Estimating Heat Requirement for Flowering in Peach Germplasm
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Phenotyping
2.3. Statistical Analyses
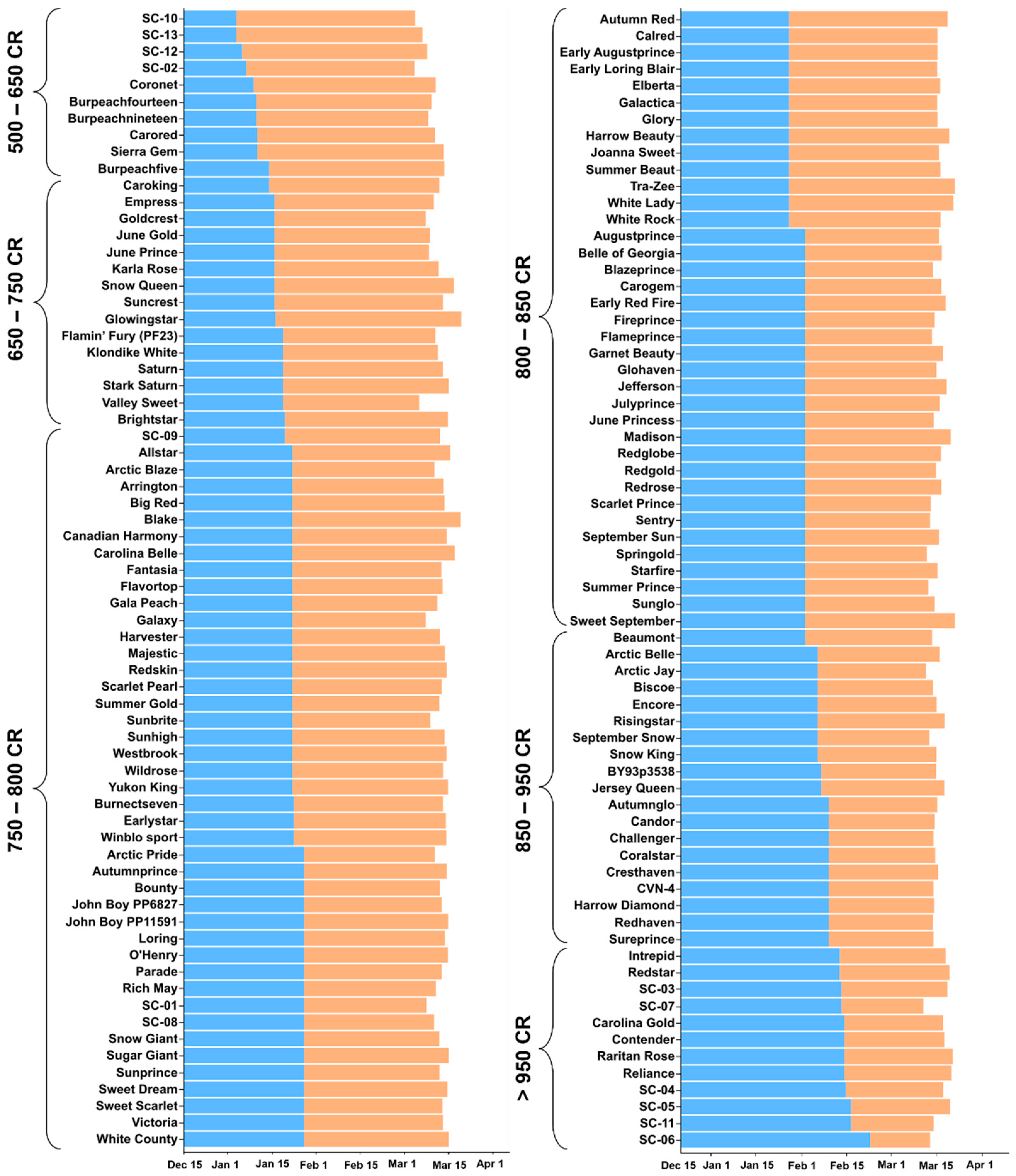
3. Results
3.1. Bloom Date and Chilling Requirement
3.2. Heat Requirement
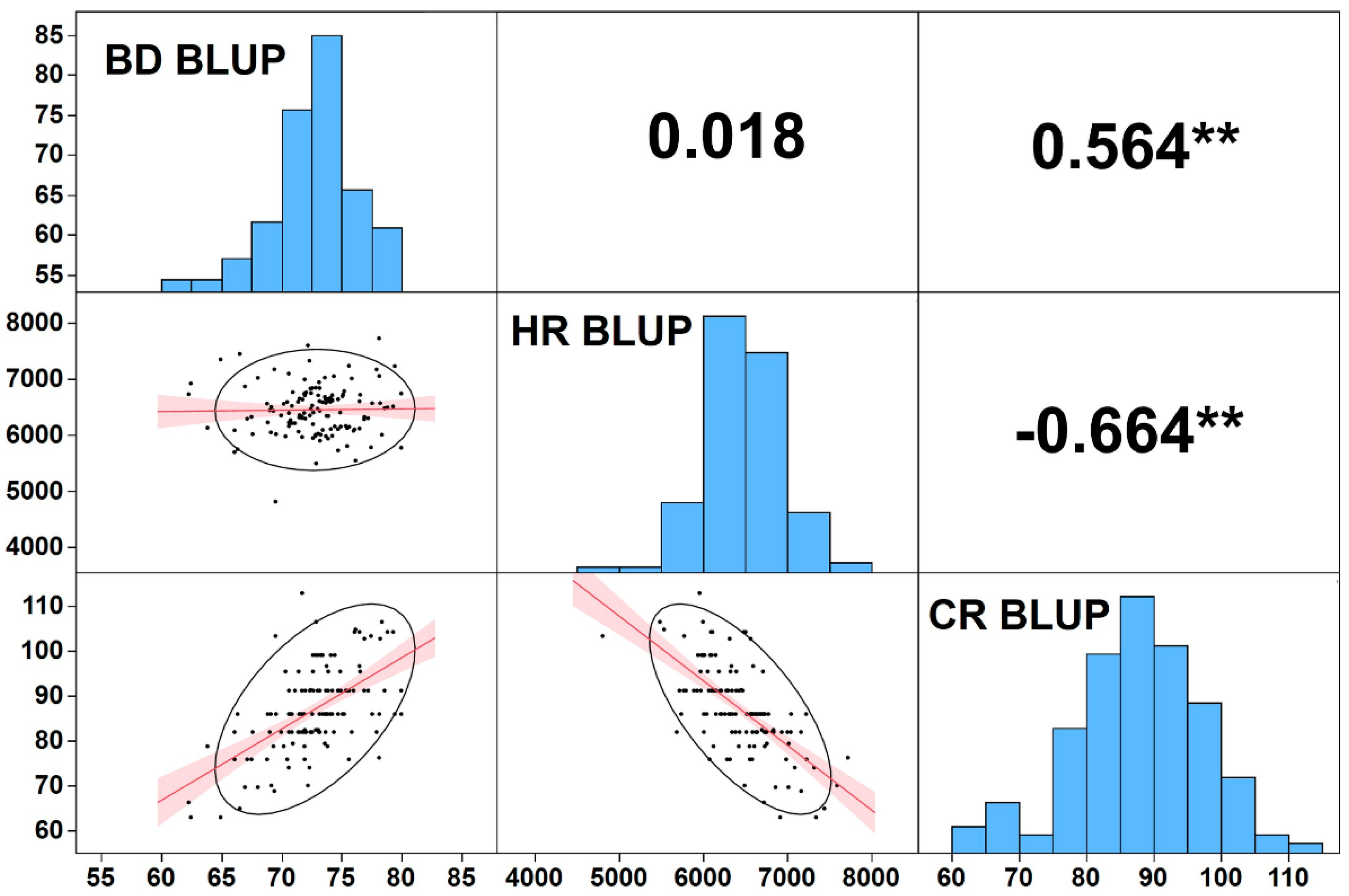
3.3. Trait Correlations and CR and HR Effect on BD
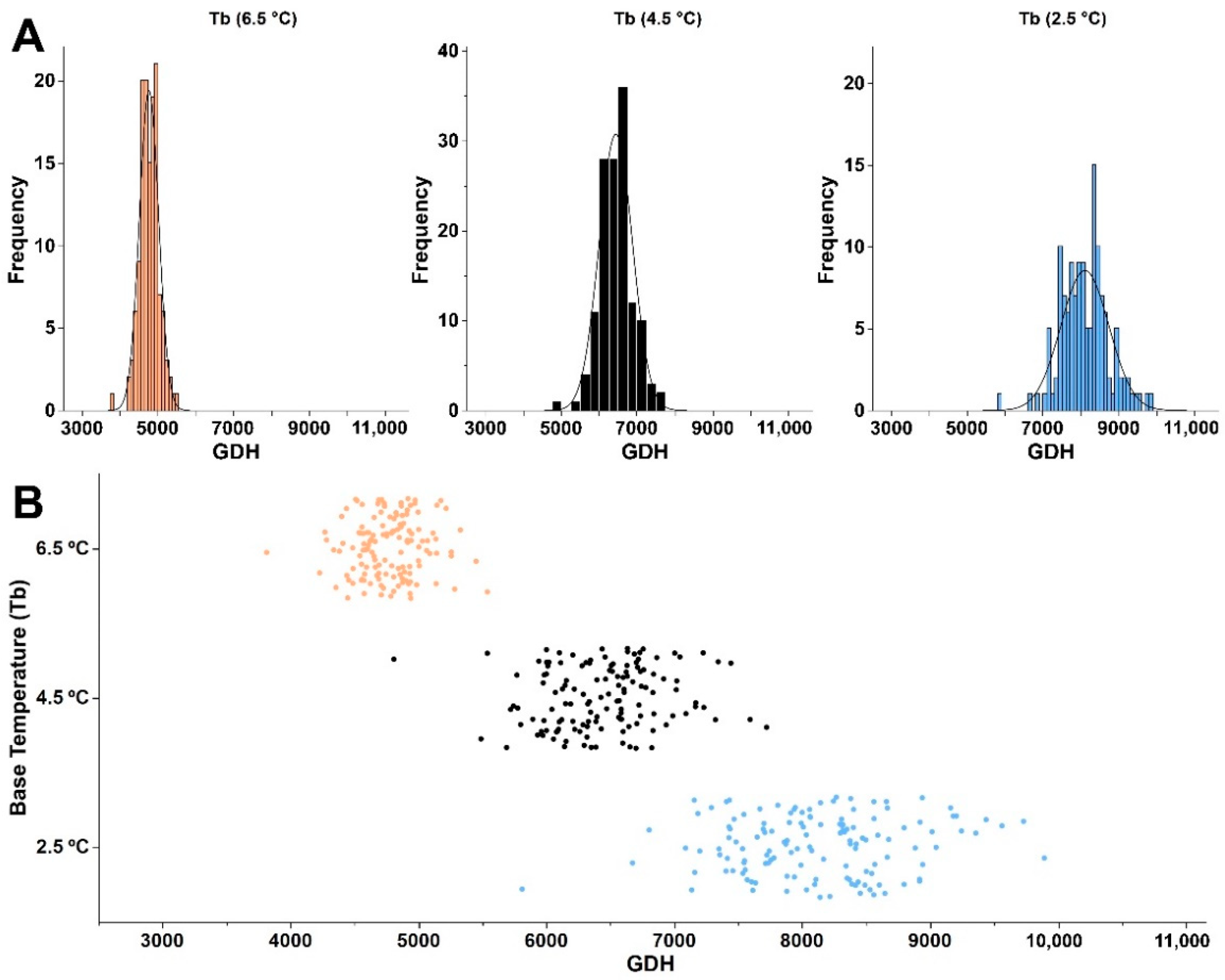
3.4. Simulating HR Using Different Base Temperatures
4. Discussion
4.1. Heat Requirement Variation in the Germplasm
4.2. Correlations between Dormancy-Related Traits
4.3. HR and BD in Response to Annual Environmental Conditions
4.4. Modeling Heat Requirement
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pio, R.; de Souza, F.B.M.; Kalcsits, L.; Bisi, R.B.; da Hora Farias, D. Advances in the production of temperate fruits in the tropics. Acta Sci. Agron. 2019, 41, e39549. [Google Scholar] [CrossRef]
- Prudencio, A.S.; Martínez-Gómez, P.; Dicenta, F. Evaluation of breaking dormancy, flowering and productivity of extra-late and ultra-late flowering almond cultivars during cold and warm seasons in South-East of Spain. Sci. Hortic. 2018, 235, 39–46. [Google Scholar] [CrossRef]
- Rohde, A.; Bhalerao, R.P. Plant dormancy in the perennial context. Trends Plant Sci. 2007, 12, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Lang, G.A.; Early, J.; Martin, G.C.; Darnell, R.L. Endo-, para-, and ecodormancy: Physiological terminology and classification for dormancy research. HortScience 1987, 22, 371–377. [Google Scholar]
