Ectomycorrhizal Mushrooms as a Natural Bio-Indicator for Assessment of Heavy Metal Pollution
Abstract
:1. Introduction
2. Ectomycorrhizal Fungi
3. The Role of Ectomycorrhizas in Heavy Metal Stress Tolerance of Host Plants
3.1. Heavy Metal Deposition in EM Fungi
3.2. Heavy Metal Deposition in Edible EM Mushrooms
4. Assessment of Heavy Metals in ECM Mushrooms
5. Mechanisms of Symbiotic Mycorrhizal Tolerance to Heavy Metal
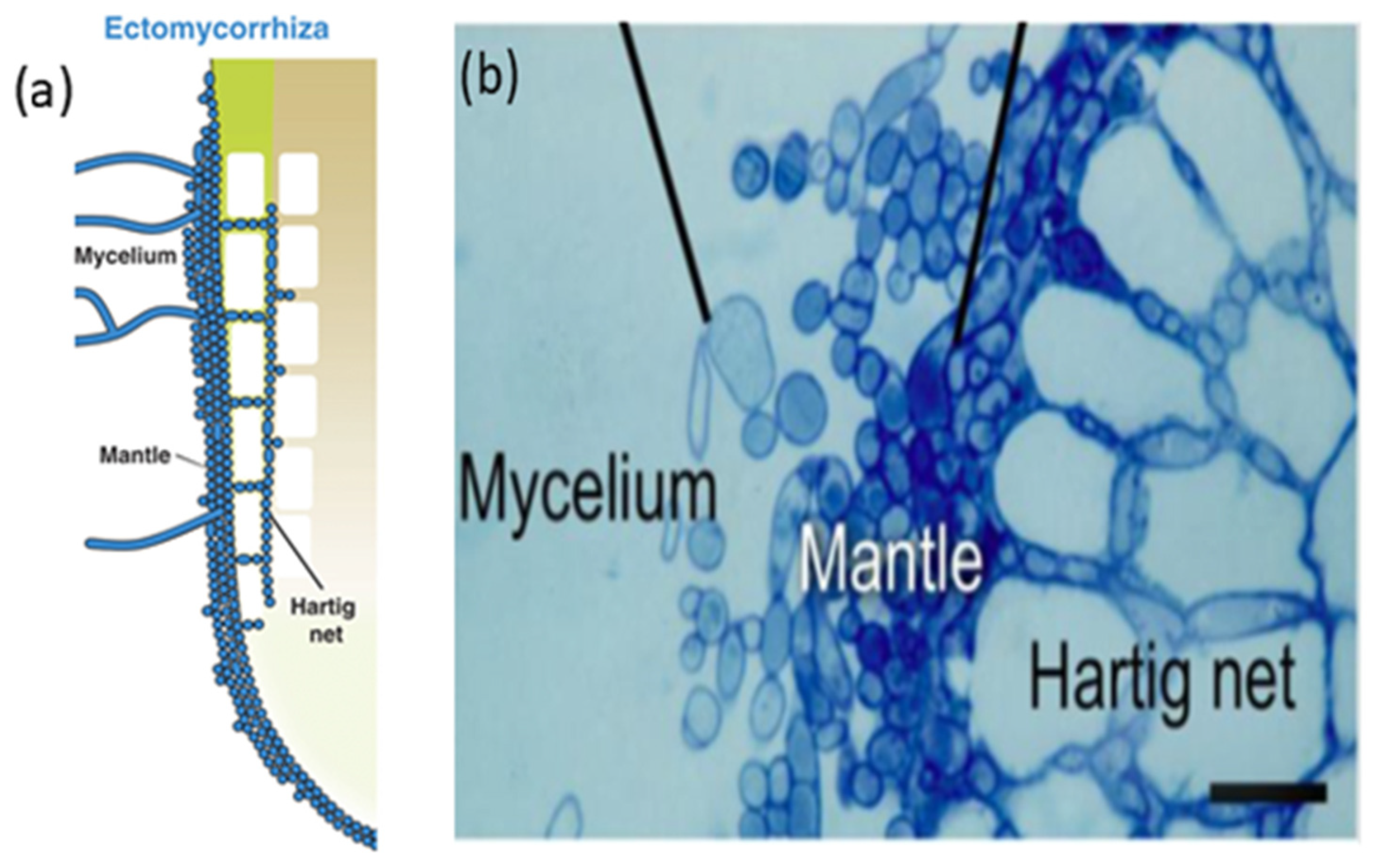
5.1. Anatomical Mechanism of Mycorrhizal Symbiosis
5.2. The Molecular Biological Mechanism of Plants Regulated by EM Fungi
5.3. Extracellular and Cellular Mechanisms to Sustain Metal Tolerance in Ectomycorrhizal Fungi
5.3.1. Metal Homeostasis in Ectomycorrhizal Fungi
5.3.2. Mechanism of Avoidance
5.3.3. Mechanism of Tolerance
6. ECM Mushrooms vs. Saprobic Mushrooms as Bio-Indicators
7. Benefits of Using ECM as a Natural Bio-Indicator of Metal Pollution
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Singh, N.; Gupta, V.K.; Kumar, A.; Sharma, B. Synergistic Effects of Heavy Metals and Pesticides in Living Systems. Front. Chem. 2017, 5, 70. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Jyotis, J.; Sood, A.; Sood, R.; Sidhu, C.; Kaur, G. Microbes as Potential Tool for Remediation of Heavy Metals: A Review. J. Microb. Biochem. Technol. 2016, 8, 364–372. [Google Scholar] [CrossRef] [Green Version]
- Chaturvedi, R.; Favas, P.; Pratas, J.; Varun, M.; Paul, M.S. Assessment of edibility and effect of arbuscular mycorrhizal fungi on Solanum melongena L. grown under heavy metal(loid) contaminated soil. Ecotoxicol. Environ. Saf. 2018, 148, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Grant, C.A.; Sheppard, S.C. Fertilizer Impacts on Cadmium Availability in Agricultural Soils and Crops. Hum. Ecol. Risk Assess. Int. J. 2008, 14, 210–228. [Google Scholar] [CrossRef]
- Szarlowicz, K.; Reczynski, W.; Misiak, R.; Kubica, B. Radionuclides and heavy metal concentrations as complementary tools for studying the impact of industrialization on the environment. J. Radioanal. Nucl. Chem. 2013, 298, 1323–1333. [Google Scholar] [CrossRef] [Green Version]
- Yap, C.K.; Al-Mutairi, K.A. Ecological-Health Risk Assessments of Heavy Metals (Cu, Pb, and Zn) in Aquatic Sediments from the ASEAN-5 Emerging Developing Countries: A Review and Synthesis. Biology 2022, 11, 7. [Google Scholar] [CrossRef]
- Caridi, F.; Testagrossa, B.; Acri, G. Elemental composition and natural radioactivity of refractory materials. Environ. Earth Sci. 2021, 80, 170. [Google Scholar] [CrossRef]
- Tangahu, B.V.; Sheikh Abdullah, S.R.; Basri, H.; Idris, M.; Anuar, N. A Review on Heavy Metals (As, Pb, and Hg) Uptake by Plants through Phytoremediation. Int. J. Chem. Eng. 2011, 2011, 939161. [Google Scholar] [CrossRef]
- Alloway, B.J. Cadmium. In Heavy Metals in Soils; Alloway, B.J., Ed.; Blackie and Son Ltd.: Glasgow, Scotland, 1990; pp. 100–124. [Google Scholar]
- Nriagu, J.O. A global assessment of natural sources of atmospheric trace metals. Nature 1989, 338, 47–49. [Google Scholar] [CrossRef]
- Chandrajith, R.; Seneviratna, S.; Wickramaarachchi, K.; Attanayake, T.; Aturaliya, T.N.C.; Dissanayake, C.B. Natural radionuclides and trace elements in rice field soils in relation to fertilizer application: Study of a chronic kidney disease area in Sri Lanka. Environ. Earth Sci. 2009, 60, 193–201. [Google Scholar] [CrossRef]
