High-Throughput Virus Screening in Crosses of South American and African Cassava Germplasm Reveals Broad-Spectrum Resistance against Viruses Causing Cassava Brown Streak Disease and Cassava Mosaic Virus Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cassava Viruses
2.2. Cassava Seeds and Seedlings
2.3. Virus Infections
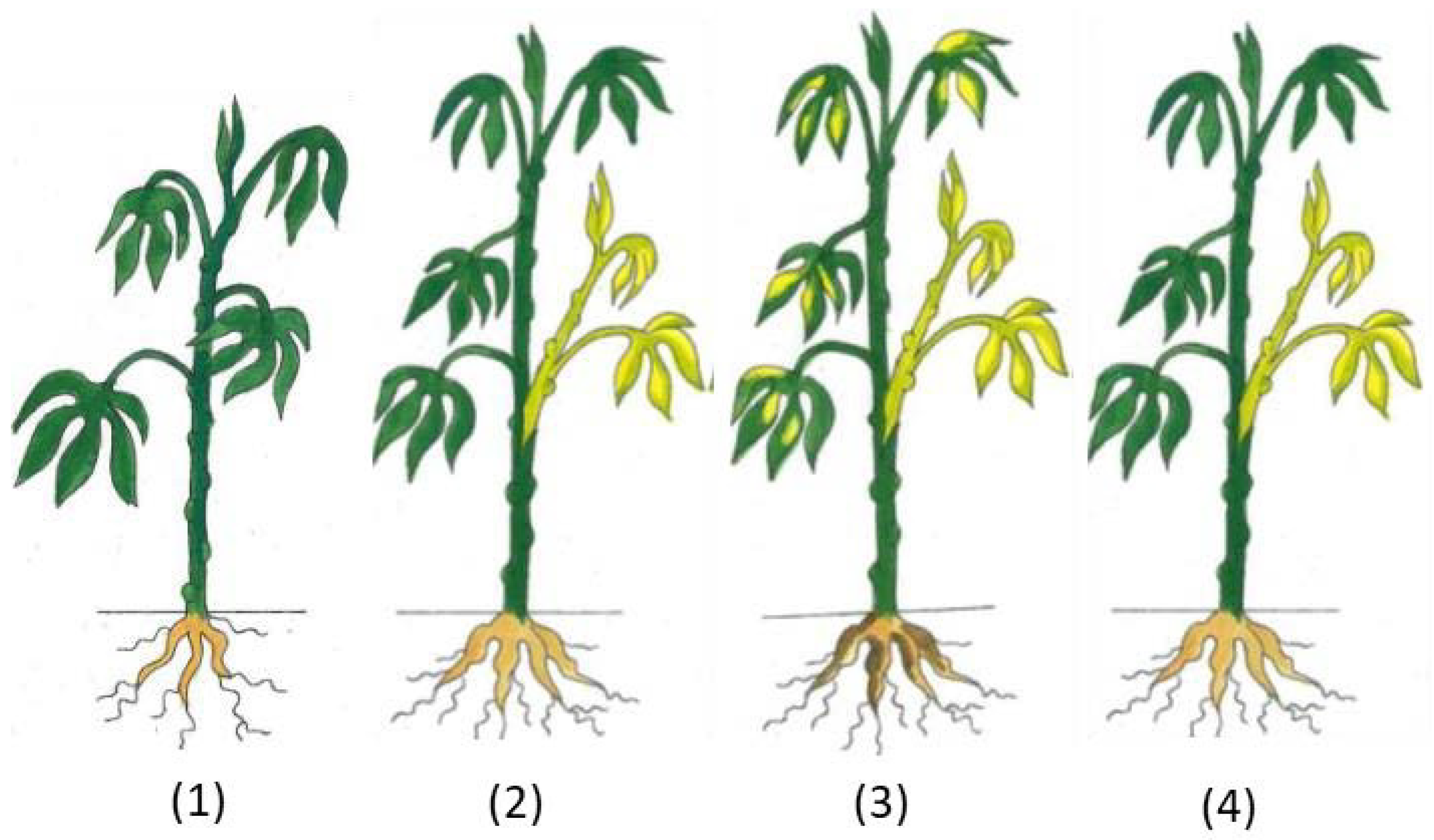
2.4. Symptom Assessment and Virus Detection
2.5. Resistance Screening Protocol
3. Results
3.1. Infection of Cassava Seedlings with CBSV, UCBSV, and EACMV-UG
3.2. Mounting a Precise and Efficient Screening Strategy to Identify Virus Resistance in Cassava
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jennings, D.L. Further Studies in Breeding Cassava for Virus Resistance. East Afr. Agric. J. 1957, 22, 213–219. [Google Scholar] [CrossRef]
- Hillocks, R.J.; Jennings, D.L. Cassava brown streak disease: A review of present knowledge and research needs. Int. J. Pest Manag. 2003, 49, 225–234. [Google Scholar] [CrossRef]
- Akano, A.O.; Dixon, A.G.O.; Mba, C.; Barrera, E.; Fregene, M. Genetic mapping of a dominant gene conferring resistance to cassava mosaic disease. Theor. Appl. Genet. 2002, 105, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Atiri, G.I.; Ogbe, F.O.; Dixon, A.G.O.; Winter, S.; Ariyo, O. Status of cassava mosaic virus diseases and cassava Begomoviruses in sub-Saharan Africa. J. Sustain. Agric. 2004, 24, 5–35. [Google Scholar] [CrossRef]
- Ariyo, O.A.; Atiri, G.I.; Dixon, A.G.O.; Winter, S. The use of biolistic inoculation of cassava mosaic begomoviruses in screening cassava for resistance to cassava mosaic disease. J. Virol. Methods 2006, 137, 43–50. [Google Scholar] [CrossRef]
- Ariyo, O.A.; Koerbler, M.; Dixon, A.G.O.; Atiri, G.I.; Winter, S. Molecular variability and distribution of Cassava mosaic begomoviruses in Nigeria. J. Phytopathol. 2005, 153, 226–231. [Google Scholar] [CrossRef]
- Rabbi, I.Y.; Hamblin, M.T.; Kumar, P.L.; Gedil, M.A.; Ikpan, A.S.; Jannink, J.L.; Kulakow, P.A. High-resolution mapping of resistance to cassava mosaic geminiviruses in cassava using genotyping-by-sequencing and its implications for breeding. Virus Res. 2014, 186, 87–96. [Google Scholar] [CrossRef] [Green Version]
- Codjia, E.D.; Olasanmi, B.; Agre, A.P.; Uwugiaren, R.; Ige, A.D.; Rabbi, I.Y. Selection for resistance to cassava mosaic disease in African cassava germplasm using single nucleotide polymorphism markers. S. Afr. J. Sci. 2022, 118. [Google Scholar] [CrossRef]