- Cooke, J.E.; Eriksson, M.E.; Junttila, O. The dynamic nature of bud dormancy in trees: Environmental control and molecular mechanisms. Plant Cell Environ. 2012, 35, 1707–1728. [Google Scholar] [CrossRef]
- Abbott, A.G.; Zhebentyayeva, T.; Barakat, A.; Liu, Z. Chapter Six—The genetic control of bud-break in trees. Adv. Bot. Res. 2015, 74, 201–228. [Google Scholar] [CrossRef]
- Castède, S.; Campoy, J.A.; García, J.Q.; Dantec, L.; Lafargue, M.; Barreneche, T.; Wenden, B.; Dirlewanger, E. Genetic determinism of phenological traits highly affected by climate change in Prunus avium: Flowering date dissected into chilling and heat requirements. New Phytol. 2014, 202, 703–715. [Google Scholar] [CrossRef]
- Citadin, I.; do Carmo Bassols Raseira, M.; Herter, F.G.; Da Silva, J.B. Heat requirement for blooming and leafing in peach. HortScience 2001, 36, 305–307. [Google Scholar] [CrossRef]
- Fan, S.; Bielenberg, D.G.; Zhebentyayeva, T.N.; Reighard, G.L.; Okie, W.R.; Holland, D.; Abbott, A.G. Mapping quantitative trait loci associated with chilling requirement, heat requirement and bloom date in peach (Prunus persica L.). New Phytol. 2010, 185, 917–930. [Google Scholar] [CrossRef]
