Salt Tolerance Strategies of Nitraria tangutorum Bobr. and Elaeagnus angustifolia Linn. Determine the Inoculation Effects of Microorganisms in Saline Soil Conditions
Abstract
:1. Introduction
2. Materials and Methods
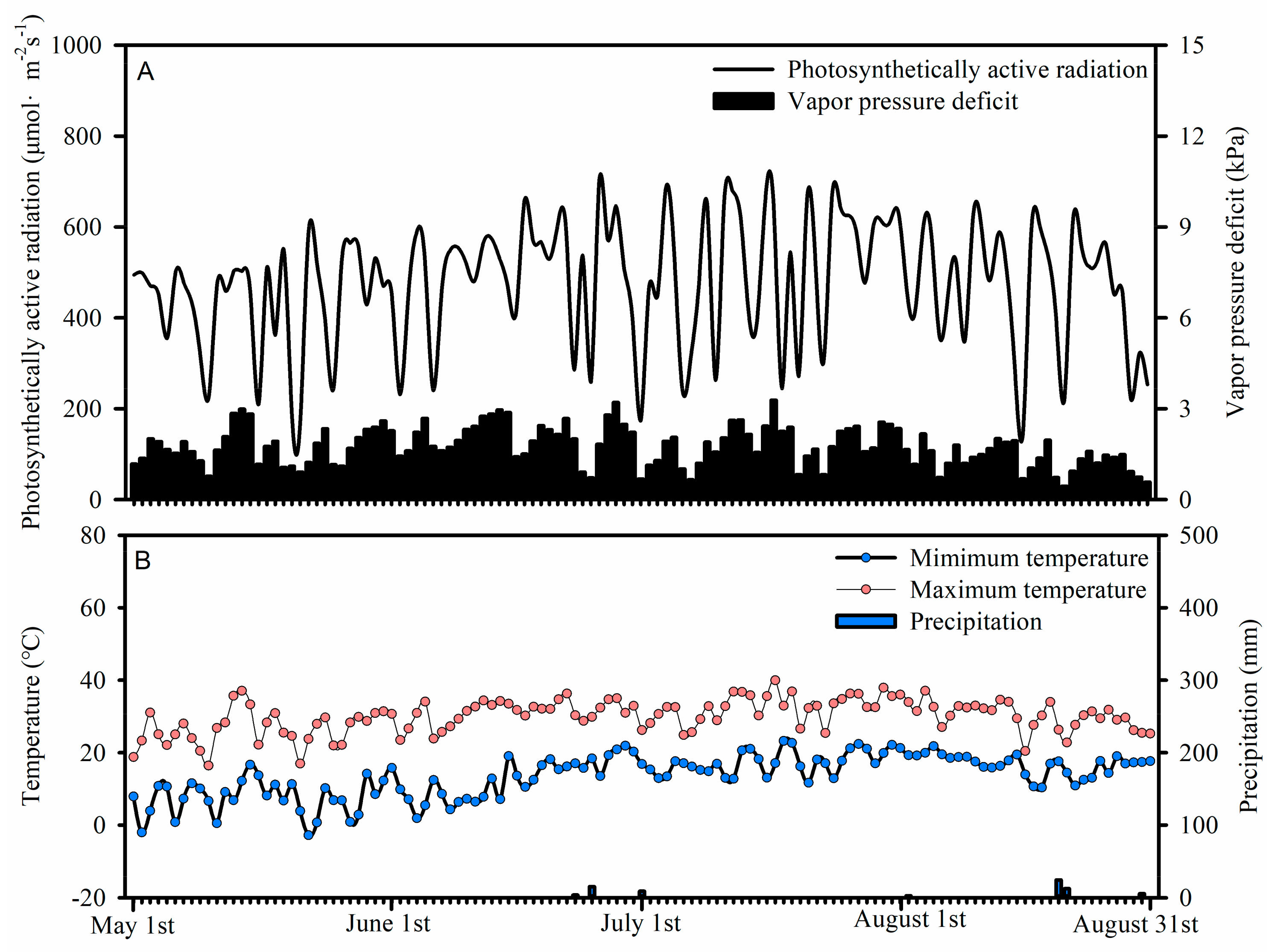
2.1. Study Site Description
2.2. Soil Sampling
2.3. Plant Materials
2.4. Microbial Inoculum and Experimental Design
2.5. Parameters Measurement
2.5.1. Measurement of Plant Biomass, AMF Colonization Rate, and Succulence Degree
2.5.2. Measurement of Mineral Nutrients Concentration
2.5.3. Measurement of Proline, Soluble Sugar, and Glycine Betaine Concentration
2.6. Calculation of the Selective Transportation Coefficient
2.7. Statistical Analysis
3. Results
3.1. Effects of AMF and PGPR Inoculation on Biomass Accumulation
3.2. Effects of AMF and PGPR Inoculation on Succulence Degree and AMF Colonization
3.3. Effects of AMF and PGPR Inoculation on N and P Concentrations
3.4. Effects of AMF and PGPR Inoculation on K+, Ca2+, and Na+ Concentrations
3.5. Effects of AMF and PGPR Inoculation on K+/Na+ Ratio, Ca2+/Na+ Ratio and STC
3.6. Effects of AMF and PGPR Inoculation on Compatible Osmolyte Concentrations
4. Discussion
4.1. “Plant-Microbe Specificity” Leads to Different Biomass Accumulation Responses between N. tangutorum and E. angustifolia Seedlings Inoculated with AMF and PGPR
4.2. Distinctive Strategies of Na+ Acquisition and Distribution in N. tangutorum and E. angustifolia Affect the Salt Tolerance Strategies and Succulence Degree after Microbial Inoculations
4.3. Mechanisms of Nutrient Acquisition and Ion Homeostasis in Organ-Level Are Different between N. tangutorum and E. angustifolia Seedlings with Microbial Inoculation
4.4. Inorganic Ions and Organic Solutes Play Different Roles in the Osmoregulation of N. tangutorum and E. angustifolia Seedlings
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saidi, S.; Cherif-Silini, H.; Bouket, A.C.; Silini, A.; Eshelli, M.; Luotakova, L.; Alenezi, F.N.; Belbahri, L. Improvement of Medicago sativa crops productivity by the co-inoculation of Sinorhizobium meliloti–Actinobacteria under salt stress. Curr. Microbiol. 2021, 78, 1344–1357. [Google Scholar] [CrossRef] [PubMed]
- Qian, T.; Tsunekawa, A.; Peng, F.; Masunaga, T.; Wang, T.; Li, R. Derivation of salt content in salinized soil from hyperspectral reflectance data: A case study at Minqin Oasis, Northwest China. J. Arid Land 2019, 11, 111–122. [Google Scholar] [CrossRef] [Green Version]
- Zhao, K.; Song, J.; Feng, G.; Zhao, M.; Liu, J. Species, types, distribution, and economic potential of halophytes in China. Plant Soil 2011, 342, 495–509. [Google Scholar] [CrossRef]
- Nouri, H.; Borujeni, S.C.; Nirola, R.; Hassanli, A.; Beecham, S.; Alaghmand, S.; Saint, C.; Mulcahy, D. Application of green remediation on soil salinity treatment: A review on halophytoremediation. Proc. Saf. Env. Prot. 2017, 107, 94–107. [Google Scholar] [CrossRef]