- Okogbenin, E.; Egesi, C.N.; Olasanmi, B.; Ogundapo, O.; Kahya, S.; Hurtado, P.; Marin, J.; Akinbo, O.; Mba, C.; Gomez, H.; et al. Molecular Marker Analysis and Validation of Resistance to Cassava Mosaic Disease in Elite Cassava Genotypes in Nigeria. Crop Sci. 2012, 52, 2576–2586. [Google Scholar] [CrossRef] [Green Version]
- Dixon, A.G.O.; Ogbe, F.O.; Okechukwu, R.U. Cassava mosaic disease in Sub-Saharan Africa: A feasible solution for an unsolved problem. Outlook Agric. 2010, 39, 89–94. [Google Scholar] [CrossRef]
- Lokko, Y.; Dixon, A.G.O.; Offei, S.K.; Danquah, E.Y. Genetic relationships among improved cassava accessions and landraces for resistance to the cassava mosaic disease. J. Food Agric. Environ. 2009, 7, 156–162. [Google Scholar]
- Sheat, S.; Fuerholzner, B.; Stein, B.; Winter, S. Resistance Against Cassava Brown Streak Viruses From Africa in Cassava Germplasm From South America. Front. Plant Sci. 2019, 10, 567. [Google Scholar] [CrossRef] [PubMed]
- Ariyo, O.A.; Dixon, A.G.; Atiri, G.I. Whitefly Bemisia tabaci (Homoptera: Aleyrodidae) infestation on cassava genotypes grown at different ecozones in Nigeria. J. Econ. Entomol. 2005, 98, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Nzuki, I.; Katari, M.S.; Bredeson, J.V.; Masumba, E.; Kapinga, F.; Salum, K.; Mkamilo, G.S.; Shah, T.; Lyons, J.B.; Rokhsar, D.S.; et al. QTL Mapping for Pest and Disease Resistance in Cassava and Coincidence of Some QTL with Introgression Regions Derived from Manihot glaziovii. Front. Plant Sci. 2017, 8, 1168. [Google Scholar] [CrossRef] [Green Version]
- Hillocks, R.J.; Raya, M.; Thresh, J.M. The association between root necrosis and above-ground symptoms of brown streak virus infection of cassava in southern Tanzania. Int. J. Pest Manag. 1996, 42, 285–289. [Google Scholar] [CrossRef]
- Kawuki, R.S.; Kaweesi, T.; Esuma, W.; Pariyo, A.; Kayondo, I.S.; Ozimati, A.; Kyaligonza, V.; Abaca, A.; Orone, J.; Tumuhimbise, R.; et al. Eleven years of breeding efforts to combat cassava brown streak disease. Breed. Sci. 2016, 66, 560–571. [Google Scholar] [CrossRef] [Green Version]
- Thuy, C.T.L.; Lopez-Lavalle, L.A.B.; Vu, N.A.; Hy, N.H.; Nhan, P.T.; Ceballos, H.; Newby, J.; Tung, N.B.; Hien, N.T.; Tuan, L.N.; et al. Identifying New Resistance to Cassava Mosaic Disease and Validating Markers for the C2 Locus. Agriculture 2021, 11, 829. [Google Scholar] [CrossRef]
