ. Sci 2016, 16, 449–454. [Google Scholar] [CrossRef]
- Tipple, B.J.; Berke, M.A.; Doman, C.E.; Khachaturyan, S.; Ehleringer, J.R. Leaf-wax n-alkanes record the plant-water environment at leaf flush. Proc. Natl. Acad. Sci. USA 2013, 110, 2659–2664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of the degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Warncke, D.; Brown, J. Potassium and Other Basic Cations. Recomm. Chem. Soil Test Proced. North Cent. Reg. NCR 1998, 1001, 31–33. [Google Scholar]
- Bertsch, P.; Bloom, P. Aluminum. In Methods of Soil Analysis; 1996; pp. 526–527. Available online: https://acsess.onlinelibrary.wiley.com/doi/abs/10.2136/sssabookser5.3.c18 (accessed on 1 March 2022).
- Sadzawka, A.; Carrasco, M.; Grez, R.; Mora, M.; Flores, H.; Neaman, A. Metodos de análisis recomendados para lo suelos de Chile. Int. J. Autom. Comput. 2006, 163, 1–14. [Google Scholar]
- Durán, P.; Acuña, J.J.; Jorquera, M.A.; Azcón, R.; Paredes, C.; Rengel, Z.; de la Luz Mora, M. Endophytic bacteria from selenium-supplemented wheat plants could be useful for plant-growth promotion, biofortification and Gaeumannomyces graminis biocontrol in wheat production. Biol. Fertil. Soils 2014, 50, 983–990. [Google Scholar] [CrossRef]
- Durán, P.; Jorquera, M.; Viscardi, S.; Carrion, V.J. Screening and Characterization of Potentially Suppressive Soils against Gaeumannomyces graminis under Extensive Wheat Cropping by Chilean Indigenous Communities. Front. Microbiol. 2017, 8, 1152. [Google Scholar] [CrossRef]
- Duran, P.; Tortella, G.; Viscardi, S.; Barra, P.J.; Carrion, V.J.; Mora, M.D.L.L.; Pozo, M.J. Microbial Community Composition in Take-All Suppressive Soils. Front. Microbiol. 2018, 9, 2198. [Google Scholar] [CrossRef]
- Méndez, I.; Fallard, A.; Soto, I.; Tortella, G.; Mora, M.D.L.L.; Valentine, A.J.; Barra, P.J.; Duran, P. Efficient biocontrol of gaeumannomyces graminis var. Tritici in wheat: Using bacteria isolated from suppressive soils. Agronomy 2021, 11, 2008. [Google Scholar] [CrossRef]
- Durán, P.; Barra, P.J.; Jorquera, M.A.; Viscardi, S.; Fernandez, C.; Paz, C.; Mora, M.D.L.L.; Bol, R. Occurrence of Soil Fungi in Antarctic Pristine Environments. Front. Bioeng. Biotechnol. 2019, 7, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, F.; Babalola, O.O.; Tak, H.I. Potential of MALDI-TOF mass spectrometry as a rapid detection technique in plant pathology: Identification of plant-associated microorganisms. Anal. Bioanal. Chem. 2012, 404, 1247–1255. [Google Scholar] [CrossRef]
- Santos, C.; Ventura, J.A.; Lima, N. New Insights for Diagnosis of Pineapple Fusariosis by MALDI-TOF MS Technique. Curr. Microbiol. 2016, 73, 206–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sagar, R.; Sharma, G. Measurement of alpha divers ity using Simpson index (1/λ): The jeopardy. Environ. Skept. Crit. 2012, 1, 23–24. [Google Scholar]