- Rabie, G.H.; Aboul-Nasr, M.B.; Al-Humiany, A. Increased salinity tolerance of cowpea plants by dual inoculation of an arbuscular mycorrhizal fungus Glomus clarum and a nitrogen-fixer Azospirillum brasilense. Mycobiology 2005, 33, 51–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qurashi, A.W.; Sabri, A.N. Bacterial exopolysaccharide and biofilm formation stimulate chickpea growth and soil aggregation under salt stress. Braz. J. Microbiol. 2012, 43, 1183–1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Wu, X.; Li, G.; Qin, P. Interactions between arbuscular mycorrhizal fungi and phosphate-solubilizing fungus (Mortierella sp.) and their effects on Kostelelzkya virginica growth and enzyme activities of rhizosphere and bulk soils at different salinities. Biol. Fert. Soils 2011, 47, 543–554. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Gao, H.; Liang, Y.; Cao, Y. Full-length transcriptome analysis of asparagus roots reveals the molecular mechanism of salt tolerance induced by arbuscular mycorrhizal fungi. Environ. Exp. Bot. 2021, 185, 104402. [Google Scholar] [CrossRef]
- Santos, S.S.; Rask, K.A.; Vestergård, M.; Johansen, J.L.; Priemé, A.; Frøslev, T.G.; González, A.M.M.; He, H.; Ekelund, F. Specialized microbiomes facilitate natural rhizosphere microbiome interactions counteracting high salinity stress in plants. Environ. Exp. Bot. 2021, 186, 104430. [Google Scholar] [CrossRef]
- Klironomos, J.N. Variation in plant response to native and exotic arbuscular mycorrhizal fungi. Ecology 2003, 84, 2292–2301. [Google Scholar] [CrossRef]
- Kumar, A.; Verma, J.P. The role of microbes to improve crop productivity and soil health. In Ecological Wisdom Inspired Restoration Engineering; Achal, V., Mukherjee, A., Eds.; Springer: Singapore, 2019; pp. 249–265. [Google Scholar]
- Ilangumaran, G.; Smith, D.L. Plant growth promoting rhizobacteria in amelioration of salinity stress: A systems biology perspective. Front. Plant Sci. 2017, 8, 1768. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, M.; Boughattas, S.; Hu, S.; Oh, S.H.; Sa, S.H. A meta-analysis of arbuscular mycorrhizal effects on plants grown under salt stress. Mycorrhiza 2014, 24, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Porcel, R.; Aroca, R.; Ruiz-Lozano, J.M. Salinity stress alleviation using arbuscular mycorrhizal fungi. A review. Agron. Sustain. Dev. 2012, 32, 181–200. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Wang, C.; Lu, T.; Zheng, Y. Cooperation between arbuscular mycorrhizal fungi and earthworms promotes the physiological adaptation of maize under a high salt stress. Plant Soil 2018, 423, 125–140. [Google Scholar] [CrossRef]
