Polyploid Miscanthus Lutarioriparius: A Sustainable and Scalable Biomass Feedstock for Cellulose Nanocrystal Preparation in Biorefinery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling and Measurements
2.2. Flow Cytometry
2.3. Sample Pretreatment
2.4. CNC Preparation
2.5. Biomass Feedstock Chemical Contents Measurement
2.6. CNC Physical Property Measurement
2.7. Statistical Analysis
3. Results and Discussion
3.1. Chromosome Number Identification of PML
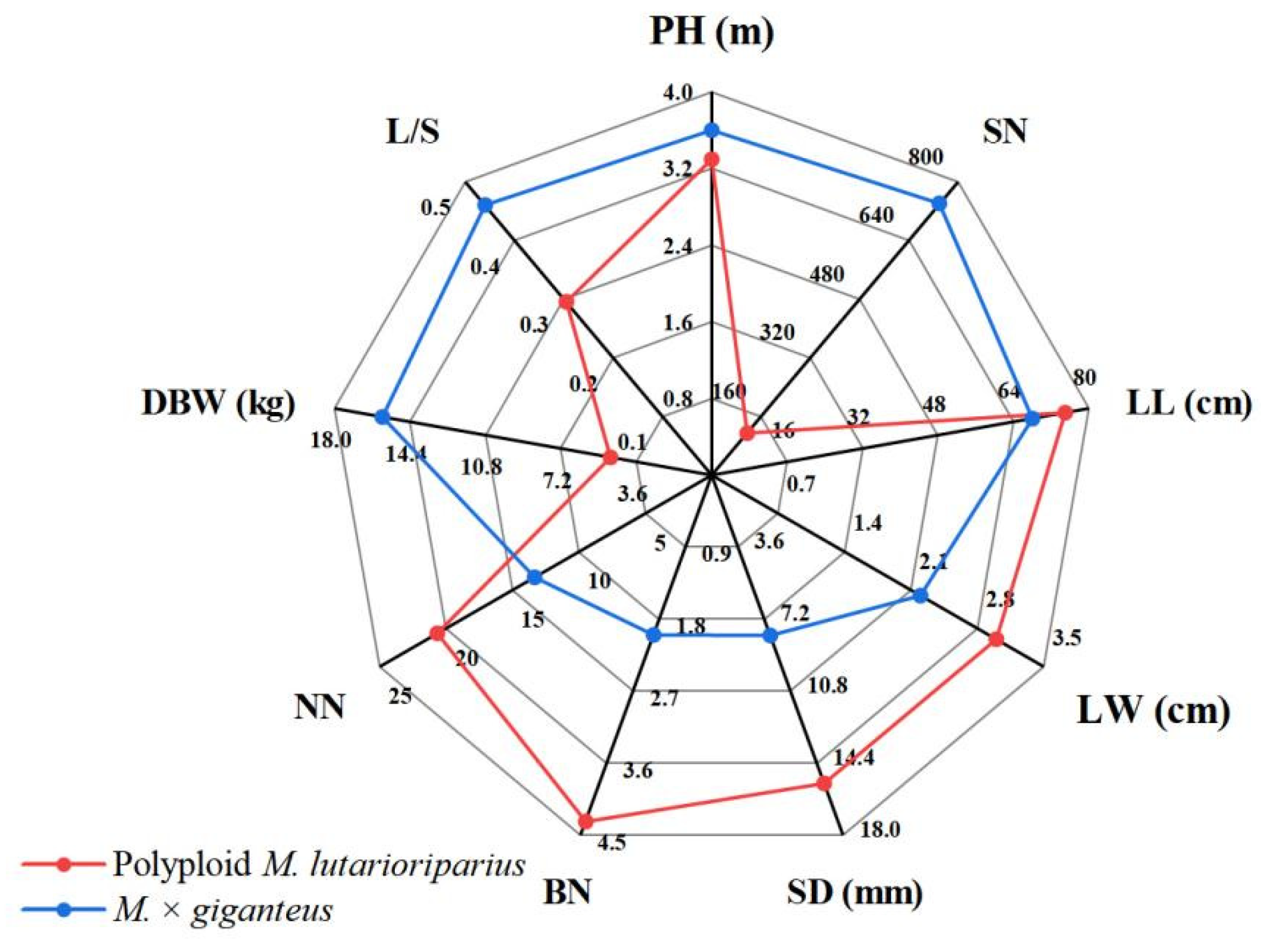
3.2. Significant Differences in Agronomic Traits between PML and MG
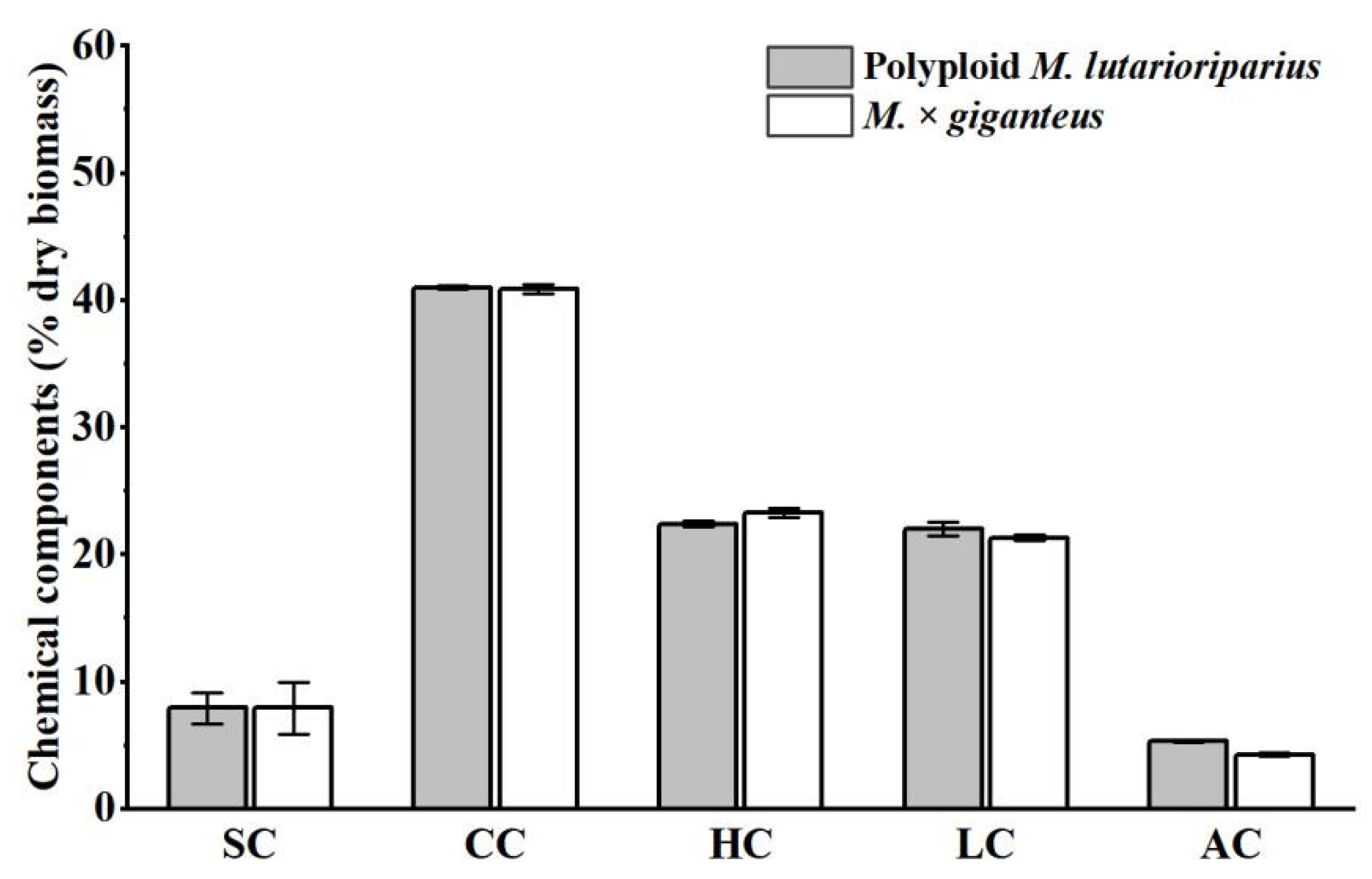
3.3. Comparison of Chemical Components and Physical Features of PML and MG
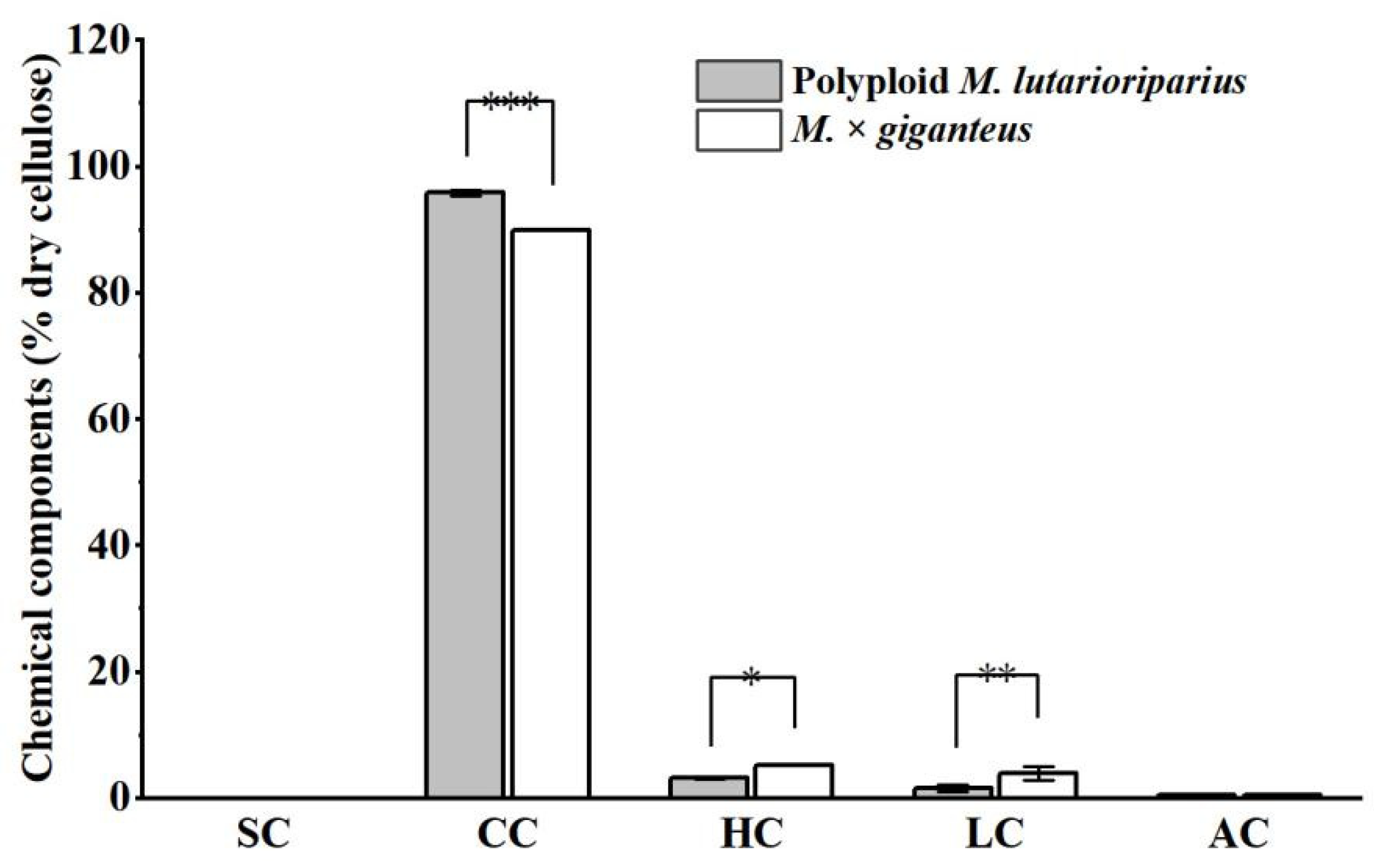
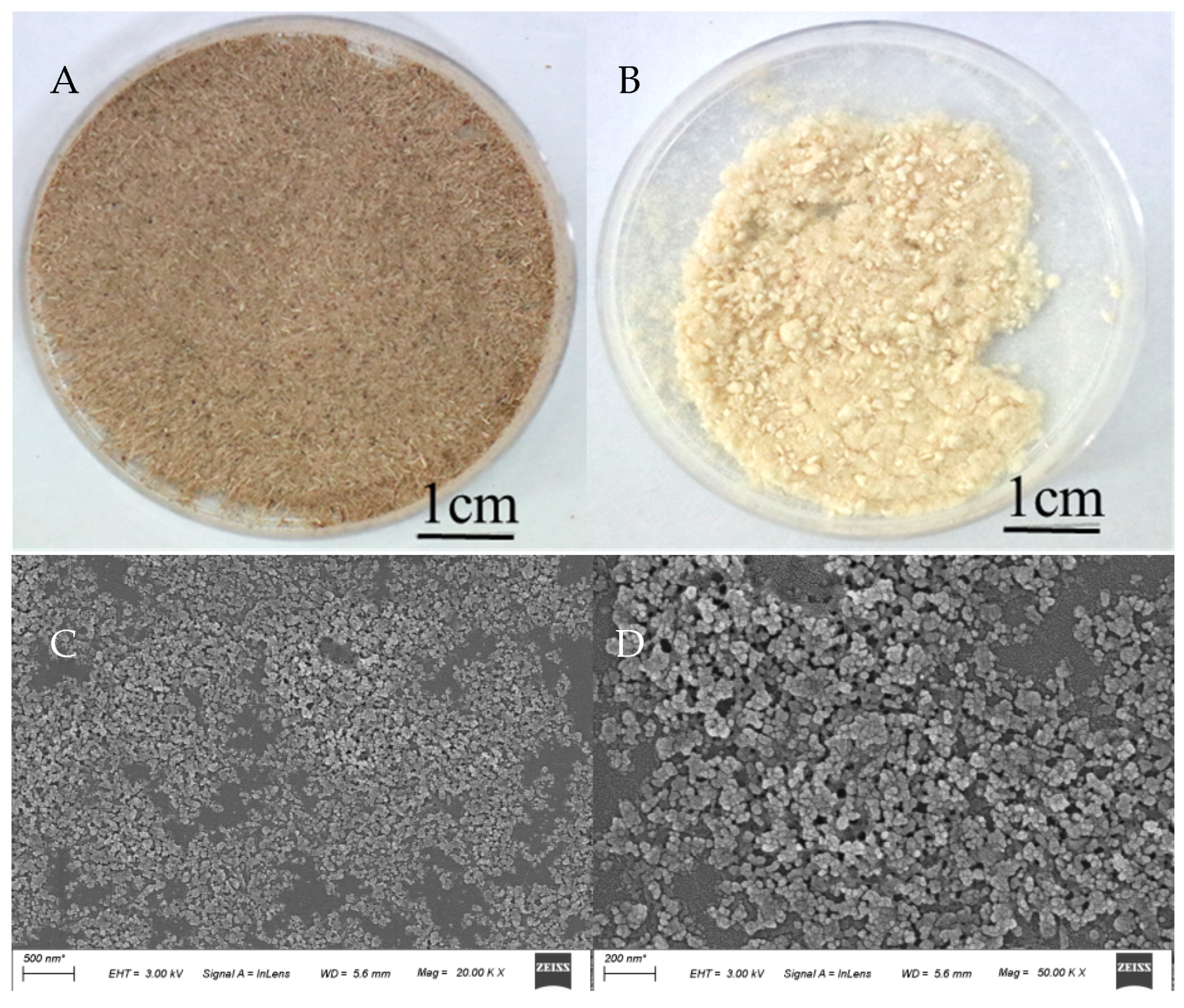
3.4. Isolation of Crude Cellulose from Raw Biomass of PML and MG by Modified Alkaline Peroxide Pretreatment
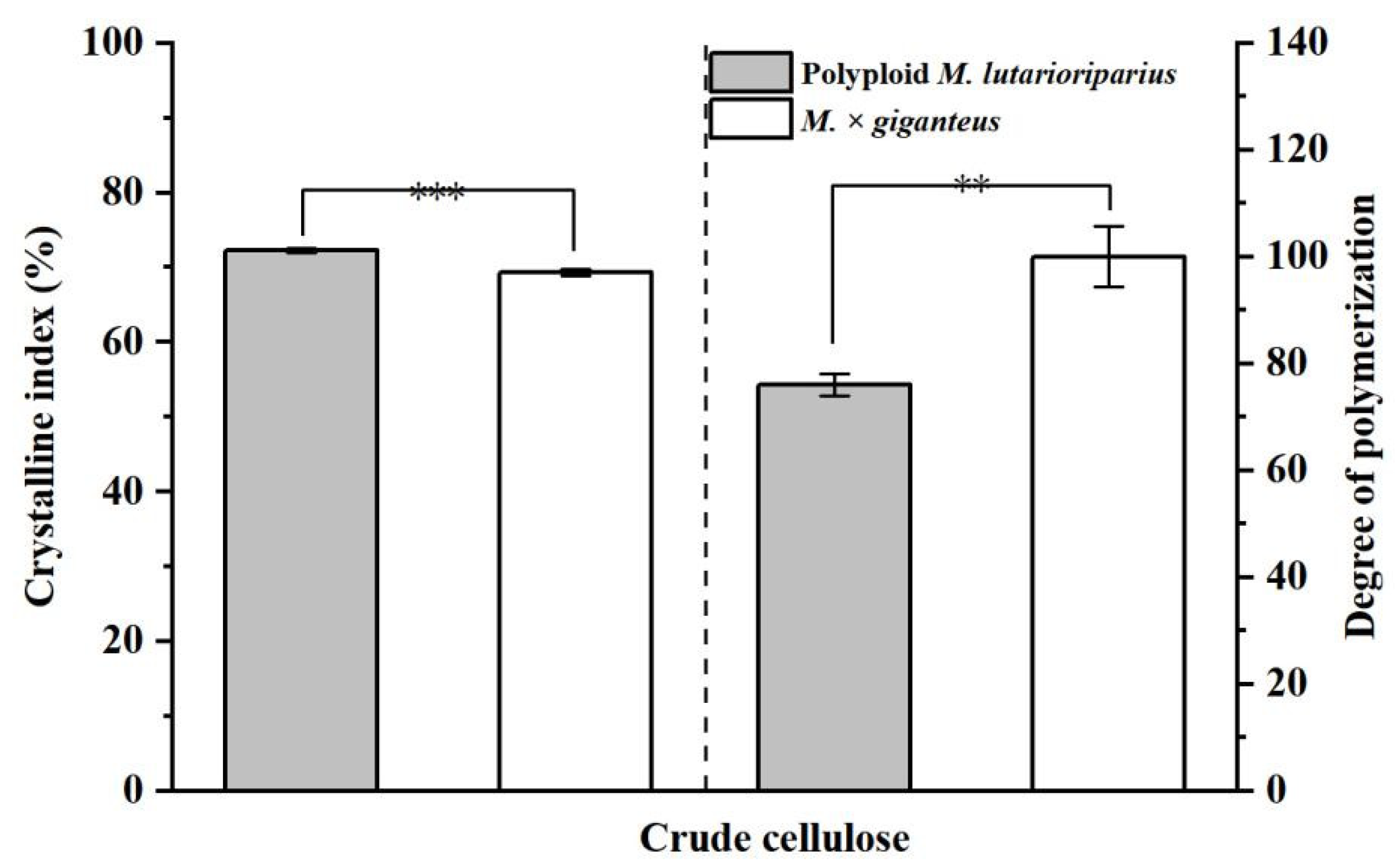
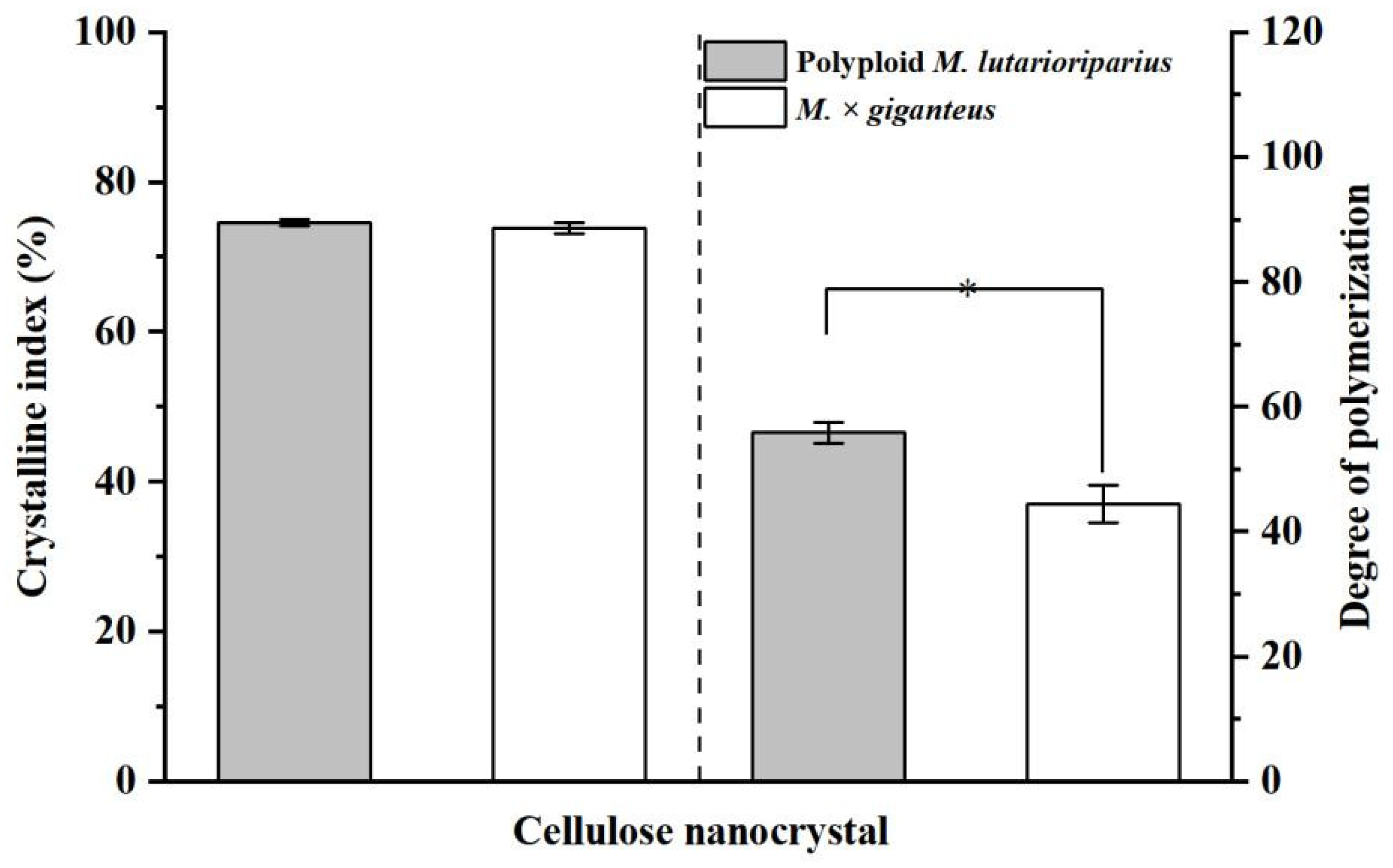
3.5. Comparative Analysis of CNC from Crude Cellulose of PML and MG Extracted by Sulfuric Acid Hydrolysis
