Joint Effects of Developmental Stage and Water Deficit on Essential Oil Traits (Content, Yield, Composition) and Related Gene Expression: A Case Study in Two Thymus Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Growth, Flowering and Harvest Index
2.3. Essential Oil Content
2.4. Essential Oil Composition
2.5. Leaf Trichome Density
2.6. Leaf Water Status
2.7. Leaf Chlorophyll and Carotenoid Content
2.8. Leaf Proline Content
2.9. Leaf Hydrogen Peroxide Content
2.10. Leaf Lipid Peroxidation
2.11. Antioxidant Defense Elements
2.11.1. Total Phenolic Content
2.11.2. Activity of Five Antioxidant Enzymes
2.12. QPCR Expression Analysis
2.13. Statistical Analysis
3. Results
3.1. Growth, Flowering and Harvest Index
3.2. Essential Oil Content, Yield and Composition
3.3. Principal Component Analysis in Plant Growth and Yield Traits
3.4. Leaf Trichome Density
3.5. Leaf Water Status
3.6. Leaf Chlorophyll, and Proline Contents
3.7. Leaf Hydrogen Peroxide Content and Lipid Peroxidation Level
3.8. Enzymatic and Non-Enzymatic Antioxidant Defense Elements
3.9. Interplay of Physiological Traits
3.10. Relative Expression of Terpene Synthesis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Paschalidis, K.; Fanourakis, D.; Tsaniklidis, G.; Tzanakakis, V.A.; Bilias, F.; Samara, E.; Kalogiannakis, K.; Debouba, F.J.; Ipsilantis, I.; Tsoktouridis, G.; et al. Pilot cultivation of the vulnerable Cretan endemic Verbascum arcturus L. (Scrophulariaceae): Effect of fertilization on growth and quality features. Sustainability 2021, 13, 14030. [Google Scholar] [CrossRef]
- Hassanvand, F.; Rezaei Nejad, A.; Fanourakis, D. Morphological and physiological components mediating the silicon-induced enhancement of geranium essential oil yield under saline conditions. Ind. Crops Prod. 2019, 134, 19–25. [Google Scholar] [CrossRef]
- Taheri-Garavand, A.; Mumivand, H.; Fanourakis, D.; Fatahi, S.; Taghipour, S. An artificial neural network approach for non-invasive estimation of essential oil content and composition through considering drying processing factors: A case study in Mentha aquatica. Ind. Crops Prod. 2021, 171, 113985. [Google Scholar] [CrossRef]