- Zhu, X.; Song, F.; Liu, S.; Liu, F. Role of arbuscular mycorrhiza in alleviating salinity stress in wheat (Triticum aestivum L.) grown under ambient and elevated CO2. J. Agron. Crop Sci. 2016, 202, 486–496. [Google Scholar] [CrossRef]
- Liu, J.; Hu, J.; Cheng, Z.; Li, M.; Liu, Z.; Wang, J.; Lin, X. Can phosphorus (P)-releasing bacteria and earthworm (Eisenia fetida L.) co-enhance soil P mobilization and mycorrhizal P uptake by maize (Zea mays L.)? J. Soil Sediments 2020, 21, 842–852. [Google Scholar] [CrossRef]
- Barnawal, D.; Bharti, N.; Pandey, S.S.; Pandey, A.; Chanotiya, C.S.; Kalra, A. Plant growth-promoting rhizobacteria enhance wheat salt and drought stress tolerance by altering endogenous phytohormone levels and TaCTR1/TaDREB2 expression. Physiol. Plant. 2017, 161, 502–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atzori, G. The Potential of Edible Halophytes as New Crops in Saline Agriculture. In Future of Sustainable Agriculture in Saline Environments; Negacz, K., Vellinga, P., Barrett-Lennard, E., Choukr-Allah, R., Elzenga, T., Eds.; CSC Press: St. Paul, MN, USA, 2021; pp. 443–460. [Google Scholar]
- Koyro, H.W.; Lieth, H.; Gul, B.; Ansari, R.; Huchzermeyer, B.; Abideen, Z.; Hussain, T.; Ajhman Khan, M. Importance of the Diversity within the Halophytes to Agriculture and Land Management in Arid and Semiarid Countries. In Sabkha Ecosystems: Volume IV: Cash Crop Halophyte and Biodiversity Conservation; Khan, M.A., Böer, B., Öztürk, M., et al., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 175–198. [Google Scholar]
- Qin, S.; Zhang, Y.; Yuan, B.; Xu, P.Y.; Xing, K.; Wang, J.; Jiang, J.H. Isolation of ACC deaminase-producing habitat-adapted symbiotic bacteria associated with halophyte Limonium sinense (Girard) Kuntze and evaluating their plant growth-promoting activity under salt stress. Plant Soil 2014, 374, 753–766. [Google Scholar] [CrossRef]
- Rueda-Puente, E.O.; García-Hernández, J.L.; Preciado-Rangel, P.; Murillo-Amador, B.; Tarazón-Herrera, M.A.; Flores-Hernández, A.; Holguin-Peña, J.; Aybar, A.N.; Barrón Hoyos, J.M.; Weimers, D.; et al. Germination of Salicornia bigelovii ecotypes under stressing conditions of temperature and salinity and ameliorative effects of plant growth-promoting bacteria. J. Agron. Crop Sci. 2007, 193, 167–176. [Google Scholar] [CrossRef]
- Liu, C.; Dai, Z.; Cui, M.; Lu, W.; Sun, H. Arbuscular mycorrhizal fungi alleviate boron toxicity in Puccinellia tenuiflora under the combined stresses of salt and drought. Environ. Pollut. 2018, 240, 557–565. [Google Scholar] [CrossRef]