3.6. The Yield Potential of Crude Cellulose and CNC
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malherbe, S.; Cloete, T.E. Lignocellulose biodegradation: Fundamentals and applications. Rev. Environ. Sci. Biotechnol. 2002, 1, 105–114. [Google Scholar] [CrossRef]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic Acid Hydrogels for Biomedical Applications. Adv. Mater. 2011, 23, H41–H56. [Google Scholar] [CrossRef] [PubMed]
- Lunardi, V.B.; Soetaredjo, F.E.; Putro, J.N.; Santoso, S.P.; Yuliana, M.; Sunarso, J.; Ju, Y.; Ismadji, S. Nanocelluloses: Sources, Pretreatment, Isolations, Modification, and Its Application as the Drug Carriers. Polymers 2021, 13, 2052. [Google Scholar] [CrossRef] [PubMed]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef]
- Tasnim, R.; Calderwood, L.; Tooley, B.; Wang, L.; Zhang, Y. Are Foliar Fertilizers Beneficial to Growth and Yield of Wild Lowbush Blueberries? Agronomy 2022, 12, 470. [Google Scholar] [CrossRef]
- Mateo, S.; Peinado, S.; Morillas-Gutiérrez, F.; La Rubia, M.D.; Moya, A.J. Nanocellulose from Agricultural Wastes: Products and Applications—A Review. Processes 2021, 9, 1594. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Mariano, M.; Huang, J.; Lin, N.; Ahmad, I.; Dufresne, A.; Thomas, S. Recent developments on nanocellulose reinforced polymer nanocomposites: A review. Polymer 2017, 132, 368–393. [Google Scholar] [CrossRef]
- Lucia, L.A.; Rojas, O.J. Fiber nanotechnology: A new platform for "green" research and technological innovations. Cellulose 2007, 14, 539–542. [Google Scholar] [CrossRef]
- Adelantado, C.; Ríos, Á.; Zougagh, M. Magnetic nanocellulose hybrid nanoparticles and ionic liquid for extraction of neonicotinoid insecticides from milk samples prior to determination by liquid chromatography-mass spectrometry. Food Addit. Contaminants. Part A Chem. Anal. Control. Expo. Risk Assess. 2018, 35, 1755–1766. [Google Scholar] [CrossRef]
- El-Samahy, M.A.; Mohamed, S.A.A.; Rehim, M.H.A.; Mohram, M.E. Synthesis of hybrid paper sheets with enhanced air barrier and antimicrobial properties for food packaging. Carbohydr. Polym. Sci. Technol. Asp. Ind. Important Polysacch. 2017, 168, 212–219. [Google Scholar] [CrossRef]