- Winter, S.; Koerbler, M.; Stein, B.; Pietruszka, A.; Paape, M.; Butgereitt, A. Analysis of cassava brown streak viruses reveals the presence of distinct virus species causing cassava brown streak disease in East Africa. J. Gen. Virol. 2010, 91, 1365–1372. [Google Scholar] [CrossRef]
- Otti, G.; Bouvaine, S.; Kimata, B.; Mkamillo, G.; Kumar, P.L.; Tomlins, K.; Maruthi, M.N. High-throughput multiplex real-time PCR assay for the simultaneous quantification of DNA and RNA viruses infecting cassava plants. J. Appl. Microbiol. 2016, 120, 1346–1356. [Google Scholar] [CrossRef] [Green Version]
- Sheat, S.; Margaria, P.; Winter, S. Differential Tropism in Roots and Shoots of Resistant and Susceptible Cassava (Manihot esculenta Crantz) Infected by Cassava Brown Streak Viruses. Cells 2021, 10, 1221. [Google Scholar] [CrossRef]
- Wolfe, M.D.; Rabbi, I.Y.; Egesi, C.; Hamblin, M.; Kawuki, R.; Kulakow, P.; Lozano, R.; Del Carpio, D.P.; Ramu, P.; Jannink, J.L. Genome-Wide Association and Prediction Reveals Genetic Architecture of Cassava Mosaic Disease Resistance and Prospects for Rapid Genetic Improvement. Plant Genome 2016, 9, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Wolfe, M.D.; Del Carpio, D.P.; Alabi, O.; Ezenwaka, L.C.; Ikeogu, U.N.; Kayondo, I.S.; Lozano, R.; Okeke, U.G.; Ozimati, A.A.; Williams, E.; et al. Prospects for Genomic Selection in Cassava Breeding. Plant Genome 2017, 10, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Ceballos, H.; Rojanaridpiched, C.; Phumichai, C.; Becerra, L.A.; Kittipadakul, P.; Iglesias, C.; Gracen, V.E. Excellence in Cassava Breeding: Perspectives for the Future. Crop Breed. Genet. Genom. 2020, 2, e200008. [Google Scholar] [CrossRef] [Green Version]
- Joaqui Barandica, O.; Perez, J.C.; Lenis, J.I.; Calle, F.; Morante, N.; Pino, L.; Hershey, C.H.; Ceballos, H. Cassava Breeding II: Phenotypic Correlations through the Different Stages of Selection. Front. Plant Sci. 2016, 7, 1649. [Google Scholar] [CrossRef] [Green Version]
- Hillocks, R.J.; Thresh, J.M. Cassava mosaic and cassava brown streak virus diseases in Africa: A comparative guide to symptoms and aetiologies. Root 2000, 7, 1–8. [Google Scholar]