- Mora, M.L.; Alfaro, M.A.; Jarvis, S.C.; Demanet, R.; Cartes, P. Soil aluminium availability in Andisols of southern Chile and its effect on forage production and animal metabolism. Soil Use Manag. 2006, 22, 95–101. [Google Scholar] [CrossRef]
- Belisario, A.; Santori, A. Gray necrosis of hazelnut fruit: A fungal disease causing fruit drop. Acta Hortic. 2009, 845, 501–506. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Venturi, V. Synergisms between microbial pathogens in plant disease complexes: A growing trend. Front. Plant Sci. 2015, 6, 385. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Luo, L.; Tan, X.; Kong, X.; Yang, J.; Wang, D.; Zhang, D.; Jin, D.; Liu, Y. Pumpkin powdery mildew disease severity influences the fungal diversity of the phyllosphere. PeerJ. 2018, 6, e4559. [Google Scholar] [CrossRef] [Green Version]
- Trivedi, P.; He, Z.; Van Nostrand, J.D.; Albrigo, G.; Zhou, J.; Wang, N. Huanglongbing alters the structure and functional diversity of microbial communities associated with citrus rhizosphere. ISME J. 2012, 6, 363–383. [Google Scholar] [CrossRef] [Green Version]
- Liew, Y.A.; Omar, S.S.; Husni, M.H.A.; Zainal, A.M.A.; Ashikin, P.N. Effects of Foliar Applied Copper and Boron on Fungal Diseases and Rice Yield on Cultivar MR219. Pertanika J. Trop. Agric. Sci. 2012, 35, 339–349. [Google Scholar]
- Qin, G.; Zong, Y.; Chen, Q.; Hua, D.; Tian, S. Inhibitory effect of boron against Botrytis cinerea on table grapes and its possible mechanisms of action. Int. J. Food Microbiol. 2010, 138, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Mazzali de Ilja, R. Notas sobre Bioseguridad Nivel 3 de Bioseguridad. Biosecurity Level-3. Rev. Soc. Venez. Microbiol. 2004, 24, 105–107. [Google Scholar]
- Duffy, B.K.; Défago, G. Zinc Improves Biocontrol of Fusarium Crown and Root Rot of Tomato by Pseudomonas fluorescens and Represses the Production of Pathogen Metabolites Inhibitory to Bacterial Antibiotic Biosynthesis. Phytopathology 1997, 87, 1250–1257. [Google Scholar] [CrossRef] [Green Version]
- Simoglou, K.B.; Dordas, C. Effect of foliar applied boron, manganese and zinc on tan spot in winter durum wheat. Crop Prot. 2006, 25, 657–663. [Google Scholar] [CrossRef]
- Battilani, P.; Chiusa, G.; Arciuolo, R.; Somenzi, M.; Fontana, M.; Castello, G.; Spigolon, N. Diaporthe as the main cause of hazelnut defects in the Caucasus region. Phytopathol. Mediterr. 2015, 54, 241–252. [Google Scholar] [CrossRef]
- Gao, H.; Pan, M.; Tian, C.; Fan, X. Cytospora and Diaporthe Species Associated With Hazelnut Canker and Dieback in Beijing, China. Front. Cell. Infect. Microbiol. 2021, 11, 664366. [Google Scholar] [CrossRef]
- Guerrero, J.C.; Pérez, S.F.; Ferrada, E.Q.; Cona, L.Q.; Bensch, E.T. Phytopathogens of hazelnut (Corylus avellana L.) in southern Chile. Acta Hortic. 2014, 1052, 269–274. [Google Scholar] [CrossRef]