- Kitamura, Y.; Habu, T.; Yamane, H.; Nishiyama, S.; Kajita, K.; Sobue, T.; Kawai, T.; Numaguchi, K.; Nakazaki, T.; Kitajima, A.; et al. Identification of QTLs controlling chilling and heat requirements for dormancy release and bud break in Japanese apricot (Prunus mume). Tree Genet. Genomes 2018, 14, 33. [Google Scholar] [CrossRef]
- Sánchez-Pérez, R.; Dicenta, F.; Martínez-Gómez, P. Inheritance of chilling and heat requirements for flowering in almond and QTL analysis. Tree Genet. Genomes 2012, 8, 379–389. [Google Scholar] [CrossRef]
- Bielenberg, D.G.; Gasic, K. Peach [Prunus persica (L.) Batsch] cultivars differ in apparent base temperature and growing degree hour requirement for floral bud break. Front. Plant Sci. 2022, 13, 801606. [Google Scholar] [CrossRef] [PubMed]
- Pawasut, A.; Fujishige, N.; Yamane, K.; Yamaki, Y.; Honjo, H. Relationships between chilling and heat requirement for flowering in ornamental peaches. J. Jpn. Soc. Hortic. Sci. 2004, 73, 519–523. [Google Scholar] [CrossRef][Green Version]
- Ruíz, D.; Campoy, J.A.; Egea, J. Chilling and heat requirements of apricot cultivars for flowering. Environ. Exp. Bot. 2007, 61, 254–263. [Google Scholar] [CrossRef]
- Ruíz, D.; Egea, J.; Salazar, J.A.; Campoy, J.A. Chilling and heat requirements of Japanese plum cultivars for flowering. Sci. Hortic. 2018, 242, 164–169. [Google Scholar] [CrossRef]
- Alburquerque, N.; García-Montiel, F.; Carrillo, A.; Burgos, L. Chilling and heat requirements of sweet cherry cultivars and the relationship between altitude and the probability of satisfying the chill requirements. Environ. Exp. Bot. 2008, 64, 162–170. [Google Scholar] [CrossRef]