- Maruthi, M.N.; Hillocks, R.J.; Mtunda, K.; Raya, M.D.; Muhanna, M.; Kiozia, H.; Rekha, A.R.; Colvin, J.; Thresh, J.M. Transmission of Cassava brown streak virus by Bemisia tabaci (Gennadius). J. Phytopathol. 2005, 153, 307–312. [Google Scholar] [CrossRef]
- Fondong, V.N. The Search for Resistance to Cassava Mosaic Geminiviruses: How Much We Have Accomplished, and What Lies Ahead. Front. Plant Sci. 2017, 8, 408. [Google Scholar] [CrossRef] [Green Version]
- Mukiibi, D.R.; Alicai, T.; Kawuki, R.; Okao-Okuja, G.; Tairo, F.; Sseruwagi, P.; Ndunguru, J.; Ateka, E.M. Resistance of advanced cassava breeding clones to infection by major viruses in Uganda. Crop Prot. 2019, 115, 104–112. [Google Scholar] [CrossRef]
- Masumba, E.A.; Kapinga, F.; Mkamilo, G.; Salum, K.; Kulembeka, H.; Rounsley, S.; Bredeson, J.V.; Lyons, J.B.; Rokhsar, D.S.; Kanju, E.; et al. QTL associated with resistance to cassava brown streak and cassava mosaic diseases in a bi-parental cross of two Tanzanian farmer varieties, Namikonga and Albert. Theor. Appl. Genet. 2017, 130, 2069–2090. [Google Scholar] [CrossRef] [Green Version]
- Kayondo, S.I.; Pino Del Carpio, D.; Lozano, R.; Ozimati, A.; Wolfe, M.; Baguma, Y.; Gracen, V.; Offei, S.; Ferguson, M.; Kawuki, R.; et al. Genome-wide association mapping and genomic prediction for CBSD resistance in Manihot esculenta. Sci. Rep. 2018, 8, 1549. [Google Scholar] [CrossRef] [Green Version]
- Maruthi, M.N.; Jeremiah, S.C.; Mohammed, I.U.; Legg, J.P. The role of the whitefly, Bemisia tabaci (Gennadius), and farmer practices in the spread of cassava brown streak ipomoviruses. J. Phytopathol. 2017, 165, 707–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Cassava Line | CBSV | UCBSV | EACMV |
|---|---|---|---|
| COL 40/DSC 118 | resistant | resistant | recovery |
| COL 2182/DSC 167 | resistant | resistant | susceptible |
| PER 221/DSC 250 | resistant | susceptible | susceptible |
| COL 144/DSC 120 | resistant | susceptible | susceptible |
| PER 353/DSC 260 | resistant/root restr. | resistant/root restr. | susceptible |
| C19 | susceptible | susceptible | susceptible |
| C33 | susceptible | susceptible | resistant |
| C39 | susceptible | susceptible | ? |
| C243 | susceptible | susceptible | susceptible |
| C413 | susceptible | susceptible | resistant |
| GM7673-3 | susceptible | susceptible | ? |
| GM1055B-1 | susceptible | susceptible | ? |
| GM10055B-2 | susceptible | susceptible | ? |
| Entry | Mother | Father | Seeds |