- Mumivand, H.; Shayganfar, A.; Tsaniklidis, G.; Emami Bistgani, Z.; Fanourakis, D.; Nicola, S. Pheno-Morphological and Essential Oil Composition Responses to UVA Radiation and Protectants: A Case Study in Three Thymus Species. Horticulturae 2022, 8, 31. [Google Scholar] [CrossRef]
- Tohidi, B.; Rahimmalek, M.; Trindade, H. Review on essential oil, extracts composition, molecular and phytochemical properties of Thymus species in Iran. Ind. Crops Prod. 2019, 134, 89–99. [Google Scholar] [CrossRef]
- Christensen, L.P.; Grevsen, K. Effect of development stage at harvest on the composition and yield of essential oils from thyme and oregano. Dev. Food Sci. 2006, 43, 261–264. [Google Scholar]
- Abu-Darwish, M.S.; Alu’datt, M.H.; Al-Tawaha, A.R.; Ereifej, K.; Almajwal, A.; Odat, N.; Al Khateeb, W. Seasonal variation in essential oil yield and composition from Thymus vulgaris L. during different growth stages in the south of Jordan. Nat. Prod. Res. 2012, 26, 1310–1317. [Google Scholar] [CrossRef]
- Amiri, H.; Dousty, B.; Hosseinzedeh, S.R. Water stress-induced changes of morphological, physiological and essential oil compounds in Thymus eriocalyx from Iran. J. Essent. Oil-Bear. Plants. 2018, 21, 1210–1223. [Google Scholar] [CrossRef]
- Ahmadi-Majd, M.; Mousavi-Fard, S.; Rezaei Nejad, A.; Fanourakis, D. Carbon nanotubes in the holding solution stimulate flower opening and prolong vase life in carnation. Chem. Biol. Technol. Agric. 2022, 9, 15. [Google Scholar] [CrossRef]
- Ahmadi-Majd, M.; Rezaei Nejad, A.; Mousavi-Fard, S.; Fanourakis, D. Postharvest application of single, multi-walled carbon nanotubes and nanographene oxide improves rose keeping quality. J. Hortic. Sci. Biotechnol. 2021, 1–15. [Google Scholar] [CrossRef]
- Chen, Y.; Fanourakis, D.; Tsaniklidis, G.; Aliniaeifard, S.; Yang, Q.; Li, T. Low UVA intensity during cultivation improves the lettuce shelf-life, an effect that is not sustained at higher intensity. Postharvest Biol. Technol. 2021, 172, 111376. [Google Scholar] [CrossRef]