- Ko, Y.J.; Kim, J.S.; Kim, K.D.; Jeun, Y.C. Microscopical observation of inhibition-behaviors against Diaporthe citri by pre-treated with Pseudomonas putida strain THJ609-3 on the leaves of citrus plants. J. Microbiol. 2014, 52, 879–883. [Google Scholar] [CrossRef]
- Hernández-León, R.; Rojas-Solís, D.; Contreras, M.; Orozco-Mosqueda, M.D.C.; Macías-Rodríguez, L.I.; de la Cruz, H.R.; Valencia-Cantero, E.; Santoyo, G. Characterization of the antifungal and plant growth-promoting effects of diffusible and volatile organic compounds produced by Pseudomonas fluorescens strains. Biol. Control 2015, 81, 83–92. [Google Scholar] [CrossRef]
- Pusey, P.L.; Stockwell, V.O.; Reardon, C.L.; Smits, T.H.M.; Duffy, B. Antibiosis activity of Pantoea agglomerans biocontrol strain E325 against Erwinia amylovora on apple flower stigmas. Phytopathology 2011, 101, 1234–1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trotel-Aziz, P.; Couderchet, M.; Biagianti, S.; Aziz, A. Characterization of new bacterial biocontrol agents Acinetobacter, Bacillus, Pantoea and Pseudomonas spp. mediating grapevine resistance against Botrytis cinerea. Environ. Exp. Bot. 2008, 64, 21–32. [Google Scholar] [CrossRef]
- Cañamás, T.P.; Viñas, I.; Usall, J.; Torres, R.; Anguera, M.; Teixidó, N. Control of postharvest diseases on citrus fruit by preharvest applications of biocontrol agent Pantoea agglomerans CPA-2: Part II. Effectiveness of different cell formulations. Postharvest Biol. Technol. 2008, 49, 96–106. [Google Scholar] [CrossRef]
- Jiang, L.; Jeong, J.C.; Lee, J.S.; Park, J.M.; Yang, J.W.; Lee, M.H.; Choi, S.H.; Kim, C.Y.; Kim, D.H.; Kim, S.W.; et al. Potential of Pantoea dispersa as an effective biocontrol agent for black rot in sweet potato. Sci. Rep. 2019, 9, 16354. [Google Scholar] [CrossRef] [Green Version]
- Morales, H.; Sanchis, V.; Usall, J.; Ramos, A.J.; Marín, S. Effect of biocontrol agents Candida sake and Pantoea agglomerans on Penicillium expansum growth and patulin accumulation in apples. Int. J. Food Microbiol. 2008, 122, 61–67. [Google Scholar] [CrossRef]
- Mohamed, B.F.F.; Sallam, N.M.A.; Alamri, S.A.M.; Abo-Elyousr, K.A.M.; Mostafa, Y.S.; Hashem, M. Approving the biocontrol method of potato wilt caused by Ralstonia solanacearum (Smith) using Enterobacter cloacae PS14 and Trichoderma asperellum T34. Egypt. J. Biol. Pest Control 2020, 30, 61. [Google Scholar] [CrossRef]
- Guo, D.J.; Singh, R.K.; Singh, P.; Li, D.P.; Sharma, A.; Xing, Y.X.; Song, X.P.; Yang, L.T.; Li, Y.R. Complete Genome Sequence of Enterobacter roggenkampii ED5, a Nitrogen Fixing Plant Growth Promoting Endophytic Bacterium With Biocontrol and Stress Tolerance Properties, Isolated From Sugarcane Root. Front. Microbiol. 2020, 11, 2270. [Google Scholar] [CrossRef]
- Anzalone, A.; Di Guardo, M.; Bella, P.; Ghadamgahi, F.; Dimaria, G.; Zago, R.; Cirvilleri, G.; Catara, V. Bioprospecting of Beneficial Bacteria Traits Associated With Tomato Root in Greenhouse Environment Reveals That Sampling Sites Impact More Than the Root Compartment. Front. Plant Sci. 2021, 12, 637582. [Google Scholar] [CrossRef]