- Jabeen, F.; Chaudhry, A.S. Environmental impacts of anthropogenic activities on the mineral uptake in Oreochromis mossambicus from Indus River in Pakistan. Environ. Monit. Assess. 2010, 166, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.; Verma, D.K.; Dhakerya, R.; Rais, S.; Alam, M.; Ansari, F.A. Bio-indicator: A comparative study on uptake and accumulation of heavy metals in some plant leaves. Res. J. Environ. Earth Sci. 2012, 4, 1060–1070. [Google Scholar]
- Rieuwerts, J.S.; Thornton, I.; Farago, M.E.; Ashmore, M.R. Factors influencing metal bioavailability in soils: Preliminary investigations for the development of a critical loads approach for metals. Chem. Speciat. Bioavailab. 1998, 10, 61–75. [Google Scholar] [CrossRef] [Green Version]
- Crane, S.; Dighton, J.; Barkay, T. Growth responses to and accumulation of mercury by ectomycorrhizal fungi. Fungal Biol. 2010, 114, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Falandysz, J.; Frankowska, A.; Jarzyńska, G.; Dryżałowska, A. Survey on composition and bioconcentration potential of 12 metallic elements in King Bolete (Boletus edulis) mushroom that emerged at 11 spatially distant sites. J. Environ. Sci. Health Part B 2011, 46, 231–246. [Google Scholar] [CrossRef] [PubMed]
- Falandysz, J.; Borovička, J. Macro and trace mineral constituents and radionuclides in mushrooms: Health benefits and risks. Appl. Microbiol. Biotechnol. 2012, 97, 477–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowakowski, P.; Renata, M.; Jolanta, S.; Puścion-Jakubik, A.; Mielcarek, K.; Borawska, M.; Socha, K. Evaluation of toxic element content and health risk assessment of edible wild mushrooms. J. Food Compos. Anal. 2020, 96, 103698. [Google Scholar] [CrossRef]
- Kuldeep, S.; Prodyut, B. Lichen as a Bio-indicator tool for assessment of climate and air pollution vulnerability: Review. Int. Res. J. Environ. Sci. 2015, 4, 107–117. [Google Scholar]
- Attaullah, M.; Nawaz, M.A.; Ilahi, I.; Ali, H.; Jan, T.; Khwaja, S.; Hazrat, A.; Ullah, I.; Ullah, Z.; Ullah, S.; et al. Honey as a bioindicator of environmental organochlorine insecticides contamination. Braz. J. Microbiol. 2021, 83, e250373. [Google Scholar] [CrossRef]
- O’Connor, P.J.; Sally, E.S.; Smith, A.F. Arbuscular mycorrhizal associations in the southern Simpson Desert. Aust. J. Bot. 2001, 49, 493–499. [Google Scholar] [CrossRef]
- Brundrett, M.C. Coevolution of roots and mycorrhizas of land plants. New Phytol. 2002, 154, 275–304. [Google Scholar] [CrossRef] [PubMed]
- Massicotte, H.B.; Melville, L.H.; Peterson, R.L. Scanning Electron Microscopy of Ectomycorrhizae Potential and Limitations. Scanning Microsc. 1987, 3, 58. [Google Scholar]
- Kalac, P.; Svoboda, L. Review of trace element concentrations in edible mushrooms. Food Chem. 2000, 69, 273–281. [Google Scholar] [CrossRef]
- Vetter, J. Arsenic content of some edible mushroom species. Eur. Food Res. Technol. 2004, 219, 71–74. [Google Scholar] [CrossRef]
- Svoboda, L.; Havlickova, B.; Kalač, P. Contents of cadmium, mercury and lead in edible mushrooms growing in a historical silver mining area. Food Chem. 2006, 96, 580–585. [Google Scholar] [CrossRef]
- Amaranthus, M. The Importance and Conservation of Ectomycorrhizal Fungal Diversity in Forest Ecosystems; United States Department of Agriculture, Forest Service, Pacific Northwest Research Station: Portland, OR, USA, 1998.