- Egea, J.; Ortega, E.; Martínez-Gómez, P.; Dicenta, F. Chilling and heat requirements of almond cultivars for flowering. Environ. Exp. Bot. 2003, 50, 79–85. [Google Scholar] [CrossRef]
- Alonso, J.M.; Ansón, J.M.; Espiau, M.T.; Socias i Company, R. Stability of the almond blooming date in a changing climate. Acta Hortic. 2011, 912, 337–342. [Google Scholar] [CrossRef]
- Scorza, R.; Okie, W.R. Peaches (Prunus). In Genetic Resources of Temperate Fruit and Nut Crops, 1st ed.; Moore, J.M., Ballington, J.R., Eds.; Acta Horticulturae: Leuven, Belgium, 1991; Volume 290, pp. 177–234. [Google Scholar] [CrossRef]
- Fadón, E.; Rodrigo, J.; Luedeling, E. Cultivar-specific responses of sweet cherry flowering to rising temperatures during dormancy. Agric. For. Meteorol. 2021, 307, 108486. [Google Scholar] [CrossRef]
- Bielenberg, D.G.; Rauh, B.; Fan, S.; Gasic, K.; Abbott, A.G.; Reighard, G.L.; Okie, W.R.; Wells, C.E. Genotyping by sequencing for SNP-based linkage map construction and QTL analysis of chilling requirement and bloom date in peach [Prunus persica (L.) Batsch]. PLoS ONE 2015, 10, e0139406. [Google Scholar] [CrossRef]
- Demirel, G.; Calle, A.; Atagul, O.; Lawton, J.M.; Gasic, K. Enabling DNA Informed Breeding for Chilling Requirement in Peach. J. Am. Pomol. Soc. 2022, 76, 11–19. Available online: http://www.pubhort.org/aps/76/v76_n1_a2.htm (accessed on 8 February 2022).