- Chang, W.; Sui, X.; Fan, X.X.; Jia, T.T.; Song, F.Q. Arbuscular mycorrhizal symbiosis modulates antioxidant response and ion distribution in salt-stressed Elaeagnus angustifolia seedlings. Front. Microbiol. 2018, 9, 652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, S.; Li, H.; Paré, P.W.; Aziz, M.; Wang, S.M.; Shi, H.; Li, J.; Han, Q.Q.; Guo, S.Q.; Li, J.; et al. Induced growth promotion and higher salt tolerance in the halophyte grass Puccinellia tenuiflora by beneficial rhizobacteria. Plant Soil 2016, 407, 217–230. [Google Scholar] [CrossRef]
- Maimaiti, A.; Yunus, Q.; Iwanaga, F.; Mori, N.; Tanaka, K.; Yamanaka, N. Effects of salinity on growth, photosynthesis, inorganic and organic osmolyte accumulation in Elaeagnus oxycarpa seedlings. Acta Physiol. Plant. 2014, 36, 881–892. [Google Scholar] [CrossRef]
- Pan, J.; Peng, F.; Tedeschi, A.; Xue, X.; Wang, T.; Liao, J.; Zhang, W.; Huang, C. Do halophytes and glycophytes differ in their interactions with arbuscular mycorrhizal fungi under salt stress? A meta-analysis. Bot. Stud. 2020, 61, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashem, A.; Abd Allah, E.F.; Alqarawi, A.A.; Al-Huqail, A.A.; Wirth, S.; Egamberdieva, D. The interaction between arbuscular mycorrhizal fungi and endophytic bacteria enhances plant growth of Acacia gerrardii under salt stress. Front. Microbiol. 2016, 7, 1089. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.; Peng, F.; Xue, X.; You, Q.; Zhang, W.; Wang, T.; Huang, C. The growth promotion of two salt-tolerant plant groups with PGPR inoculation: A meta-analysis. Sustainability 2019, 11, 378. [Google Scholar] [CrossRef] [Green Version]
- Hidri, R.; Barea, J.M.; Mahmoud, O.M.B.; Abdelly, C.; Azcón, R. Impact of microbial inoculation on biomass accumulation by Sulla carnosa provenances, and in regulating nutrition, physiological and antioxidant activities of this species under non-saline and saline conditions. J. Plant Physiol. 2016, 201, 28–41. [Google Scholar] [CrossRef]
- Walley, F.L.; Germida, J.J. Plant growth-promoting rhizobacteria alter rooting patterns and arbuscular mycorrhizal fungi colonization of field-grown spring wheat. Biol. Fert. Soils 1996, 23, 113–120. [Google Scholar]
- Seckbach, J.; Grube, M. Symbiosis and Stress; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Breckle, S.W. Salinity tolerance of different halophyte types. In Genetic Aspects of Plant Mineral Nutrition; El Bassam, N., Dambroth, M., Loughman, B.C., Eds.; Springer: Dordrecht, The Netherlands, 1990; pp. 167–175. [Google Scholar]
- Liu, L.; Wang, B. Protection of halophytes and their uses for cultivation of saline-alkali soil in China. Biology 2021, 10, 353. [Google Scholar] [CrossRef]
- Du, J.; Ping, Y.; Dong, Y. Phenological response of Nitraria tangutorum to climate change in Minqin County, Gansu Province, northwest China. Int. J. Biometeorol. 2010, 54, 583–593. [Google Scholar] [CrossRef]