- Boroujeni, D.S.; Hemmatinezhad, B. Review of Application and Importance of Ectomycorrhiza Fungi and their Role in the Stability of Ecosystems. Biosci. Biotechnol. Res. Asia 2015, 12, 153–158. [Google Scholar] [CrossRef]
- Policelli, N.; Horton, T.R.; Hudon, A.T.; Patterson, T.R.; Bhatnagar, J.M. Back to Roots: The Role of Ectomycorrhizal Fungi in Boreal and Temperate Forest Restoration. Front. For. Glob. Chang. 2020, 3, 97. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Kou, Y. Ectomycorrhizal Fungi: Participation in Nutrient Turnover and Community Assembly Pattern in Forest Ecosystems. Forests 2020, 11, 453. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 2nd ed.; Academic Press: London, UK, 1997; p. 605. [Google Scholar]
- Finlay, R.D. Ecological aspects of mycorrhizal symbiosis: With special emphasis on the functional diversity of interactions involving the extraradical mycelium. J. Exp. Bot. 2008, 59, 1115–1126. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic press: San Diego, CA, USA, 2010; p. 800. [Google Scholar]
- Buée, M.; Reich, M.; Murat, C.; Morin, E.; Nilsson, R.H.; Uroz, S.; Martin, F. 454 Pyrosequencing analyses of forest soils reveal an unexpectedly high fungal diversity. New Phytol. 2009, 184, 449–456. [Google Scholar] [CrossRef]
- Martin, F.; Nehls, U. Harnessing ectomycorrhizal genomics for ecological insights. Curr. Opin. Plant Biol. 2009, 12, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Azul, A.M.; Sousa, J.P.; Agerer, R.; Martín, M.P.; Freitas, H. Land use practices and ectomycorrhizal fungal communities from oak woodlands dominated by Quercus suber L. considering drought scenarios. Mycorrhiza 2009, 20, 73–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonfante, P.; Genre, A. Mechanisms underlying beneficial plant–fungus interactions in mycorrhizal symbiosis. Nat. Commun. 2010, 1, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; He, J.; Ma, C.; Luo, J. Ectomycorrhizas with Paxillus involutus enhance cadmium uptake and tolerance in Populus canescens. Plant Cell Environ. 2014, 37, 627–642. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Nara, K.; Lian, C.; Zong, K. Ectomycorrhizal fungal communities associated with Masson pine (Pinus massoniana Lamb.) in Pb–Zn mine sites of central south China. Mycorrhiza 2012, 22, 589–602. [Google Scholar] [CrossRef] [PubMed]
- Harrison, M.J. Signaling in the Arbuscular Mycorrhizal Symbiosis. Annu. Rev. Microbiol. 2005, 59, 19–42. [Google Scholar] [CrossRef]
- Bradshaw, B. Salinity Tolerance of Selected Ectomycorrhizal Fungi (Pisolithus tinctorius Pers.) and Ectomycorrhizal Eucalypts. B.Sc. Thesis, Edith Cowan University, Perth, Australia, June 2000. [Google Scholar]
- Perrin, R.; Garbaye, J. Influence of ectomycorrhizae on infectivity of Pythium-infested soils and substrates. Plant Soil 1983, 71, 345–351. [Google Scholar] [CrossRef]
- Jones, M.; Hutchinson, T.C. The effect of mycorrhizal infection on the response of Betula papyrifera to nickel and copper. New Phytol. 1986, 102, 429–442. [Google Scholar] [CrossRef]
- Borchers, J.G.; Perry, D.A. The influence of soil texture and aggregation on carbon and nitrogen dynamics in southwest Oregon forests and clear cuts. Can. J. For. Res. 1992, 22, 298–305. [Google Scholar] [CrossRef]
- Godbold, D.L.; Jentschke, G.; Winter, S.; Marschner, P. Ectomycorrhizas and amelioration of metal stress in forest trees. Chemosphere 1998, 36, 757–762. [Google Scholar] [CrossRef]
- Jentschke, G.; Godbold, D.L. Metal toxicity and ectomycorrhizas. Physiol. Plant. 2000, 109, 107–116. [Google Scholar] [CrossRef] [Green Version]
- Reddy, M.S.; Kour, M.; Aggarwal, S.; Ahuja, S. Metal induction of a Pisolithus albus metallothionein and its potential involvement in heavy metal tolerance during mycorrhizal symbiosis. Environ. Microbiol. 2016, 18, 2446–2454. [Google Scholar] [CrossRef]
- Sousa, N.R.; Ramos, M.A.; Marques, A.P.; Castro, P.M. The effect of ectomycorrhizal fungi forming symbiosis with Pinus pinaster seedlings exposed to cadmium. Sci. Total Environ. 2012, 414, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Krznaric, E.; Verbruggen, N.; Wevers, J.H.L.; Carleer, R. Cd tolerant Suillus luteus: A fungal insurance for pines exposed to cadmium. Environ. Pollut. 2009, 157, 1581–1588. [Google Scholar] [CrossRef]