- Fanourakis, D.; Papadakis, V.M.; Psyllakis, E.; Tzanakakis, V.A.; Nektarios, P.A. The role of water relations and oxidative stress in the vase life response to prolonged storage: A case study in chrysanthemum. Agriculture 2022, 12, 185. [Google Scholar] [CrossRef]
- Fanourakis, D.; Kazakos, P.; Nektarios, P.A. Allometric individual leaf area estimation in chrysanthemum. Agronomy 2021, 11, 795. [Google Scholar] [CrossRef]
- Javadi Asayesh, E.; Aliniaeifard, S.; Askari, N.; Roozban, M.R.; Sobhani, M.; Tsaniklidis, G.; Woltering, E.J.; Fanourakis, D. Supplementary light with increased blue fraction accelerates emergence and improves development of the inflorescence in Aechmea, Guzmania and Vriesea. Horticulturae 2021, 7, 485. [Google Scholar] [CrossRef]
- Fanourakis, D.; Papadopoulou, E.; Valla, A.; Tzanakakis, V.A.; Nektarios, P.A. Partitioning of transpiration to cut flower organs and its mediating role on vase life response to dry handling: A case study in chrysanthemum. Postharvest Biol. Technol. 2021, 181, 111636. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation Carol Stream: Carol Stream, IL, USA, 2007. [Google Scholar]
- Seif, M.; Aliniaeifard, S.; Arab, M.; Mehrjerdi, M.Z.; Shomali, A.; Fanourakis, D.; Li, T.; Woltering, E. Monochromatic red light during plant growth decreases the size and improves the functionality of stomata in chrysanthemum. Funct. Plant Biol. 2021, 48, 515–528. [Google Scholar] [CrossRef]
- Taheri-Garavand, A.; Rezaei Nejad, A.; Fanourakis, D.; Fatahi, S.; Ahmadi-Majd, M. Employment of artificial neural networks for non-invasive estimation of leaf water status using color features: A case study in Spathiphyllum wallisii. Acta Physiol. Plant. 2021, 43, 78. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef] [Green Version]
- Bates, L.; Waldren, R.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Narwal, S.S.; Bogatek, R.; Zagdanska, B.M.; Sampietro, D.A.; Vattuone, M.A. Plant Biochemistry; Studium Press LLC: Houston, TX, USA, 2009. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Bistgani, Z.E.; Hashemi, M.; DaCosta, M.; Craker, L.; Maggi, F.; Morshedloo, M.R. Effect of salinity stress on the physiological characteristics, phenolic compounds and antioxidant activity of Thymus vulgaris L. and Thymus daenensis Celak. Ind. Crops Prod. 2019, 135, 311–320. [Google Scholar] [CrossRef]