- Ghrab, M.; Mimoun, M.B.; Masmoudi, M.M.; Mechlia, N.B. Chilling trends in a warm production area and their impact on flowering and fruiting of peach trees. Sci. Hortic. 2014, 178, 87–94. [Google Scholar] [CrossRef]
- Jiménez, S.; Reighard, G.L.; Bielenberg, D.G. Gene expression of DAM5 and DAM6 is suppressed by chilling temperatures and inversely correlated with bud break rate. Plant Mol. Biol. 2010, 73, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Parker, L.E.; Abatzoglou, J.T. Warming winters reduce chill accumulation for peach production in the Southeastern United States. Climate 2019, 7, 94. [Google Scholar] [CrossRef]
- Yamane, H.; Ooka, T.; Jotatsu, H.; Hosaka, Y.; Sasaki, R.; Tao, R. Expressional regulation of PpDAM5 and PpDAM6, peach (Prunus persica) dormancy-associated MADS-box genes, by low temperature and dormancy-breaking reagent treatment. J. Exp. Bot. 2011, 62, 3481–3488. [Google Scholar] [CrossRef]
- Richardson, E.A.; Seeley, S.D.; Walker, D.R.; Anderson, J.L.; Ashcroft, G.L. Pheno-climatography of spring peach bud development. HortScience 1975, 10, 236–237. [Google Scholar]
- Fishman, S.; Erez, A.; Couvillon, G.A. The temperature dependence of dormancy breaking in plants: Mathematical analysis of a two-step model involving a cooperative transition. J. Theor. Biol. 1987, 124, 473–483. [Google Scholar] [CrossRef]
- Richardson, E.A.; Seeley, S.D.; Walker, D.R. A model for estimating the completion of rest for “Redhaven” and “Elberta” peach trees. HortScience 1974, 9, 331–332. [Google Scholar]
- Weinberger, J.H. Chilling requirements of peach varieties. Proc. Am. Soc. Hortic. Sci. 1950, 56, 122–128. [Google Scholar]
- Anderson, J.; Richardson, E.; Kesner, C. Validation of chill unit and flower bud phenology models for “Montmorency” sour cherry. Acta Hortic. 1986, 184, 71–78. [Google Scholar] [CrossRef]
- Ashcroft, G.L.; Richardson, E.A.; Seeley, S.D. A statistical method of determining chill unit and growing degree hour requirements for deciduous fruit trees. HortScience 1977, 12, 347–348. [Google Scholar]
- Ramírez, L.; Sagredo, K.X.; Reginato, G.H. Prediction models for chilling and heat requirements to estimate full bloom of almond cultivars in the Central Valley of Chile. Acta Hortic. 2010, 872, 107–112. [Google Scholar] [CrossRef]
- Okie, W.R.; Blackburn, B. Increasing chilling reduces heat requirement for floral budbreak in peach. HortScience 2011, 46, 245–252. [Google Scholar] [CrossRef]
- Carrillo-Navarro, A.; Guevara-Gázquez, A.; García-Montiel, F.; López-Ortiz, D.; Fuentes-Denia, A.; López-Soto, M.B.; Caballero-Hernández, C.M.; Ruiz-García, L.; Cos-Terrer, J. Caracterización fenotípica y molecular de variedades del programa de mejora de melocotonero del IMIDA. In Proceedings of the IX Congreso de Mejora Genética de Plantas, Murcia, Spain, 18–20 September 2018. [Google Scholar]
- Kwon, J.; Nam, E.; Yun, S.; Kim, S.; Song, S.; Lee, J.; Hwang, K. Chilling and heat requirement of peach cultivars and changes in chilling accumulation spectrums based on 100-year records in Republic of Korea. Agric. For. Meteorol. 2020, 288–289, 108009. [Google Scholar] [CrossRef]
- Maulión, E.; Valentini, G.H.; Kovalevski, L.; Prunello, M.; Monti, L.L.; Daorden, M.E.; Quaglino, M.; Cervigni, G.D.L. Comparison of methods for estimation of chilling and heat requirements of nectarine and peach genotypes for flowering. Sci. Hortic. 2014, 177, 112–117. [Google Scholar] [CrossRef]
- Mounzer, O.H.; Conejero, W.; Nicolás, E.; Abrisqueta, I.; Garcia-Orellana, Y.V.; Tapia, L.M.; Vera, J.; Abrisqueta, J.M.; del Carmen Ruiz-Sánchez, M. Growth pattern and phenological stages of early-maturing peach trees under a Mediterranean climate. HortScience 2008, 43, 1813–1818. [Google Scholar] [CrossRef]