- Kułdo, E.; Jarzyńska, G.; Gucia, M.; Falandysz, J. Mineral constituents of edible parasol mushroom Macrolepiota procera (Scop. ex Fr.) Sing and soils beneath its fruiting bodies collected from a rural forest area. Chem. Pap. 2014, 68, 484–492. [Google Scholar] [CrossRef]
- Milenge, K.H.; Nshimba, S.M.H.; Masumbuko, N.C.; Nabahungu, N.L.; Degreef, J.; De Kesel, A. Host plants and edaphic factors influence the distribution and diversity of ectomycorrhizal fungal fruiting bodies within rainforests Tshopo, Democratic Republic of the Congo. Afr. J. Ecol. 2019, 57, 247–259. [Google Scholar] [CrossRef]
- Leake, J.R. Is diversity of ECM fungi important for ecosystem function? New Phytol. 2001, 152, 1–8. [Google Scholar] [CrossRef]
- Kernaghan, K. Mycorrhizal diversity: Cause and effect? Pedobiologia 2005, 49, 511–520. [Google Scholar] [CrossRef]
- Seeger, R. Toxische schwermetalle in Pilzen. Dtsch. Apoth. Ztg. 1982, 122, 1835–1844. [Google Scholar]
- Svoboda, L.; Zimmermannová, K.; Kalač, P. Concentrations of mercury, cadmium, lead and copper in fruiting bodies of edible mushrooms in an emission area of a copper smelter and a mercury smelter. Sci. Total Environ. 2000, 246, 61–67. [Google Scholar] [CrossRef]
- Chen, Y.P.; Liu, Q.; Liu, Y.J.; Jia, F.A.; He, X.H. Responses of soil microbial activity to cadmium pollution and elevated CO2. Sci. Rep. 2014, 4, 4287. [Google Scholar] [CrossRef]
- García, M.A.; Alonso, J.; Fernández, M.I.; Melgar, M.J. Lead Content in Edible Wild Mushrooms in Northwest Spain as Indicator of Environmental Contamination. Arch. Environ. Contam. Toxicol. 1998, 34, 330–335. [Google Scholar] [CrossRef]
- Demirbaş, A. Concentrations of 21 metals in 18 species of mushrooms growing in the East Black Sea region. Food Chem. 2001, 75, 453–457. [Google Scholar] [CrossRef]
- Melgar, M.J.; Alonso, J.; Pérez-López, M.; Garcia, M.A. Influence of some factors in toxicity and accumulation of cadmium from edible wild macrofungi in NW Spain. J. Environ. Sci. Health Part B 1998, 33, 439–455. [Google Scholar] [CrossRef] [PubMed]
- Falandysz, J.; Brzostowski, A.; Nosewicz, M.; Danisiewicz, D.; Frankowska, A.; Apanasewicz, D.; Bielawski, L. Mercury in edible mushrooms from the area of the Trojmiejski Landscape Park. Bromatol. Chem. Toksykol. 2000, 33, 177–182. [Google Scholar]
- Ruhling, A.; Baath, E.; Nordgren, A.; Soderstrom, B. Fungi in metal contaminated soil near the Gusum Brass Mill, Sweden. J. Hum. Environ. Stud. 1984, 13, 34–36. [Google Scholar]
- Fomina, M.; Alexander, I.; Colpaert, J.V.; Gadd, G. Solubilization of toxic metal minerals and metal tolerance of mycorrhizal fungi. Soil Biol. Biochem. 2005, 37, 851–866. [Google Scholar] [CrossRef]
- Kalač, P.; Svoboda, L.; Havlíčková, B. Contents of cadmium and mercury in edible mushrooms. J. Appl. Biomed. 2004, 2, 15–20. [Google Scholar] [CrossRef] [Green Version]
- Akin, C.; Munevver, C.; Mahmut, C. The heavy metal content of wild edible mushroom samples collected in Canakkale Province, Turkey. Biol. Trace Elem. Res. 2010, 134, 212–219. [Google Scholar]
- Poitou, M.; Oliveier, J.M. Effect of copper on mycelium of three edible ectomycorrhizal fungi. Agric. Ecosyst. Environ. 1990, 28, 403–408. [Google Scholar] [CrossRef]
- Colpaert, J.V.; Van, A.J.A. Zinc toxicity in ectomycorrhizal Pinus sylvestris. Plant Soil 1992, 143, 201–211. [Google Scholar] [CrossRef]
- Denny, H.J.; Wilkins, D.A. Zinc tolerance in Betula spp. IV. The mechanism of ectomycorrhizal amelioration of zinc toxicity. New Phytol. 1987, 106, 545–553. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Zhang, Q.; Huang, J.; Wang, F.; Xu, J. Mercury Distribution and Deposition in Glacier Snow over Western China. Environ. Sci. Technol. 2008, 46, 5404–5413. [Google Scholar] [CrossRef] [PubMed]