- Pan, J.; Huang, C.; Peng, F.; Zhang, W.; Luo, J.; Ma, S.; Xue, X. Effect of arbuscular mycorrhizal fungi (AMF) and plant growth-promoting bacteria (PGPR) inoculations on Elaeagnus angustifolia L. in saline soil. Appl. Sci. 2020, 10, 945. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Ma, Q.; Jin, H.; Fan, B.; Wang, D.; Lin, H. Change in characteristics of soil carbon and nitrogen during the succession of Nitraria Tangutorum in an arid desert area. Sustainability 2019, 11, 1146. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Feng, Q.; Cheng, A. Spatial distribution of soil water and salt contents and reasons of saline soils’ development in the Minqin Oasis. J. Arid Land Res. Environ. 2013, 27, 99–105. [Google Scholar]
- Liu, W.; Yuan, X.; Zhang, Y.; Xuan, Y.; Yan, Y. Effects of salt stress and exogenous Ca2+ on Na+ compartmentalization, ion pump activities of tonoplast and plasma membrane in Nitraria tangutorum Bobr. leaves. Acta Physiol. Plant. 2014, 36, 2183–2193. [Google Scholar] [CrossRef]
- Liang, B.B.; Wang, W.J.; Fan, X.X.; Kurakov, A.V.; Liu, Y.F.; Song, F.; Chang, W. Arbuscular mycorrhizal fungi can ameliorate salt stress in Elaeagnus angustifolia by improving leaf photosynthetic function and ultrastructure. Plant Biol. 2020, 23, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Hao, H.; Lu, X.; Zhao, X.; Wang, Y.; Zhang, Y.; Xie, Z.; Wang, R. Transcriptome profiling of genes involved in induced systemic salt tolerance conferred by Bacillus amyloliquefaciens FZB42 in Arabidopsis thaliana. Sci. Rep. 2017, 7, 10795. [Google Scholar] [CrossRef] [Green Version]
- Weremijewicz, J.; Da Silveira Lobo, O.; Janos, D.P. Arbuscular common mycorrhizal networks mediate intra- and interspecific interactions of two prairie grasses. Mycorrhiza 2018, 28, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Giovannetti, M.; Mosse, B. An evaluation of techniques for measuring vesicular-arbuscular mycorrhizal infection in roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- Biermann, B.; Linderman, R. Quantifying vesicular-arbuscular mycorrhizae: A proposed method towards standardization. New Phytol. 1981, 87, 63–67. [Google Scholar] [CrossRef]
- Han, Y.; Wang, W.; Sun, J.; Ding, M.; Zhao, R.; Deng, S.; Wang, F.; Hu, Y.; Wang, Y.; Lu, Y.; et al. Populus euphratica XTH overexpression enhances salinity tolerance by the development of leaf succulence in transgenic tobacco plants. J. Exp. Bot. 2013, 64, 4225–4238. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Lai, L.; Zhou, J.; Li, Q.; Yi, S.; Sun, Q.; Zheng, Y. Changes in levels of enzymes and osmotic adjustment compounds in key species and their relevance to vegetation succession in abandoned croplands of a semiarid sandy region. Ecol. Evol. 2020, 10, 2269–2280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, T.; Richard, J.; Chen, S.; Lv, H.; Zhou, J.; Li, C. Infection by the fungal endophyte Epichlo bromicola enhances the tolerance of wild barley (Hordeum brevisubulatum) to salt and alkali stresses. Plant Soil 2018, 428, 353–370. [Google Scholar] [CrossRef]