- Fontenot, K.R.; Edwards, J.V.; Haldane, D.; Pircher, N.; Liebner, F.; Condon, B.D.; Qureshi, H.; Yager, D. Designing cellulosic and nanocellulosic sensors for interface with a protease sequestrant wound-dressing prototype: Implications of material selection for dressing and protease sensor design. J. Biomater. Appl. 2017, 32, 622–637. [Google Scholar] [CrossRef] [PubMed]
- Bacakova, L.; Pajorova, J.; Bacakova, M.; Skogberg, A.; Kallio, P.; Kolarova, K.; Svorcik, V. Versatile Application of Nanocellulose: From Industry to Skin Tissue Engineering and Wound Healing. Nanomaterials 2019, 9, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lahiji, R.R.; Xu, X.; Reifenberger, R.; Raman, A.; Rudie, A.; Moon, R.J. Atomic Force Microscopy Characterization of Cellulose Nanocrystals. Langmuir 2010, 26, 4480–4488. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, Q.; Li, Y.; Zhang, Q.; Huang, J.; Wu, Q.; Wang, S. Fabrication of Cellulose Nanocrystal-g-Poly(Acrylic Acid-Co-Acrylamide) Aerogels for Efficient Pb(II) Removal. Polymers 2020, 12, 333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García, A.; Gandini, A.; Labidi, J.; Belgacem, N.; Bras, J. Industrial and crop wastes: A new source for nanocellulose biorefinery. Ind. Crops Prod. 2016, 93, 26–38. [Google Scholar] [CrossRef]
- Zhang, K.; Sun, P.; Liu, H.; Shang, S.; Song, J.; Wang, D. Extraction and comparison of carboxylated cellulose nanocrystals from bleached sugarcane bagasse pulp using two different oxidation methods. Carbohydr. Polym. 2016, 138, 237–243. [Google Scholar] [CrossRef]
- Barbash, V.A.; Yashchenko, O.V.; Vasylieva, O.A. Preparation and application of nanocellulose from Miscanthus × giganteus to improve the quality of paper for bags. SN Appl. Sci. 2020, 2, 727. [Google Scholar] [CrossRef] [Green Version]
- Atakhanov, A.; Turdikulov, I.; Mamadiyorov, B.; Abdullaeva, N.; Nurgaliev, I.; Khaydar, Y.; Rashidova, S. Isolation of Nanocellulose from Cotton Cellulose and Computer Modeling of Its Structure. Open J. Polym. Chem. 2019, 09, 117–129. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Liu, G.; Nges, I.A.; Liu, J. Enhanced biomethane production from Miscanthus lutarioriparius using steam explosion pretreatment. Fuel 2016, 179, 267–273. [Google Scholar] [CrossRef]
- Zheng, C.; Iqbal, Y.; Labonte, N.; Sun, G.; Feng, H.; Yi, Z.; Xiao, L. Performance of switchgrass and Miscanthus genotypes on marginal land in the Yellow River Delta. Ind. Crops Prod. 2019, 141, 111773. [Google Scholar] [CrossRef]
- Sai, Y.; Liang, X.; Zuan, W.; Zi-li, Y.I. Principal Components Analysis and Comprehensive Evaluation of Agronomy and Quality Traits of Miscanthus lutarioriparius. Chin. J. Grassl. 2016, 38, 26–33. [Google Scholar]
- Yang, S.; Xue, S.; Kang, W.; Qian, Z.; Yi, Z. Genetic diversity and population structure of Miscanthus lutarioriparius, an endemic plant of China. PLoS ONE 2019, 14, e211471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Junyi, D.; Yanlan, S.; Yuhuan, L.; Hongmei, H.; Qingbo, L.; Zili, Y.I.; Zhiyong, C. Characteristics of inorganic salt ion absorption of Miscanthus lutarioriparius polyploid under NaCl stress. Acta Prataculturae Sin. 2018, 35, 2893–2902. [Google Scholar]
- Nguyen, V.T.H.; Kraska, T.; Winkler, W.; Aydinlik, S.; Jackson, B.E.; Pude, R. Primary Mechanical Modification to Improve Performance of Miscanthus as Stand-Alone Growing Substrates. Agronomy 2022, 12, 420. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass. Available online: https://www.nrel.gov/docs/gen/fy13/42618.pdf (accessed on 18 March 2022).
- Peng, S.J. The Development of Artificial Polyploid in Miscanthus Lutarioriparius and Its Identification Technique. Master’s Thesis, Hunan Agricultural University, Changsha, China, 2016. [Google Scholar]
- Gabriel, T.; Belete, A.; Hause, G.; Neubert, R.H.H.; Gebre-Mariam, T. Isolation and Characterization of Cellulose Nanocrystals from Different Lignocellulosic Residues: A Comparative Study. J. Polym. Environ. 2021, 29, 2964–2977. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Ash in Biomass. Available online: https://www.nrel.gov/docs/gen/fy08/42622.pdf (accessed on 18 March 2022).