- Işiloğlu, M.M.M.; Merdivan, M.; Yilmaz, F. Heavy Metal Contents in Some Macrofungi Collected in the Northwestern Part of Turkey. Arch. Environ. Contam. Toxicol. 2001, 41, 1–7. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, T.; Pei, M.A.; Ying, L.; Pengcheng, S.U. Bioaccumulation of Heavy Metal in Wild Growing Mushrooms from Liangshan Yi Nationality Autonomous Prefecture, China. Wuhan Univ. J. Nat. Sci. 2008, 13, 267–272. [Google Scholar] [CrossRef]
- Zhu, X.; Song, F.; Liu, F. Arbuscular Mycorrhizal Fungi and Tolerance of Temperature Stress in Plants. In Arbuscular Mycorrhizas and Stress Tolerance of Plants; Wu, Q.S., Ed.; Springer: Singapore, 2017; pp. 163–194. [Google Scholar] [CrossRef]
- Kalac, P.; Wittingerova, M.; Staskova, I. The contents of seven biogenic trace elements in edible mushrooms. Potravin. Vedy 1989, 7, 131–136. [Google Scholar]
- Michelot, D.; Siobud, E.; Dore, J.C.; Viel, C. Update on Metal Content Profiles in mushrooms-toxicological implycations and tentative approach to the mechanisms of bioaccumulation. Toxicon 1998, 36, 1997–2012. [Google Scholar] [CrossRef]
- Stamets, P. Mycelium Running: How Mushrooms Can Help Save the World; Ten Speed Press, Penguin Random House LLC: New York, NY, USA, 2005. [Google Scholar]
- Kalač, P.; Burda, J.; Stašková, I. Concentrations of lead, cadmium, mercury and copper in mushrooms in the vicinity of a lead smelter. Sci. Total Environ. 1991, 105, 109–119. [Google Scholar] [CrossRef]
- Kalac, P.; Niznaska, M.; Staskova, I. Concentration of Mercury, Copper Cadmium and Lead in Fruiting Bodies of Edible Mushrooms in the Vicinity of Mercury and Copper Smelter. Sci. Total Environ. 1996, 177, 251–258. [Google Scholar] [CrossRef]
- Tüzen, M.; Özdemir, M.; Demirbaş, A. Study of heavy metals in some cultivated and uncultivated mushrooms of Turkish origin. Food Chem. 1998, 63, 247–251. [Google Scholar] [CrossRef]
- Yilmaz, F.; Isiloglu, M.; Merdivan, M. 2003—Heavy metal levels in some macrofungi. Turk. J. Bot. 2003, 27, 45–56. [Google Scholar]
- Durkan, N.; Ugulu, I.; Unver, M.C.; Dogan, Y.; Baslar, S. Concentrations of trace elements aluminum, boron, cobalt and tin in various wild edible mushroom species from Buyuk Menderes River Basin of Turkey by ICP-OES. Trace Elem. Electrolytes 2011, 28, 242–248. [Google Scholar] [CrossRef]
- Mleczek, M.; Siwulski, M.; Mikołajczak, P.; Gąsecka, M. Differences in Cu content in selected mushroom species growing in the same unpolluted areas in Poland. J. Environ. Sci. Health 2015, 50, 659–666. [Google Scholar] [CrossRef]
- Ouzouni, P.; Riganakos, K. Nutritional value and metal content profile of Greek wild edible fungi. Acta Aliment. 2007, 36, 99–110. [Google Scholar] [CrossRef]
- Soylak, M.; Saracoglu, S.; Tuzen, M.; Mendil, D. Determination of trace metals in mushroom samples from Kayseri, Turkey. Food Chem. 2005, 92, 649–652. [Google Scholar] [CrossRef]
- Yamada, A. Utility of mycorrhizal mushrooms as food resources in Japan. J. Fac. Agric. Shinshu Univ. 2002, 1, 1–7. [Google Scholar]
- Kamalebo, H.M.; De Kesel, A. Wild edible ectomycorrhizal fungi: An underutilized food resource from the rainforests of Tshopo province (Democratic Republic of the Congo). J. Ethnobiol. Ethnomed. 2020, 16, 8. [Google Scholar] [CrossRef] [Green Version]
- Arvay, J.; Tomáš, J.; Hauptvogl, M.; Massányi, P.; Harangozo, L.; Tóth, T.; Stanovič, R.; Bryndzová, S.; Bumbalová, M. Human exposure to heavy metals and possible public health risks via consumption of wild edible mushrooms from Slovak paradise national park, Slovakia. J. Environ. Sci. Health. 2015, 50, 833–843. [Google Scholar] [CrossRef]
- Omeljaniuk, W.J.; Socha, K.; Soroczynska, J.; Charkiewicz, A.E.; Laudanski, T.; Kulikowski, M.; Kobylec, E.; Borawska, M.H. Cadmium and lead in women who miscarried. Clin Lab. 2018, 64, 59–67. [Google Scholar] [CrossRef]
- Jablonska, E.; Socha, K.; Reszka, E.; Wieczorek, E.; Skokowski, J.; Kalinowski, L.; Fendler, W.; Seroczynska, B.; Wozniak, M.; Borawska, M.; et al. Cadmium, arsenic, selenium and iron- Implications for tumor progression in breast cancer. Environ. Toxicol. Pharmacol. 2017, 53, 151–157. [Google Scholar] [CrossRef]
- WHO. Evaluation of certain contaminants in food. World Health Organ Tech. Rep. Ser. 2011, 959, 1–105. [Google Scholar]