- D’rose, V.; Johny, T.K.; Bhat, S. Comparative analysis of metagenomic DNA extraction methods from gut microbiota of zebrafish (Danio rerio) for downstream next-generation sequencing. J. Appl. Biol. Biotechnol. 2019, 7, 11–15. [Google Scholar] [CrossRef] [Green Version]
- Solís-oba, M.M.; Villegas-aparicio, Y.; Solís-oba, A. Cocksfood forage yield inoculated with PGPB bacteria. Rev. Mex. Cienc. Agríc. 2020, 11, 129–139. [Google Scholar] [CrossRef] [Green Version]
- Ponpandian, L.N.; Rim, S.O.; Shanmugam, G.; Jeon, J.; Park, Y.H.; Lee, S.K.; Bae, H. Phylogenetic characterization of bacterial endophytes from four Pinus species and their nematicidal activity against the pine wood nematode. Sci. Rep. 2019, 9, 12457. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.; Back, C.G.; Choi, H.; Cho, W.K. Comparative microbiome study of mummified peach fruits by metagenomics and metatranscriptomics. Plants 2020, 9, 1052. [Google Scholar] [CrossRef] [PubMed]

| Application | Microbial Group | Primer Set | Sequence | Reference |
|---|---|---|---|---|
| DGGE | Total fungi | fNS1/rNS8 NS7-GC/f1Ra | 5′-GTA GTC ATA TGC TTG TCT C-3′/5′-TCC GCA GGT TCA CCT ACG GA-3′ 5′-GAG GCA ATA ACA GGT CTG TGA TGC-3, GC-clamp: CGC CCG GGG CGC GCC CCG GGC GGG GCG GGG GCA CGG GGG/5′-CTT TTA CTT CCT CTA AAT GAC C-3′ | [32] |
| DGGE | Total bacteria | EUBf933-GC/EUBr1387 | 5′-GCA CAA GCG GTG GAG CAT GTG G-3′/5′-GCC CGG GAA CGT ATT CAC CG-3′ | [32] |
| Microbial identification | fungi | fITS9/ITS4 | 5′-GAACGCAGCRAAIIGYG-3′/5′-TCCTCCGCTTATTGATATGC-3′ | [35] |
| Microbial identification | bacteria | 27F/1492r | 5′-AGA GTT TGATCC TGG CTC AG-3′/5′-TAC GGY TAC CTT GTT ACG ACT T-3′ | [34] |
| Soil Parameter | Soil Content |
|---|---|
| N (mg kg−1) | 23.0 (±2.0) |
| P (mg kg−1) | 7.0 (±0.6) |
| K (mg kg−1) | 203.0 (±8.3) |
| pH (H2O) | 6.05 (±0.89) |
| Organic matter (%) | 21.0 (±1.0) |
| K (cmol + kg−1) | 0.52 (±0.03) |
| Na (cmol + kg−1) | 0.04 (±0.00) |
| Ca (cmol + kg−1) | 6.41 (±1.01) |
| Mg (cmol + kg−1) | 2.37 (±0.09) |
| Al (cmol + kg−1) | 0.03 (±0.00) |
| Al sat (%) † | 0.32 (±0.00) |
| CICE (cmol + kg−1) | 9.37 (±1.14) |
| Σ basis (cmol + kg−1) | 9.34 (±1.13) |
| B (mg kg−1) | 0.39 (±0.01) |
| Zn (mg kg−1) | 0.69 (±0.02) |
| Cu (mg kg−1) | 6.65 (±0.89) |
| Fe (mg kg−1) | 61 (±2.03) |
| Mn (mg kg−1) | 3.28 (±0.09) |
| S (mg kg−1) | 27.00 (±1.03) |
| Ext. Al (mg kg−1) | 967.0 (±23.1) |
| pH (CaCl2) | 5.17 (±0.03) |
| BCNc | BCNf | |
|---|---|---|
| B (mg kg−1) | 19.8 ± 3.04 | 70.6 ± 13.4 * |
| Zn (mg kg−1) | 34.4 ± 8.71 | 136 ± 31.5 * |
| Ca (mg kg−1) | 0.66 ± 0.04 | 0.62 ± 0.03 |
| Mg (mg kg−1) | 0.19 ± 0.01 | 0.17 ± 0.00 |
| Na (mg kg−1) | 0.02 ± 0.00 | 0.02 ± 0.00 |
| K (mg kg−1) | 1.14 ± 0.06 | 1.15 ± 0.03 |
| (A) | |||
|---|---|---|---|
| Parameter | Fungi | Bacteria | |