- Asghari, B.; Mafakheri, S.; Rejali, F. Assessment of morphological, physiological, and biochemical characteristics of thymus kotschyanus bioss. And hohen under different bio and chemical fertilizers. J. Agric. Sci. Technol. 2021, 23, 1309–1325. [Google Scholar]
- Moradi, P.; Ford-Lloyd, B.; Pritchard, J. Plant-water responses of different medicinal plant thyme (Thymus spp.) species to drought stress condition. Aust. J. Crop Sci. 2014, 8, 666. [Google Scholar]
- Ashrafi, M.; Azimi-Moqadam, M.-R.; MohseniFard, E.; Shekari, F.; Jafary, H.; Moradi, P.; Pucci, M.; Abate, G.; Mastinu, A. Physiological and Molecular Aspects of Two Thymus Species Differently Sensitive to Drought Stress. BioTech 2022, 11, 8. [Google Scholar] [CrossRef]
- Spinoni, J.; Barbosa, P.; Bucchignani, E.; Cassano, J.; Cavazos, T.; Christensen, J.H.; Christensen, O.B.; Coppola, E.; Evans, J.; Geyer, B.; et al. Future global meteorological drought hotspots: A study based on CORDEX data. J. Clim. 2020, 33, 3635–36561. [Google Scholar] [CrossRef]
- Turner, G.; Gershenzon, J.; Nielson, E.E.; Froehlich, J.E.; Croteau, R. Limonene synthase, the enzyme responsible for monoterpene biosynthesis in peppermint, is localized to leucoplasts of oil gland secretory cells. Plant Physiol. 1999, 120, 879–886. [Google Scholar] [CrossRef] [Green Version]
- Kowalski, R.; Kowalska, G.; Jankowska, M.; Nawrocka, A.; Kałwa, K.; Pankiewicz, U.; Włodarczyk-Stasiak, M. Secretory Structures and Essential Oil Composition of Selected Industrial Species of Lamiaceae. Acta Sci. Pol. Hortorum Cultus 2019, 18, 53–69. [Google Scholar] [CrossRef]
- Wiese, N.; Fischer, J.; Heidler, J.; Lewkowski, O.; Degenhardt, J.; Erler, S. The terpenes of leaves, pollen, and nectar of thyme (Thymus vulgaris) inhibit growth of bee disease-associated microbes. Sci. Rep. 2018, 8, 14634. [Google Scholar] [CrossRef] [Green Version]