- Leonhardt, T.; Borovicka, J.; Sacký, J.; Santrucek, J.; Kameník, J.; Kotrba, P. Zn over accumulating Russula species clade together and use the same mechanism for the detoxification of excess Zn. Chemosphere 2019, 225, 618–626. [Google Scholar] [CrossRef]
- Majorel, C.; Hannibal, L.; Soupe, M.; Carriconde, F.; Ducousso, M.; Lebrun, M.; Jourand, P. Tracking nickel-adaptive biomarkers in Pisolithus albus from New Caledonia using a tran-scriptomic approach. Mol Ecol. 2012, 21, 2208–2223. [Google Scholar] [CrossRef] [PubMed]
- Jourand, P.; Hannibal, L.; Majorel, C.; Mengant, S.; Ducousso, M.; Lebrun, M. Ectomycorrhizal Pisolithus albus inoculation of Acacia spirorbis and Eucalyptus globulus grown in ultramafic topsoil enhances plant growth and mineral nutrition while limits metal uptake. J. Plant Physiol. 2014, 171, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Beneš, V.; Leonhardt, T.; Sácký, J.; Kotrba, P. Two P1B-1-ATPases of Amanita strobiliformis with Distinct Properties in Cu/Ag Transport. Front. Microbiol. 2018, 23, 747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colpaert, J.; Wevers, J.; Krznaric, E.; Adriaensen, K. How metal-tolerant ecotypes of ectomycorrhizal fungi protect plants from heavy metal pollution. Ann. For. Sci. 2011, 68, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Vrålstad, T.; Schumacher, T.; Taylor, A.F.S. Mycorrhizal synthesis between fungal strains of the Hymenoscyphus ericae aggregate and potential ectomycorrhizal and ericoid hosts. New Phytol. 2002, 153, 143–152. [Google Scholar] [CrossRef]
- Johansson, L.; Xydas, C.; Messios, N.; Stoltz, E.; Greger, M. Growth and Cu accumulation by plants grown on Cu containing mine tailings in Cyprus. Appl. Geochem. 2005, 20, 101–107. [Google Scholar] [CrossRef]
- Adriaensen, K.; Vangronsveld, J.; Colpaert, J.V. Zinc-tolerant Suillus bovinus improves growth of Zn-exposed Pinus sylvestris seedlings. Mycorrhiza 2006, 16, 553–558. [Google Scholar] [CrossRef]
- Wilkinson, D.M.; Dickinson, N.M. Metal Resistance in Trees: The Role of Mycorrhizae. Oikos 1995, 72, 298–300. [Google Scholar] [CrossRef]
- Bellion, M.; Courbot, M.; Jacob, C.; Blaudez, D.; Chalot, M.; Courbot, M. Extracellular and cellular mechanisms sustaining metal tolerance in ectomycorrhizal fungi. FEMS Microbiol. Lett. 2006, 254, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Fogarty, R.V.; Tobin, J.M. Fungal melanins and their interactions with metals. Enzym. Microb. Technol. 1996, 19, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Blaudez, D.; Botton, B.; Chalot, M. Cadmium uptake and subcellular compartmentation in the ectomycorrhizal fungus Paxillus involutus. Microbiology 2000, 146, 1109–1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahonen-Jonnarth, U.; Patrick, A.W.; Van, H.; Lundstrom, U.S.; Finlay, R.D. Organic acids produced by mycorrhizal Pinus sylvestris exposed to elevated aluminium and heavy metal concentrations. New Phytol. 2000, 146, 557–567. [Google Scholar] [CrossRef]
- Morselt, A.F.W.; Smits, W.T.M.; Limonard, T. Histochemical demonstration of heavy metal tolerance in ectomycorrhizal fungi. Plant Soil 1986, 96, 417–420. [Google Scholar] [CrossRef]
- Reddy, M.S.; Prasanna, L.; Marmeisse, R.; Fraissinet-Tachet, L. Differential expression of metallothioneins in response to heavy metals and their involvement in metal tolerance in the symbiotic basidiomycete Laccaria bicolor. Microbiology 2014, 160, 2235–2242. [Google Scholar] [CrossRef] [Green Version]
- Ramesh, G.; Podila, G.K.; Gay, G.; Marmeisse, R.; Reddy, M.S. Differential pattern of regulation for the copper and cadmium induced metallothioneins of the ectomycorrhizal fungus Hebeloma cylindrosporum. Appl. Environ. Microbiol. 2009, 75, 2266–2274. [Google Scholar] [CrossRef] [Green Version]
- Courbot, M.; Diez, L.; Ruotolo, R.; Chalot, M.; Leroy, P. Cadmium-Responsive Thiols in the Ectomycorrhizal Fungus Paxillus involutus. Appl. Environ. Microbiol. 2004, 70, 7413–7417. [Google Scholar] [CrossRef] [Green Version]