- Razavi, F.; Hajilou, J.; Tabatabaei, S.J.; Dadpour, M.R. Comparison of chilling and heat requirement in some peach and apricot cultivars. Res. Plant Biol. 2011, 1, 40–47. [Google Scholar]
- Sawamura, Y.; Suesada, Y.; Sugiura, T.; Yaegaki, H. Chilling requirements and blooming dates of leading peach cultivars and a promising early maturing peach selection, Momo Tsukuba 127. Hortic. J. 2017, 86, 426–436. [Google Scholar] [CrossRef]
- Valentini, N.; Me, G.; Spanna, F.; Lovisetto, M. Chilling and heat requirement in apricot and peach varieties. Acta Hortic. 2004, 636, 199–203. [Google Scholar] [CrossRef]
- Alonso, J.M.; Ansón, J.M.; Espiau, M.T.; Socias i Company, R. Determination of endodormancy break in almond flower buds by a correlation model using the average temperature of different day intervals and its application to the estimation of chill and heat requirements and blooming date. J. Am. Soc. Hortic. Sci. 2005, 130, 308–318. [Google Scholar] [CrossRef]
- Benmoussa, H.; Ghrab, M.; Ben Mimoun, M.; Luedeling, E. Chilling and heat requirements for local and foreign almond (Prunus dulcis Mill.) cultivars in a warm Mediterranean location based on 30 years of phenology records. Agric. For. Meteorol. 2017, 239, 34–46. [Google Scholar] [CrossRef]
- Díez-Palet, I.; Funes, I.; Savé, R.; Biel, C.; de Herralde, F.; Miarnau, X.; Vargas, F.; Ávila, G.; Carbó, J.; Aranda, X. Blooming under Mediterranean climate: Estimating cultivar-specific chill and heat requirements of almond and apple trees using a statistical approach. Agronomy 2019, 9, 760. [Google Scholar] [CrossRef]
- Campoy, J.A.; Ruiz, D.; Allderman, L.; Cook, N.; Egea, J. The fulfilment of chilling requirements and the adaptation of apricot (Prunus armeniaca L.) in warm winter climates: An approach in Murcia (Spain) and the Western Cape (South Africa). Eur. J. Agron. 2012, 37, 43–55. [Google Scholar] [CrossRef]
- García, E.G.; Guerriero, R.; Monteleone, P. Apricot bud chilling and heat requirements in two different climatic areas: Murcia and the Tuscan Maremma. Acta Hortic. 1997, 488, 289–294. [Google Scholar] [CrossRef]
- Viti, R.; Andreini, L.; Ruiz, D.; Egea, J.; Bartolini, S.; Iacona, C.; Campoy, J.A. Effect of climatic conditions on the overcoming of dormancy in apricot flower buds in two Mediterranean areas: Murcia. Sci. Hortic. 2010, 124, 217. [Google Scholar] [CrossRef]
- Gao, Z.; Zhuang, W.; Wang, L.; Shao, J.; Luo, X.; Cai, B.; Zhang, Z. Evaluation of chilling and heat requirements in Japanese apricot with three models. HortScience 2012, 47, 1826–1831. [Google Scholar] [CrossRef]
- Kuden, A.B.; Imrak, B.; Bayazıt, S.; Çömlekçıoğlu, S.; Kuden, A. Chilling requirements of cherries grown under subtropical conditions of Adana. Middle East J. Sci. Res. 2012, 12, 1497–1501. [Google Scholar] [CrossRef]
- Luedeling, E.; Kunz, A.; Blanke, M.M. Identification of chilling and heat requirements of cherry trees—A statistical approach. Int. J. Biometeorol. 2013, 57, 679–689. [Google Scholar] [CrossRef]
- Fadón, E.; Fernandez, E.; Behn, H.; Luedeling, E. A conceptual framework for winter dormancy in deciduous trees. Agronomy 2020, 10, 241. [Google Scholar] [CrossRef]
- NOAA National Centers for Environmental Information, State of the Climate: Global Climate Report for Annual 2020, Online January 2021. Available online: https://www.ncdc.noaa.gov/sotc/global/202013 (accessed on 15 March 2021).
- Longstroth, M. Critical Spring Temperatures for Tree Fruit Bud Development Stages. Michigan State University Extension Bulletin Online March 2021. Available online: https://www.canr.msu.edu/resources/picture-table-critical-spring-temperatures-for-tree-fruit-bud-development-stages (accessed on 8 February 2022).