- Chakraborty, K.; Mondal, S.; Ray, S.; Samal, P.; Pradhan, B.; Chattopadhyay, K.; Kar, M.K.; Swain, P.; Sarkar, R.K. Tissue tolerance coupled with ionic discrimination can potentially minimize the energy cost of salinity tolerance in rice. Front. Plant Sci. 2020, 11, 265. [Google Scholar] [CrossRef] [PubMed]
- Orhan, F. Alleviation of salt stress by halotolerant and halophilic plant growth-promoting bacteria in wheat (Triticum aestivum). Braz. J. Microbiol. 2016, 47, 621–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Zhang, Y.; Yuan, X.; Xuan, Y.; Gao, Y.; Yan, Y. Exogenous salicylic acid improves salinity tolerance of Nitraria tangutorum. Russ. J. Plant Physiol. 2016, 63, 132–142. [Google Scholar] [CrossRef]
- Zheng, X.; Lin, J.; Xin, J.; Li, L.; Qi, Y.; Chen, M. Response to NaCl stress and salinity threshold by two species of Elaeagnus from the same provenance. Crops 2017, 4, 143–149. [Google Scholar]
- Cobian, G.M.; Egan, C.P.; Amend, A.S. Plant–microbe specificity varies as a function of elevation. ISME J. 2019, 13, 2778–2788. [Google Scholar] [CrossRef]
- Drew, G.C.; Stevens, E.J.; King, K.C. Microbial evolution and transitions along the parasite–mutualist continuum. Nat. Rev. Microbiol. 2021, 19, 623–638. [Google Scholar] [CrossRef]
- Diagne, N.; Ndour, M.; Djighaly, P.I.; Ngom, D.; Ngom, M.C.N.; Ndong, G.; Svistoonoff, S.; Cherif-Silini, H. Effect of plant growth promoting rhizobacteria (PGPR) and arbuscular mycorrhizal fungi (AMF) on salt stress tolerance of Casuarina obesa (Miq.). Front. Sustain. Food Syst. 2020, 4, 601004. [Google Scholar] [CrossRef]
- Brundrett, M. Mycorrhizas in natural ecosystems. Adv. Ecol. Res. 1991, 21, 171–313. [Google Scholar]
- Porter, S.S.; Bantay, R.; Friel, C.A.; Garoutte, A.; Gdanetz, K.; Ibarreta, K.; Moore, B.M.; Shetty, P.; Siler, E.; Friesen, M.L. Beneficial microbes ameliorate abiotic and biotic sources of stress on plants. Funct. Ecol. 2020, 34, 2075–2086. [Google Scholar] [CrossRef] [Green Version]
- Hao, H.; Zhao, X.; Shang, Q.; Wang, Y.; Guo, Z.H.; Zhang, Y.B.; Xie, Z.K.; Wang, R.Y. Comparative digital gene expression analysis of the Arabidopsis response to volatiles emitted by Bacillus amyloliquefaciens. PLoS ONE 2016, 11, e0158621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leifheit, E.F.; Veresoglou, S.D.; Lehmann, A.; Morris, E.K.; Rillig, M.C. Multiple factors influence the role of arbuscular mycorrhizal fungi in soil aggregation-a meta-analysis. Plant Soil 2014, 374, 523–537. [Google Scholar] [CrossRef]