| B | 0.001 ** | 0.001 ** | |
| Zn | 0.001 ** | 0.002 ** | |
| Ca | 0.435 | 0.462 | |
| Mg | 0.190 | 0.400 | |
| Na | 0.535 | 0.796 | |
| K | 0.951 | 0.945 | |
| (B) | |||
| Corr. | Fungi | Correlation | Bacteria |
| 0.737 | B | 0.729 | B |
| 0.668 | B, Zn | 0.673 | B, Zn |
| 0.659 | B, Zn, Mg | 0.592 | B, Zn, Ca |
| 0.654 | B, Zn, Ca, Mg | 0.580 | Zn |
| 0.609 | B, Mg | 0.578 | B, Zn, Mg |
| 0.598 | B, Zn, Ca | 0.556 | B, Zn, Ca, Mg |
| 0.591 | B, Zn, Mg, Ca | 0.550 | B, Zn, K |
| 0.564 | Zn | 0.521 | B, Zn, Na |
| 0.554 | B, Zn, K | 0.506 | B, Zn, Ca, K |
| 0.551 | B, Ca, Mg | 0.504 | B, Ca |
| Isolate | Closest Relatives or Cloned Sequences (Accession n°) † | Similarity | Accession N° |
|---|---|---|---|
| Fungi from BCNc | |||
| Alternaria sp. HAB1.4 | Alternaria alternata fungi colonizing after dieback (MF509751) | 100% | MF629825 |
| Phomopsis sp. HAB2.1 | Phomopsis sp. dieback of Pinus nigra (KJ482539) | 99% | MF629824 |
| Neofusicoccum sp. HAB2.2 | Neofusicoccum arbuti stem cancer and dieback on blueberries (EU856062) | 99% | MF629823 |
| Diaporthe sp. HAB2.3 | Diaporthe rudis anamoph: Phoma ridis phytopatogen (KY964222) | 99% | MF629822 |
| Fusarium sp. HAB2.4 | Fusarium sporotrichioides dieback in Abies alba (KU516465) | 99% | MF629821 |
| Diaporthe sp. HAB3.1 | Diaporthe australafricana dieback in kiwi fruit (KU679315) | 99% | MF629820 |
| Bacteria from BCNc | |||
| Enterobacter sp. BAG1.2 | Enterobacter sp. phytate degrading bacteria, PGPR (JQ864388) | 99% | MF623059 |
| Bacteria from BCNf | |||
| Pseudomonas sp. BAB1.2 | Pseudomonas poae biocontrol against soybean cyst nematodes (KT695820) | 99% | MF623063 |
| Ewingella sp. BAB1.3 | Ewingella americana endophytic bacteria with biocontrol activity (KY203802) | 99% | MF623062 |
| Pantoea sp. BAB2.1 | Pantoea agglomerans biocontrol of fire blight of apple and pear (KY357286) | 99% | MF623061 |
| Enterobacter sp. BAG1.3 | Enterobacter sp. biocontrol against Rhizoctonia solani (KM589029) | 99% | MF623058 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duran, P.; Barra, P.J.; Mora, M.d.l.L.; Nunes-Nesi, A.; Merino-Gergichevich, C. Boron and Zinc Diminish Grey Necrosis Incidence by the Promotion of Desirable Microorganisms on Hazelnut Orchards. Agronomy 2022, 12, 868. https://doi.org/10.3390/agronomy12040868
Duran P, Barra PJ, Mora MdlL, Nunes-Nesi A, Merino-Gergichevich C. Boron and Zinc Diminish Grey Necrosis Incidence by the Promotion of Desirable Microorganisms on Hazelnut Orchards. Agronomy. 2022; 12(4):868. https://doi.org/10.3390/agronomy12040868
Chicago/Turabian StyleDuran, Paola, Patricio Javier Barra, María de la Luz Mora, Adriano Nunes-Nesi, and Cristian Merino-Gergichevich. 2022. "Boron and Zinc Diminish Grey Necrosis Incidence by the Promotion of Desirable Microorganisms on Hazelnut Orchards" Agronomy 12, no. 4: 868. https://doi.org/10.3390/agronomy12040868