- Gallie, U.; Meire, M.; Brunold, C. Effect of cadmium on non-mycorrhizal and mycorrhizal Norway spruce seedlings Picea abies (L) Karst and its ectomycorrhizal fungi Laccaria laccata (Scop ex Fr) Bk and Br- sulfate reduction, thiols and distribution of the heavy- metals. New Phytol. 1993, 125, 837–843. [Google Scholar] [CrossRef]
- Ilyas, S.; Rehman, A. Oxidative stress, glutathione level and antioxidant response to heavy metals in multi-resistant pathogen, Candida tropicalis. Environ. Monit. Assess. 2015, 187, 1–7. [Google Scholar] [CrossRef]
- Howe, D.K.; Honoré, S.; Derouin, F.; Sibley, L.D. Determination of genotypes of Toxoplasma gondii strains isolated from patients with toxoplasmosis. J. Clin. Microbiol. 1997, 35, 1411–1414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colpaert, J.; Muller, L.A.H.; Lambaerts, M.; Adriaensen, K. Evolutionary adaptation to Zn toxicity in populations of Suilloid fungi. New Phytol. 2004, 162, 549–559. [Google Scholar] [CrossRef]
- Williams, L.E.; Pittman, J.K.; Hall, J.L. Emerging mechanisms for heavy metal transport in plants. Biochim. Biophys. Acta-Biomembr. 2000, 1465, 104–126. [Google Scholar] [CrossRef]
- Hall, J.L. Cellular mechanisms for heavy metal detoxification and tolerance. J. Exp. Bot. 2002, 53, 1–11. [Google Scholar] [CrossRef] [PubMed]
- McCreight, J.D.; Schroeder, D.B. Cadmium, lead and nickel content of Lycoperdon perlatum pers. in a roadside environment. Environ. Pollut. 1977, 13, 265–268. [Google Scholar] [CrossRef]
- Laaksovirta, K.; Alakuijala, P. Lead, cadmium, and zinc contents of fungi in the parks of Helsinki. Ann. Bot. Fenn. 1978, 15, 253–257. [Google Scholar]
- Bargagli, R.; Baldi, F. Mercury and methyl mercury in higher fungi and their relation with the substrata in a cinnabar mining area. Chemosphere 1984, 13, 1059–1071. [Google Scholar] [CrossRef]
- Azul, A.M. Diversidade de fungos ectomicorrízicos emecossitemas de Montado. Ph.D. Dissertation, University of Coimbra, Coimbra, Portugal, 2002. [Google Scholar]
- Lalotra, P.; Gupta, D.; Yangdol, R.; Sharma, Y.P. Bioaccumulation of heavy metals in the sporocarps of some wild mushrooms. Curr. Res. Environ. Appl. Mycol. 2016, 6, 159–165. [Google Scholar] [CrossRef]
- Keniry, K.L. Climate Change Impacts on Ectomycorrhizal Fungi Associated with Australian Eucalypts. Ph.D. Thesis, University of Western Sydney, Hawkesbury, Australia, March 2015. [Google Scholar]
- Truong, C.; Luciano, A.G.; Adriana, C.; Alija, B.M. Ectomycorrhizal fungi and soil enzymes exhibit contrasting patterns along elevation gradients in southern Patagonia. New Phytol. 2019, 222, 1936–1950. [Google Scholar] [CrossRef]
- Bergendorf, O.; Sterner, O. The sesquiterpenes of Lactarius deliciosus and Lactarius deterrimus. Phytochemistry 1988, 27, 97–100. [Google Scholar] [CrossRef]
- Laguette, A.; Cummings, N.; Butler, R.C.; Willows, A. Lactarius deliciosus and Pinus radiata in New Zealand: Towards the development of innovative gourmet mushroom orchards. Mycorrhiza 2014, 24, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Watling, R. British fungus flora. Agarics and Boleti. 1. Boletaceae: Gomphidiaceae: Paxillaceae; H.M. Stationery Office: Edinburgh, Scotland, 1970; p. 108. [Google Scholar]
- Alessio, C.L. Un boleto non ancora noto. Xerocomus ichnusanus Alessio, Galli et Littini sp. nov. Boll. Gruppo. Micol. G Bres. 1984, 27, 166–170. [Google Scholar]
- Rochon, C.; Paré, D.; Pélardy, N.; Khasa, D.P. Ecology and productivity of Cantharellus cibarius var. roseocanus in two eastern Canadian jack pine stands. Botany 2011, 89, 663–675. [Google Scholar] [CrossRef]
- Courty, P.; Nathalie, B.; Garbaye, J. Relation between oak tree phenology and the secretion of organic matter degrading enzymes by Lactarius quietus ectomycorrhizas before and during bud break. Soil Biol. Biochem. 2007, 39, 1655–1663. [Google Scholar] [CrossRef]
- Wisitrassameewong, K.; Karunarathna, S.C.; Thongklang, N.; Zhao, R.L. Agaricus subrufescens: New records to Thailand. Chiang Mai Univ. J. Sci. 2012, 39, 281–291. [Google Scholar]
- Falandysz, J.; Kunito, T.; Kubota, R.; Gucia, M. Some mineral constituents of Parasol Mushroom (Macrole piota procera). J. Environ. Sci. Health 2008, 43, 187–192. [Google Scholar] [CrossRef]