- Seifikalhor, M.; Niknam, V.; Aliniaeifard, S.; Didaran, F.; Tsaniklidis, G.; Fanourakis, D.; Teymoorzadeh, M.; Mousavi, S.H.; Bustachi, M.; Li, T. The regulatory role of γ-Aminobutyric acid in chickpea plants depends on drought tolerance and water scarcity level. Sci. Rep. 2022, in press. [Google Scholar] [CrossRef]
- Safari, M.; Mousavi-Fard, S.; Rezaei Nejad, A.; Sorkheh, K.; Sofo, A. Exogenous salicylic acid positively affects morpho-physiological and molecular responses of Impatiens walleriana plants grown under drought stress. Int. J. Environ. Sci. Technol. 2022, 19, 969–984. [Google Scholar] [CrossRef]
- Bijalwan, P.; Sharma, M.; Kaushik, P. Review of the Effects of Drought Stress on Plants: A Systematic Approach. Preprints 2022, 2022020014. [Google Scholar] [CrossRef]
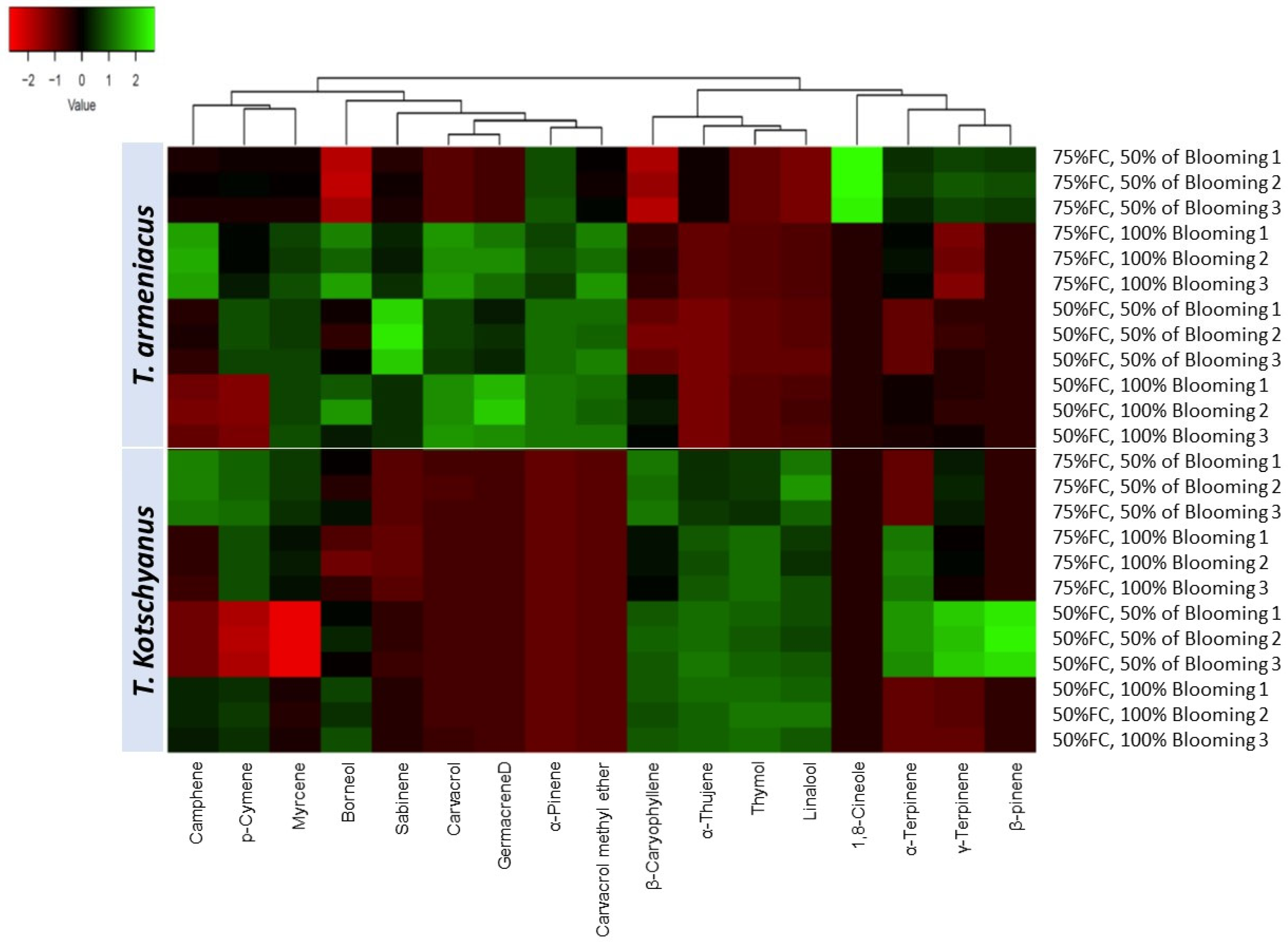
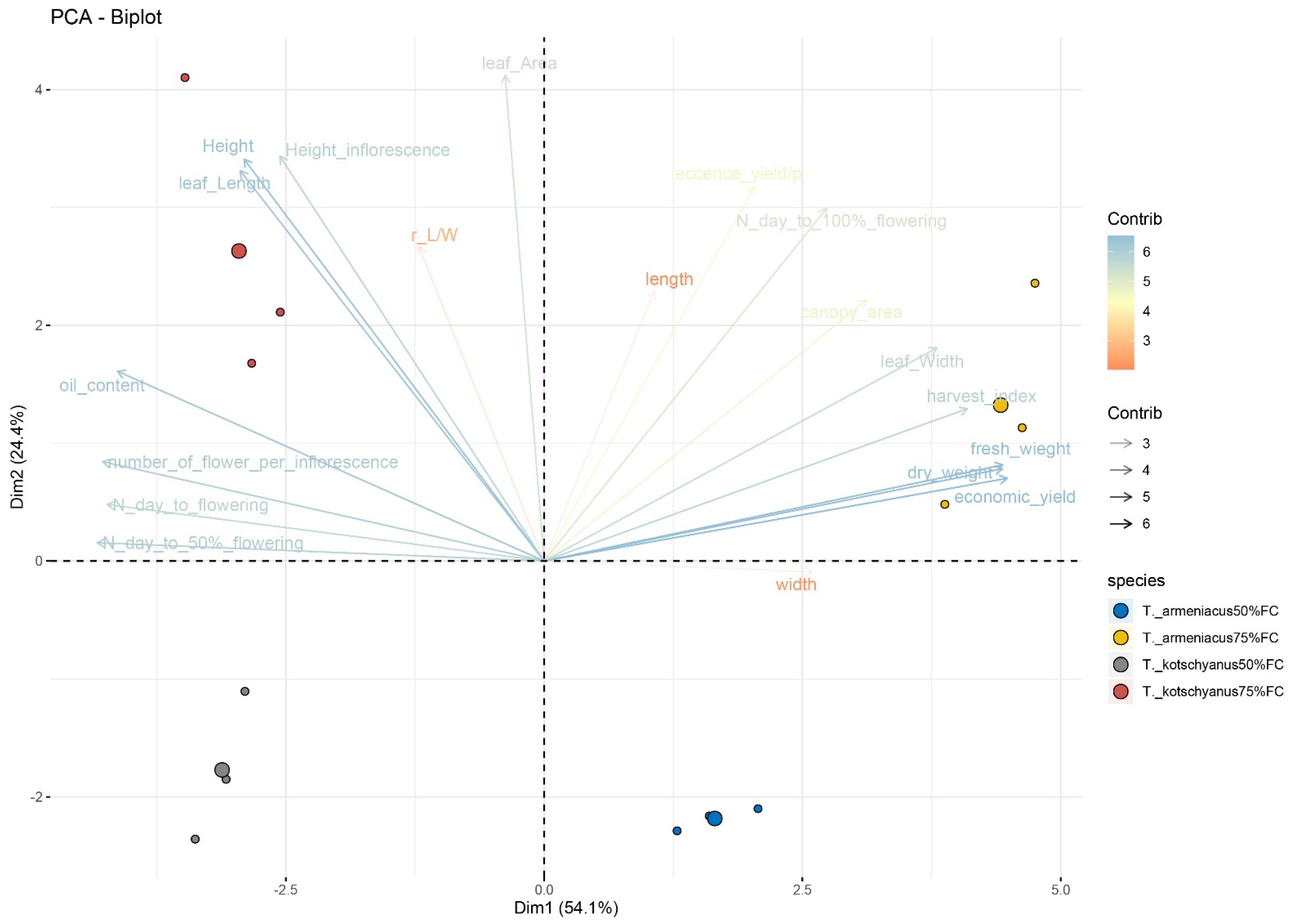

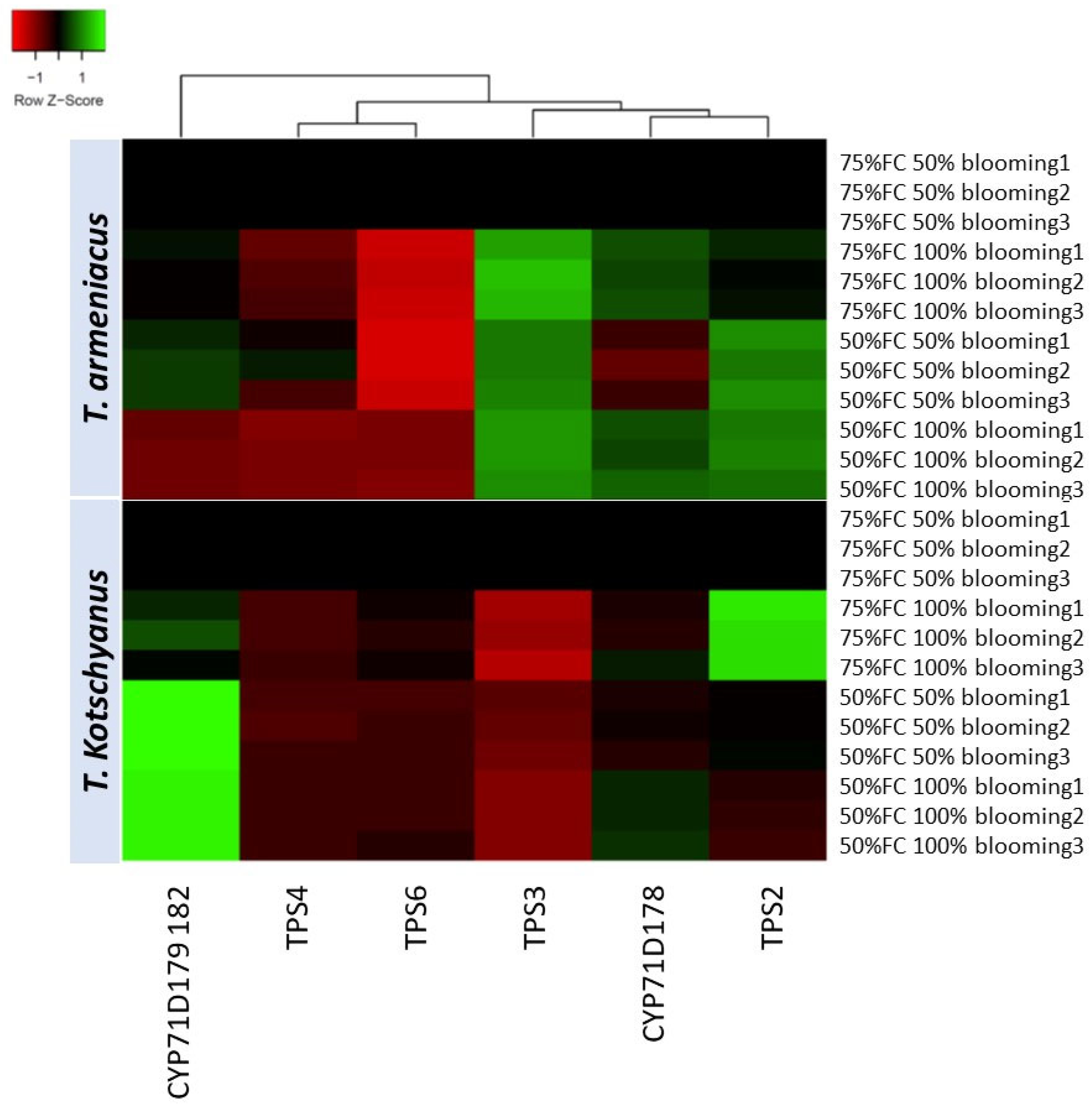
| Species | Irrigation (% of Field Capacity) | Time (d) | Inflorescence Length (cm) | Number of Flowers per Inflorescence | ||
|---|---|---|---|---|---|---|
| Onset of Flowering | 50% Flowering | 100% Flowering | ||||
| T. armeniacus | 75 | 49.3 c | 63.0 c | 120.3 a | 2.31 b | 50.22 c |
| 50 | 47.7 c | 66.3 c | 113.0 b | 1.64 c | 33.84 d | |
| T. kotschyanus | 75 | 79.3 b | 88.0 b | 114.7 ab | 4.41 a | 76.78 a |
| 50 | 88.0 a | 96.0 a | 108.7 b | 2.34 b | 63.07 b | |
| Species | Irrigation (% of Field Capacity) | Plant | Canopy Area (cm2) | Individual Leaf | ||||
|---|---|---|---|---|---|---|---|---|
| Height (cm) | Length (cm) | Width (cm) | Length (mm) | Width (mm) | Area (mm2) | |||
| T. armeniacus | 75 | 14.6 bc | 35.3 a | 31.0 a | 858 a | 12.9 b | 5.64 a | 47.4 b |
| 50 | 13.1 c | 28.8 bc | 28.3 ab | 640 b | 11.2 c | 4.89 b | 41.9 c | |
| T. kotschyanus | 75 | 20.3 a | 31.3 b | 27.3 b | 670 c | 18. 5 a | 4.71 b | 57.1 a |
| 50 | 15.9 b | 28.02 c | 29.0 ab | 637 d | 14.0 b | 3.74 c | 38.1 c | |
| Species | Irrigation (% of Field Capacity) | Plant | Harvest Index (%) | ||
|---|---|---|---|---|---|