|---|---|---|---|
| 1 | PER 353 | GM 7673-3 | 14 |
| 2 | GM10054B-1 | PER 221 | 15 |
| 3 | GM10054B-1 | PER 353 | 15 |
| 4 | GM10054B-2 | PER 353 | 15 |
| 5 | GM10055B-2 | PER 353 | 15 |
| 6 | GM10062-1 | PER 353 | 15 |
| 7 | C 33 | PER 221 | 14 |
| 8 | C 33 | PER 353 | 15 |
| 9 | C 39 | PER 353 | 12 |
| 10 | C 243 | PER 353 | 15 |
| 11 | C 413 | PER 353 | 15 |
| 12 | COL 40 | C 33 | 8 |
| 13 | COL 144 | GM 7673-3 | 15 |
| 14 | COL 144 | GM10055B-1 | 15 |
| 15 | COL 144 | GM10055B-2 | 15 |
| 16 | COL 144 | C 19 | 15 |
| 17 | COL 144 | C 33 | 15 |
| 18 | COL 144 | C 39 | 15 |
| Total | 258 |
| Entry | Family | Seedling | CBSV Resistance | CBSV/UCBSV/EACMV Resistance | |
|---|---|---|---|---|---|
| 1 MAG | 6 MAG | 6 MAG | |||
| 1 | GM13472 | 9 | 1 | 1 | 1 |
| 2 | GM13473 | 12 | 7 | 6 | |
| 3 | GM13474 | 14 | 5 | 2 | |
| 4 | GM13475 | 12 | 5 | 3 | |
| 5 | GM13477 | 9 | 3 | 2 | |
| 6 | GM13478 | 13 | 2 | 2 | |
| 7 | GM13481 | 9 | 5 | 2 | |
| 8 | GM13482 | 11 | 6 | 5 | 2 |
| 9 | GM13483 | 9 | 1 | 0 | |
| 10 | GM13484 | 12 | 5 | 2 | |
| 11 | GM13485 | 13 | 2 | 2 | |
| 12 | GM13487 | 5 | 2 | 1 | 1 |
| 13 | GM13489 | 13 | 4 | 3 | |
| 14 | GM13490 | 3 | 1 | 1 | |
| 15 | GM13491 | 6 | 0 | 0 | |
| 16 | GM13493 | 10 | 2 | 3 | |
| 17 | GM13494 | 12 | 4 | 4 | |
| 18 | GM13495 | 8 | 2 | 2 | 1 |
| Total | 180 | 57 | 41 | 5 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheat, S.; Zhang, X.; Winter, S. High-Throughput Virus Screening in Crosses of South American and African Cassava Germplasm Reveals Broad-Spectrum Resistance against Viruses Causing Cassava Brown Streak Disease and Cassava Mosaic Virus Disease. Agronomy 2022, 12, 1055. https://doi.org/10.3390/agronomy12051055
Sheat S, Zhang X, Winter S. High-Throughput Virus Screening in Crosses of South American and African Cassava Germplasm Reveals Broad-Spectrum Resistance against Viruses Causing Cassava Brown Streak Disease and Cassava Mosaic Virus Disease. Agronomy. 2022; 12(5):1055. https://doi.org/10.3390/agronomy12051055
Chicago/Turabian StyleSheat, Samar, Xiaofei Zhang, and Stephan Winter. 2022. "High-Throughput Virus Screening in Crosses of South American and African Cassava Germplasm Reveals Broad-Spectrum Resistance against Viruses Causing Cassava Brown Streak Disease and Cassava Mosaic Virus Disease" Agronomy 12, no. 5: 1055. https://doi.org/10.3390/agronomy12051055