- Augspurger, C.K. Reconstructing patterns of temperature, phenology, and frost damage over 124 years: Spring damage risk is increasing. Ecology 2013, 94, 41–50. [Google Scholar] [CrossRef]
- Ma, Q.; Huang, J.; Hänninen, H.; Berninger, F. Divergent trends in the risk of spring frost damage to trees in Europe with recent warming. Glob. Chang. Biol. 2019, 25, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Chen, Z.; Reddy, G.V.P.; Hui, C.; Huang, J.; Xiao, M. Timing of cherry tree blooming: Contrasting effects of rising winter low temperatures and early spring temperatures. Agric. For. Meteorol. 2017, 240–241, 78–89. [Google Scholar] [CrossRef]
- Bassi, D.; Monet, R. Chapter 1: Botany and taxonomy. In The Peach: Botany, Production and Uses, 1st ed.; Layne, D., Bassi, D., Eds.; CABI: Wallingford, UK, 2008; pp. 1–36. [Google Scholar] [CrossRef]
- Chen, C.; Okie, W.R.; Beckman, T.G. Peach fruit set and buttoning after spring frost. HortScience 2016, 51, 816–821. [Google Scholar] [CrossRef]
- Atkinson, C.J.; Brennan, R.M.; Jones, H.G. Declining chilling and its impact on temperate perennial crops. Environ. Exp. Bot. 2013, 91, 48–62. [Google Scholar] [CrossRef]
- Mahmood, K.; Carew, J.G.; Hadley, P.; Battey, N.H. The effect of chilling and post-chilling temperatures on growth and flowering of sweet cherry (Prunus avium L.). J. Hortic. Sci. Biotechnol. 2000, 75, 598–601. [Google Scholar] [CrossRef]
- Gasic, K.; Preece, J.E.; Karp, D. Register of new fruit and nut cultivars list 50. HortScience 2020, 55, 1164–1201. [Google Scholar] [CrossRef]
- Fadón, E.; Herrera, S.; Guerrero, B.; Guerra, M.; Rodrigo, J. Chilling and heat requirements of temperate stone fruit trees (Prunus sp.). Agronomy 2020, 10, 409. [Google Scholar] [CrossRef]
- Okie, W.R.; Ramming, D.W.; Scorza, R. Peach, nectarine, and other stone fruit breeding by the USDA in the last two decades. HortScience 1985, 20, 633–641. [Google Scholar]
- Bassi, D.; Badenes, M.; Gasic, K.; Giovanini, D.; Cirilli, M.; Foschi, S.; Iglesias, I. Chapter 7: Cultivars. In Peach and Nectarine, 1st ed.; Manganaris, G.A., Costa, G., Crisosto, C.H., Eds.; CABI: Wallingford, UK, 2018; in press. [Google Scholar]
- Peace, C.P.; Luby, J.J.; Van de Weg, W.E.; Bink, M.; Iezzoni, A.F. A strategy for developing representative germplasm sets for systematic QTL validation, demonstrated for apple, peach, and sweet cherry. Tree Genet. Genomes 2014, 10, 1679–1694. [Google Scholar] [CrossRef]
- Okie, W.R. Handbook of Peach and Nectarine Varieties: Performance in the Southeastern United States and Index of Names; Agriculture Handbook; USDA: Washington, DC, USA, 1998; Volume 714, p. 808. Available online: https://handle.nal.usda.gov/10113/CAT10853102 (accessed on 9 February 2021).
- Topp, B.L.; Sherman, W.B.; Raseira, M.C.B. Chapter 5: Low-chill cultivar development. In The Peach: Botany, Production and Uses, 1st ed.; Layne, D., Bassi, D., Eds.; CABI: Wallingford, UK, 2008; pp. 106–138. [Google Scholar] [CrossRef]
- Gannouni, T.A.; Campoy, J.A.; Quero-García, J.; Barreneche, T.; Arif, A.; Albouchi, A.; Ammari, Y. Dormancy related traits and adaptation of sweet cherry in Northern Africa: A case of study in two Tunisian areas. Sci. Hortic. 2017, 219, 272–279. [Google Scholar] [CrossRef]
- Campoy, J.A.; Ruiz, D.; Egea, J. Dormancy in temperate fruit trees in a global warming context: A review. Sci. Hortic. 2011, 130, 357–372. [Google Scholar] [CrossRef]
- Cirilli, M.; Gattolin, S.; Chiozzotto, R.; Baccichet, I.; Pascal, T.; Quilot-Turion, B.N.D.; Rossini, L.; Bassi, D. The Di2/pet variant in the PETALOSA gene underlies a major heat requirement-related QTL for blooming date in peach [Prunus persica (L.) Batsch]. Plant Cell Physiol. 2021, 62, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Zhebentyayeva, T.N.; Fan, S.; Chandra, A.; Bielenberg, D.G.; Reighard, G.L.; Okie, W.R.; Abbott, A.G. Dissection of chilling requirement and bloom date QTLs in peach using a whole genome sequencing of sibling trees from an F2 mapping population. Tree Genet. Genomes 2014, 10, 35–51. [Google Scholar] [CrossRef]
- Luedeling, E.; Brown, P.H. A global analysis of the comparability of winter chill models for fruit and nut trees. Int. J. Biometeorol. 2010, 55, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Dirlewanger, E.; Quero-García, J.; Le Dantec, L.; Lambert, P.; Ruíz, D.; Dondini, L.; Illa, E.; Quilot-Turion, B.; Audergon, J.M.; Tartarini, S.; et al. Comparison of the genetic determinism of two key phenological traits, flowering and maturity dates, in three Prunus species: Peach, apricot and sweet cherry. Heredity 2012, 109, 280–292. [Google Scholar] [CrossRef]