| Fresh Weight (g) | Dry Weight (g) | Economic Yield (g) | |||
| T. armeniacus | 75 | 116.93 a | 42.37 a | 34.1 a | 81.97 a |
| 50 | 83.78 b | 32.2 b | 23.71 b | 76.63 b | |
| T. kotschyanus | 75 | 55.26 c | 21.73 c | 15.313 c | 71.71 c |
| 50 | 42.81 d | 16.413 d | 12.167 c | 63.99 d | |
| Species | Irrigation (% of Field Capacity) | Essential Oil Content (%) | Essential Oil Yield (g) | Adaxial | Abaxial | Leaf (Adaxial + Abaxial) |
|---|---|---|---|---|---|---|
| Trichome Density (mm−2) | ||||||
| T. armeniacus | 75 | 1.180 c | 0.3870 a | 4.75 c | 5.640 c | 10.38 c |
| 50 | 1.164 c | 0.2689 b | 18.1 a | 12.74 a | 30.84 a | |
| T. kotschyanus | 75 | 2.353 a | 0.3420 a | 8.88 b | 10.32 b | 19.2 b |
| 50 | 1.988 b | 0.2578 b | 10.0 b | 11.65 ab | 21.67 b | |
| Compound | RI | T. armeniacus | T. kotschyanus | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 75% Field Capacity | 50% Field Capacity | 75% Field Capacity | 50% Field Capacity | ||||||
| 50% Flowering | 100% Flowering | 50% Flowering | 100% Flowering | 50% Flowering | 100% Flowering | 50% Flowering | 100% Flowering | ||
| α-Thujene | 928 | 5.76 e | 0.903 f | 0 g | 0 g | 9.35 d | 11.337 c | 12.893 a | 12.34 b |
| Camphene | 934 | 3.62 d | 8.12 a | 3.13 e | 1.34 g | 7.19 b | 2.82 f | 1.307 g | 4.883 c |
| α-Pinene | 934 | 12.987 b | 12.157 c | 15.42 a | 15.78 a | 0 d | 0 d | 0 d | 0 d |
| β-pinene | 967 | 2.26 b | 0 c | 0 c | 0 c | 0 c | 0 c | 5.623 a | 0 c |
| Sabinene | 971 | 4.4067 d | 6.42 c | 12.43 a | 6.873 b | 2.347 f | 2.137 f | 3.71 e | 4.06 de |
| Myrcene | 988 | 6.1 d | 8.483 ab | 8.34 ab | 8.623 a | 8.13 b | 7.22 c | 0 e | 5.73 d |
| α-Terpinene | 1018 | 4.63667 c | 3.53 d | 0 f | 2.623 e | 0 f | 7.1 b | 7.797 a | 0 f |
| p-Cymene | 1034 | 12.067 e | 13.507 d | 16.56 b | 6.703 f | 17.783 a | 16.63 b | 4.187 g | 15.267 c |
| 1,8-Cineole | 1037 | 12.53 a | 0 b | 0 b | 0 b | 0 b | 0 b | 0 b | 0 b |
| γ-Terpinene | 1073 | 23.583 b | 16.65 h | 19.21 f | 19.84 e | 21.93 c | 20.74 d | 28.11 a | 17.69 g |
| linalool | 1105 | 0 d | 0.28 c | 0.187 c | 0.29 c | 1.463 a | 1.1 b | 1.213 b | 1.347 a |