- Matinzadeha, Z.; Akhania, H.; Abedib, M.; Palacio, S. The elemental composition of halophytes correlates with key morphological adaptations and taxonomic groups. Plant Physiol. Biochem. 2019, 141, 259–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, J.; Yue, L.; Wang, S.; Zhao, W.; Bao, A. Na compound fertilizer stimulates growth and alleviates water deficit in the succulent xerophyte Nitraria tangutorum (Bobr) after breaking seed dormancy. Soil Sci. Plant Nutr. 2016, 62, 489–499. [Google Scholar] [CrossRef] [Green Version]
- Auge, R.M.; Toler, H.D.; Saxton, A.M. Arbuscular mycorrhizal symbiosis and osmotic adjustment in response to NaCl stress: A meta-analysis. Front. Plant Sci. 2014, 5, 562. [Google Scholar] [PubMed] [Green Version]
- Liu, Z.; Zhu, J.; Yang, X.; Wu, H.; Wei, Q.; Wei, H.; Zhang, H. Growth performance, organ-level ionic relations and organic osmoregulation of Elaeagnus angustifolia in response to salt stress. PLoS ONE 2018, 13, e0191552. [Google Scholar] [CrossRef] [Green Version]
- Flowers, T.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Azooz, M.M.; Prasad, M.N.V. Ecophysiology and Responses of Plants under Salt Stress; Springer: New York, NY, USA; Heidelberg, Germany; Dordrecht, The Netherlands; London, UK, 2013. [Google Scholar]
- Osman, K.T. Saline and Sodic Soils. In Management of Soil Problems; Osman, K.T., Ed.; Springer International Publishing AG, Part of Springer Nature: Cham, Switzerland, 2018; pp. 255–293. [Google Scholar]
- Xing, L.; Xue, H.; Li, Q.; Gao, T. Scaling from leaf to whole plant in biomass and nitrogen content of Nitraria tangutorum seedlings. J. Beijing For. Univ. 2018, 40, 76–82. [Google Scholar]
- Wei, Q.; Wu, H.; Liu, Z.; Li, H.; Yang, X.; Zhang, H. Biological nitrogen fixation ability and nitrogen distribution of Elaeagnus angustifolia under salt stress. For. Res. 2017, 30, 985–992. [Google Scholar]
- Govindarajulu, M.; Pfeffer, P.E.; Jin, H.; Abubaker, J.; Douds, D.D.; Allen, J.W.; Bücking, H.; Lammers, P.J.; Shachar-Hill, Y. Nitrogen transfer in the arbuscular mycorrhizal symbiosis. Nature 2005, 435, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Pfeffer, P.E.; Douds, D.D.; Piotrowski, E.; Lammers, P.J.; Shachar-Hill, Y. The uptake, metabolism, transport and transfer of nitrogen in an arbuscular mycorrhizal symbiosis. New Phytol. 2005, 168, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Tester, M. Na+ tolerance and Na+ transport in higher plants. Ann. Bot. 2003, 91, 503–527. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Ye, C.; Zhang, J.; Koziol, L.; Bever, J.D.; Li, X. Asymmetric facilitation induced by inoculation with arbuscular mycorrhizal fungi leads to overyielding in maize/faba bean intercropping. J. Plant Interact. 2019, 14, 10–20. [Google Scholar] [CrossRef]
- Ashraf, M. Some important physiological selection criteria for salt tolerance in plants. Flora 2004, 199, 361–376. [Google Scholar] [CrossRef]