- Romeu, J.F.; Monforte, A.J.; Sánchez, G.; Granell, A.; García-Brunton, J.; Badenes, M.L.; Ríos, G. Quantitative trait loci affecting reproductive phenology in peach. BMC Plant Biol. 2014, 14, 52. [Google Scholar] [CrossRef]
- Ballester, J.; I Company, R.S.; Arús, P.; De Vicente, M.C. Genetic mapping of a major gene delaying blooming time in almond. Plant Breed. 2001, 120, 268–270. [Google Scholar] [CrossRef]
- Calle, A.; Cai, L.; Iezzoni, A.; Wünsch, A. Genetic dissection of bloom time in low chilling sweet cherry (Prunus avium L.) using a multi-family QTL approach. Front. Plant Sci. 2020, 10, 1647. [Google Scholar] [CrossRef]
- Khadivi, A.; Goodarzi, S.; Sarkhosh, A. Identification of late-blooming almond (Prunus dulcis L.) genotypes with high kernel quality. Euphytica 2019, 215, 166. [Google Scholar] [CrossRef]
- Guo, L.; Dai, J.; Wang, M.; Xu, J.; Luedeling, E. Responses of spring phenology in temperate zone trees to climate warming: A case study of apricot flowering in China. Agric. For. Meteorol. 2015, 201, 1–7. [Google Scholar] [CrossRef]
- Chandler, W.H. Chilling Requirements for Opening of Buds on Deciduous Orchard Trees and Some Other Plants in California; University of California Agricultural Experiment Station Bulletin: Berkeley, CA, USA, 1937; p. 611. [Google Scholar]
- Gaeta, L.; Stellacci, A.M.; Losciale, P. Evaluation of three modelling approaches for almond blooming in Mediterranean climate conditions. Eur. J. Agron. 2018, 97, 1–10. [Google Scholar] [CrossRef]
- Lin, S.; Wang, H.; Ge, Q.; Hu, Z. Effects of chilling on heat requirement of spring phenology vary between years. Agric. For. Meteorol. 2022, 312, 108718. [Google Scholar] [CrossRef]
- Guo, L.; Dai, J.; Ranjitkar, S.; Yu, H.; Xu, J.; Luedeling, E. Chilling and heat requirements for flowering in temperate fruit trees. Int. J. Biometeorol. 2014, 58, 1195–1206. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wu, Z.; Guo, L.; He, H.S.; Ling, Y.; Wang, L.; Zong, S.; Na, R.; Du, H.; Li, M. Effects of winter chilling vs. spring forcing on the spring phenology of trees in a cold region and a warmer reference region. Sci. Total Environ. 2020, 725, 138323. [Google Scholar] [CrossRef]
- Anzanello, R.; Biasi, L.A. Base temperature as a function of genotype: A foundation for modeling phenology of temperate fruit species. Semin. Ciênc. Agrár. 2016, 37, 1811–1826. [Google Scholar] [CrossRef]
- Yan, W.; Hunt, L.A. An equation for modelling the temperature response of plants using only the cardinal temperatures. Ann. Bot. 1999, 84, 607–614. [Google Scholar] [CrossRef]
- Shafii, B.; Price, W.J. Estimation of cardinal temperatures in germination data analysis. J. Agric. Biol. Environ. Stat. 2001, 6, 356–366. [Google Scholar] [CrossRef]
- Luedeling, E.; Schiffers, K.; Fohrmann, T.; Urbach, C. PhenoFlex-an integrated model to predict spring phenology in temperate fruit trees. Agric. For. Meteorol. 2021, 307, 108491. [Google Scholar] [CrossRef]
- Chuine, I.; Régnière, J. Process-based models of phenology for plants and animals. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 159–182. [Google Scholar] [CrossRef]
- Asse, D.; Randin, C.F.; Bonhomme, M.; Delestrade, A.; Chuine, I. Process-based models outcompete correlative models in projecting spring phenology of trees in a future warmer climate. Agric. For. Meteorol. 2020, 285, 107931. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atagul, O.; Calle, A.; Demirel, G.; Lawton, J.M.; Bridges, W.C.; Gasic, K. Estimating Heat Requirement for Flowering in Peach Germplasm. Agronomy 2022, 12, 1002. https://doi.org/10.3390/agronomy12051002
Atagul O, Calle A, Demirel G, Lawton JM, Bridges WC, Gasic K. Estimating Heat Requirement for Flowering in Peach Germplasm. Agronomy. 2022; 12(5):1002. https://doi.org/10.3390/agronomy12051002
Chicago/Turabian StyleAtagul, Omer, Alejandro Calle, Gizem Demirel, John M. Lawton, William C. Bridges, and Ksenija Gasic. 2022. "Estimating Heat Requirement for Flowering in Peach Germplasm" Agronomy 12, no. 5: 1002. https://doi.org/10.3390/agronomy12051002
APA StyleAtagul, O., Calle, A., Demirel, G., Lawton, J. M., Bridges, W. C., & Gasic, K. (2022). Estimating Heat Requirement for Flowering in Peach Germplasm. Agronomy, 12(5), 1002. https://doi.org/10.3390/agronomy12051002