| Borneol | 1168 | 0.87 f | 2.78 a | 1.847 d | 2.54 ab | 1.927 d | 1.48 e | 2.067 cd | 2.37 bc |
| Carvacrol methyl ether | 1248 | 0.88 c | 2.417 a | 2.187 b | 2.153 b | 0 | 0 | 0 | 0 |
| Thymol | 1298 | 0.11 f | 0.577 de | 0.32 ef | 0.66 d | 11.41 c | 15.66 a | 14.337 b | 15.73 a |
| Carvacrol | 1313 | 0.827 d | 11.483 a | 7.657 b | 11.34 a | 1.45 c | 1.653 c | 1.52 c | 1.78 c |
| β-Caryophyllene | 1429 | 2.31333 f | 5.22 d | 3.803 e | 6.693 c | 8.917 a | 6.52 c | 8.3 b | 8.193 b |
| Germacrene D | 1484 | nd | 0.923 b | 0.48 c | 1.197 a | 0 d | 0 d | 0 d | 0 d |
| Trait | T. armeniacus | T. kotschyanus | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 75% Field Capacity | 50% Field Capacity | 75% Field Capacity | 50% Field Capacity | ||||||
| 50% Flowering | 100% Flowering | 50% Flowering | 100% Flowering | 50% Flowering | 100% Flowering | 50% Flowering | 100% Flowering | ||
| Chlorophyll content (mg g−1 FW) | a | 0.261 b | 0.31 a | 0.22 c | 0.23 c | 0.278 b | 0.28 b | 0.226 c | 0.269 b |
| b | 0.09 abc | 0.099 a | 0.082 bc | 0.086 abc | 0.086 bc | 0.094 ab | 0.058 d | 0.078 c | |
| total | 0.351 c | 0.409 a | 0.302 de | 0.316 d | 0.364 bc | 0.374 b | 0.285 e | 0.348 c | |
| Non-enzymatic | Carotenoid content (mg g−1 FW) | 2.611 c | 3.444 a | 1.596 e | 2.611 c | 2.55 c | 3.085 ab | 2.171 d | 2.769 bc |
| Total phenol content (mg GAE g−1 DW) | - | 31.422 b | - | 26.513 c | - | 34.718 a | - | 31.108 b | |
| Relative water content (%) | 74.97 b | 68.794 c | 66.394 c | 55.621 d | 76.681 b | 83.144 a | 74.467 b | 57.048 d | |
| Osmolite | Proline content (μmol g−1 FW) | 0.515 cd | 0.378 d | 0.839 bcd | 6.9 a | 0.921 bc | 0.491 cd | 1.156 b | 7.088 a |
| MDA content (nmol g−1 FW) | 10.938 f | 12.874 e | 12.018 ef | 18.707 b | 12.565 e | 16.355 c | 14.598 d | 22.323 a | |
| H2O2 content (nmol g−1 FW) | 478.57 d | 587.52 c | 663.09 b | 1154.65 a | 422.8 e | 350.25 f | 702.41 b | 677.79 b | |
| Enzymatic | APX activity (μmol AsA min−1 mg−1 protein) | 0.4641 de | 0.3968 e | 0.5707 cd | 0.6533 bc | 0.7366 b | 0.4886 de | 0.9265 a | 0.6066 c |