- Himabindu, Y.; Chakradhar, T.; Reddy, M.C.; Kanygin, A.; Redding, K.E.; Chandrasekhar, T. Salt-tolerant genes from halophytes are potential key players of salt tolerance in glycophytes. Environ. Exp. Bot. 2016, 124, 39–63. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Wei, X.; Shi, R.; Fan, Q.; An, L. Salinity-induced Physiological Modification in the Callus from Halophyte Nitraria tangutorum Bobr. J. Plant Growth Regul. 2010, 29, 465–476. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Potential biochemical indicators of salinity tolerance in plants. Plant Sci. 2004, 166, 3–16. [Google Scholar] [CrossRef]
- Borde, M.; Dudhane, M.; Kulkarni, M. Role of Arbuscular Mycorrhizal Fungi (AMF) in Salinity Tolerance and Growth Response in Plants under Salt Stress Conditions. In Mycorrhiza—Eco-Physiology, Secondary Metabolites. Nanomaterials; Varma, A., Prasad, R., Tuteja, N., Eds.; Springer: Cham, Switzerland, 2017; pp. 71–86. [Google Scholar]
- Wang, W.; Jiang, W.; Xie, Z. Effect of NaCl stress on physiological index of Nitraria tangutorum seedling. Acta Agrestia Sin. 2012, 20, 907–913. [Google Scholar]






| Soil | Unit | Value | Soil | Unit | Value |
|---|---|---|---|---|---|
| pH | 8.14 ± 0.02 | Ca2+ | g/kg | 0.36 ± 0.03 | |
| EC1:5 | μs/cm | 2015 ± 15 | Na+ | g/kg | 0.46 ± 0.09 |
| TN | g/kg | 0.61 ± 0.01 | Mg2+ | g/kg | 0.14 ± 0.04 |
| AP | mg/kg | 16.41 ± 0.81 | HCO3− | g/kg | 0.16 ± 0.01 |
| AK | mg/kg | 47.74 ± 1.60 | OC | g/kg | 9.36 ± 0.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, J.; Xue, X.; Huang, C.; Peng, F.; Liao, J.; Ma, S.; You, Q.; Wang, T. Salt Tolerance Strategies of Nitraria tangutorum Bobr. and Elaeagnus angustifolia Linn. Determine the Inoculation Effects of Microorganisms in Saline Soil Conditions. Agronomy 2022, 12, 913. https://doi.org/10.3390/agronomy12040913
Pan J, Xue X, Huang C, Peng F, Liao J, Ma S, You Q, Wang T. Salt Tolerance Strategies of Nitraria tangutorum Bobr. and Elaeagnus angustifolia Linn. Determine the Inoculation Effects of Microorganisms in Saline Soil Conditions. Agronomy. 2022; 12(4):913. https://doi.org/10.3390/agronomy12040913
Chicago/Turabian StylePan, Jing, Xian Xue, Cuihua Huang, Fei Peng, Jie Liao, Shaoxiu Ma, Quangang You, and Tao Wang. 2022. "Salt Tolerance Strategies of Nitraria tangutorum Bobr. and Elaeagnus angustifolia Linn. Determine the Inoculation Effects of Microorganisms in Saline Soil Conditions" Agronomy 12, no. 4: 913. https://doi.org/10.3390/agronomy12040913
APA StylePan, J., Xue, X., Huang, C., Peng, F., Liao, J., Ma, S., You, Q., & Wang, T. (2022). Salt Tolerance Strategies of Nitraria tangutorum Bobr. and Elaeagnus angustifolia Linn. Determine the Inoculation Effects of Microorganisms in Saline Soil Conditions. Agronomy, 12(4), 913. https://doi.org/10.3390/agronomy12040913