- Satora, L.; Pach, D.; Butryn, B.; Hydzik, P.; Balicka-Ślusarczyk, B. Fly agaric (Amanita muscaria) poisoning, case report and review. Toxicon 2005, 45, 941–943. [Google Scholar] [CrossRef]
- Falandysz, J.; Lipka, K.; Mazur, A. Mercury and its bio-concentration factors in fly agaric (Amanita muscaria) from spatially distant sites in Poland. Environ. Sci. Health 2007, 42, 1625–1630. [Google Scholar] [CrossRef]
- Tsantrizos, Y.S.; Kope, H.H.; Fortin, J.A.; Ogilvie, K.K. Antifungal antibiotics from Pisolithus tinctorius. Phytochemistry 1991, 30, 1113–1118. [Google Scholar] [CrossRef]
- Razzak, A.; Shahzad, S. Pisolithus tinctorus, a new record from Pakistan. Pak. J. Bot. 2004, 36, 449–451. [Google Scholar]
- Gawda, D.K.S.; Rajagopal, D. Association of Termitomyces spp. with fungus growing termites. Anim. Sci. 1990, 99, 311–315. [Google Scholar]
- Aryal, H.P.; Budhathoki, U. Ethnomycology of Termitomyces R. Heim in Nepal. J. Yeast Fungal Res. 2016, 7, 28–38. [Google Scholar]
- Vetter, J. Mineral element content of edible and poisonous macrofungi. Acta Aliment. 1990, 19, 27–40. [Google Scholar]
- Redhead, S.A.; Norvell, L.L.; Danell, E.; Ryman, S. (1537–1538) Proposals to conserve the names Cantharellus lutescens Fr.: Fr. and C. tubaeformis Fr.: Fr. (Basidiomycota) with conserved types. TAXON 2002, 51, 559–562. [Google Scholar] [CrossRef]
- Trappe, M.J. Habitat and Host Associations of Craterellus tubaeformis in Northwestern Oregon. Mycologia 2004, 96, 498–509. [Google Scholar] [CrossRef] [PubMed]
- Acharya, K.; Rai, M.; Pradhan, P. Agaricales of Sikkim Himalaya: A Review. Researcher 2010, 2, 29–38. [Google Scholar] [CrossRef]
- Wang, Z.-X.; Chen, J.-Q.; Chai, L.-Y.; Yang, Z.-H.; Huang, S.-H.; Zheng, Y. Environmental impact and site-specific human health risks of chromium in the vicinity of a ferro-alloy manufactory, China. J. Hazard. Mater. 2011, 190, 980–985. [Google Scholar] [CrossRef] [PubMed]
- Drewnowska, M.; Falandysz, J. Investigation on mineral composition and accumulation by popular edible mushroom common chanterelle (Cantharellus cibarius). Ecotoxicol. Environ. Saf. 2015, 113, 9–17. [Google Scholar] [CrossRef]
- Azizi, A.B.; Lim, M.P.M.; Noor, Z.M.; Abdullah, N. Vermi removal of heavy metal in sewage sludge by utilising Lumbricus rubellus. Ecotoxicol. Environ. Saf. 2013, 90, 13–20. [Google Scholar] [CrossRef]
- Khatua, S.; Dutta, A.K.; Chandra, S.; Paloi, S.; Das, K.; Acharya, K. Introducing a novel mushroom from mycophagy community with emphasis on biomedical potency. PLoS ONE 2017, 12, e0178050. [Google Scholar] [CrossRef] [Green Version]
- Tibuhwa, D.D. Cytotoxicity, antimicrobial and antioxidant activities of Boletus bicolor, a basidiomycetes mushroom indigenous to Tanzania. AJOL 2017, 43, 151–163. [Google Scholar]
- Wȩgiel, J.; Końska, G.; Guillot, J.; Muszynska, B. Isolation and antimitotic activity of Polysaccharides from fruit bodies of Xerocomus badius (FR.) kühn. ex gilib. Acta Biol. Crac. Ser. Bot. 2001, 43, 59–64. [Google Scholar]
- Kacholi, D.S.; Sahu, M. Levels and Health Risk Assessment of Heavy Metals in Soil, Water, and Vegetables of Dar es Salaam, Tanzania. J. Chem. 2018, 2018, 1402674. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ediriweera, A.N.; Karunarathna, S.C.; Yapa, P.N.; Schaefer, D.A.; Ranasinghe, A.K.; Suwannarach, N.; Xu, J. Ectomycorrhizal Mushrooms as a Natural Bio-Indicator for Assessment of Heavy Metal Pollution. Agronomy 2022, 12, 1041. https://doi.org/10.3390/agronomy12051041
Ediriweera AN, Karunarathna SC, Yapa PN, Schaefer DA, Ranasinghe AK, Suwannarach N, Xu J. Ectomycorrhizal Mushrooms as a Natural Bio-Indicator for Assessment of Heavy Metal Pollution. Agronomy. 2022; 12(5):1041. https://doi.org/10.3390/agronomy12051041
Chicago/Turabian StyleEdiriweera, Aseni Navoda, Samantha Chandranath Karunarathna, Pinnaduwage Neelamanie Yapa, Douglas Allen Schaefer, Arani Koshathaki Ranasinghe, Nakarin Suwannarach, and Jianchu Xu. 2022. "Ectomycorrhizal Mushrooms as a Natural Bio-Indicator for Assessment of Heavy Metal Pollution" Agronomy 12, no. 5: 1041. https://doi.org/10.3390/agronomy12051041