| CAT activity (μmol H2O2 min−1 mg−1 protein) | 5.446 f | 6.576 de | 9.805 a | 8.43 b | 7.105 cd | 6.23 e | 7.901 b | 7.735 bc | |
| POX activity (U mg−1 protein min−1) | 34.561 c | 35.915 c | 86.375 b | 163.423 a | 35.928 c | 33.459 c | 94.706 b | 92.627 b | |
| PPO activity (U mg−1 protein min−1) | 0.556 e | 0.666 d | 0.882 b | 0.974 ab | 0.771 c | 0.678 cd | 0.962 ab | 0.988 a | |
| SOD activity (U mg−1 protein min−1) | 11.9416 b | 6.553 d | 13.987 a | 9.941 c | 7.797 d | 7.663 d | 9.975 c | 10.350 c | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yousefzadeh, K.; Houshmand, S.; Shiran, B.; Mousavi-Fard, S.; Zeinali, H.; Nikoloudakis, N.; Gheisari, M.M.; Fanourakis, D. Joint Effects of Developmental Stage and Water Deficit on Essential Oil Traits (Content, Yield, Composition) and Related Gene Expression: A Case Study in Two Thymus Species. Agronomy 2022, 12, 1008. https://doi.org/10.3390/agronomy12051008
Yousefzadeh K, Houshmand S, Shiran B, Mousavi-Fard S, Zeinali H, Nikoloudakis N, Gheisari MM, Fanourakis D. Joint Effects of Developmental Stage and Water Deficit on Essential Oil Traits (Content, Yield, Composition) and Related Gene Expression: A Case Study in Two Thymus Species. Agronomy. 2022; 12(5):1008. https://doi.org/10.3390/agronomy12051008
Chicago/Turabian StyleYousefzadeh, Kazem, Saadollah Houshmand, Behrouz Shiran, Sadegh Mousavi-Fard, Hossein Zeinali, Nikolaos Nikoloudakis, Mohammad Mehdi Gheisari, and Dimitrios Fanourakis. 2022. "Joint Effects of Developmental Stage and Water Deficit on Essential Oil Traits (Content, Yield, Composition) and Related Gene Expression: A Case Study in Two Thymus Species" Agronomy 12, no. 5: 1008. https://doi.org/10.3390/agronomy12051008
APA StyleYousefzadeh, K., Houshmand, S., Shiran, B., Mousavi-Fard, S., Zeinali, H., Nikoloudakis, N., Gheisari, M. M., & Fanourakis, D. (2022). Joint Effects of Developmental Stage and Water Deficit on Essential Oil Traits (Content, Yield, Composition) and Related Gene Expression: A Case Study in Two Thymus Species. Agronomy, 12(5), 1008. https://doi.org/10.3390/agronomy12051008