- Li, M.; Wang, J.; Yang, Y.; Xie, G. Alkali-based pretreatments distinctively extract lignin and pectin for enhancing biomass saccharification by altering cellulose features in sugar-rich Jerusalem artichoke stem. Bioresour. Technol. 2016, 208, 31–41. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Yi, Z.; Huang, J.; Li, F.; Hao, B.; Li, M.; Hong, S.; Lv, Y.; Sun, W.; Ragauskas, A.; et al. Three lignocellulose features that distinctively affect biomass enzymatic digestibility under NaOH and H2SO4 pretreatments in Miscanthus. Bioresour. Technol. 2013, 130, 30–37. [Google Scholar] [CrossRef]
- Cudjoe, E.; Hunsen, M.; Xue, Z.; Way, A.E.; Barrios, E.; Olson, R.A.; Hore, M.J.A.; Rowan, S.J. Miscanthus Giganteus: A commercially viable sustainable source of cellulose nanocrystals. Carbohydr. Polym. 2017, 155, 230–241. [Google Scholar] [CrossRef]
- Xiang, W.; Xue, S.; Qin, S.; Xiao, L.; Liu, F.; Yi, Z. Development of a multi-criteria decision making model for evaluating the energy potential of Miscanthus germplasms for bioenergy production. Ind. Crops Prod. 2018, 125, 602–615. [Google Scholar] [CrossRef]
- Yan, J.; Chen, W.; Luo, F.; Ma, H.; Meng, A.; Li, X.; Zhu, M.; Li, S.; Zhou, H.; Zhu, W.; et al. Variability and adaptability of Miscanthus species evaluated for energy crop domestication. Glob. Change Biol. Bioenergy 2012, 4, 49–60. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, Y.; Kato, R.; Rowan, S.J. Preparation of cellulose nanofibers from Miscanthus x. Giganteus by ammonium persulfate oxidation. Carbohydr. Polym. 2019, 212, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.V.; Hamid, S.B.A.; Zain, S.K. Conversion of Lignocellulosic Biomass to Nanocellulose: Structure and Chemical Process. Sci. World J. 2014, 2014, 1–20. [Google Scholar]
- Brosse, N.; Dufour, A.; Meng, X.; Sun, Q.; Ragauskas, A. Miscanthus: A fast-growing crop for biofuels and chemicals production. Biofuels Bioprod. Biorefining 2012, 6, 580–598. [Google Scholar] [CrossRef]
- Sharma, A.; Thakur, M.; Bhattacharya, M.; Mandal, T.; Goswami, S. Commercial application of cellulose nano-composites—A review. Biotechnol. Rep. 2019, 21, e00316. [Google Scholar] [CrossRef]
- Brinchi, L.; Cotana, F.; Fortunati, E.; Kenny, J.M. Production of nanocrystalline cellulose from lignocellulosic biomass: Technology and applications. Carbohydr. Polym. 2013, 94, 154–169. [Google Scholar] [CrossRef]
- Jonoobi, M.; Oladi, R.; Davoudpour, Y.; Oksman, K.; Dufresne, A.; Hamzeh, Y.; Davoodi, R. Different preparation methods and properties of nanostructured cellulose from various natural resources and residues: A review. Cellulose 2015, 22, 935–969. [Google Scholar] [CrossRef]
- Thomas, B.; Raj, M.C.; Athira, K.B.; Rubiyah, M.H.; Sanchez, C. Nanocellulose, a Versatile Green Platform: From Biosources to Materials and Their Applications. Chem. Rev. 2018, 118, 24. [Google Scholar] [CrossRef]
- Ribeiro, R.; Pohlmann, B.C.; Calado, V.; Bojorge, N.; Pereira, N. Production of nanocellulose by enzymatic hydrolysis: Trends and challenges. Eng. Life Sci. 2019, 19, 279–291. [Google Scholar] [CrossRef] [Green Version]



| Species | X-Mean | DNA Content (pg) | Chromosomes Number |
|---|---|---|---|
| Diploid Miscantus lutarioriparius | 199.4 | 4.4 | 2n |
| Polyploid Miscantus lutarioriparius | 283.5 | 6.3 | 3n |
| Species | Purity (%) |
|---|---|
| Polyploid Miscanthus lutarioriparius | 95.6 |
| Miscanthus × giganteus | 95.0 |
| Production | Species | Yield (%, Raw Biomass) |
|---|---|---|
| Crude cellulose | Polyploid Miscanthus lutarioriparius | 32.4 |
| Crude cellulose | Miscanthus × giganteus | 33.7 |
| CNCs | Polyploid Miscanthus lutarioriparius | 10.1 |
| CNCs | Miscanthus × giganteus | 9.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Yi, Z.; Iqbal, Y.; Chen, Z.; Xue, S.; Fu, T.; Li, M. Polyploid Miscanthus Lutarioriparius: A Sustainable and Scalable Biomass Feedstock for Cellulose Nanocrystal Preparation in Biorefinery. Agronomy 2022, 12, 1057. https://doi.org/10.3390/agronomy12051057
Wang S, Yi Z, Iqbal Y, Chen Z, Xue S, Fu T, Li M. Polyploid Miscanthus Lutarioriparius: A Sustainable and Scalable Biomass Feedstock for Cellulose Nanocrystal Preparation in Biorefinery. Agronomy. 2022; 12(5):1057. https://doi.org/10.3390/agronomy12051057
Chicago/Turabian StyleWang, Sheng, Zili Yi, Yasir Iqbal, Zhiyong Chen, Shuai Xue, Tongcheng Fu, and Meng Li. 2022. "Polyploid Miscanthus Lutarioriparius: A Sustainable and Scalable Biomass Feedstock for Cellulose Nanocrystal Preparation in Biorefinery" Agronomy 12, no. 5: 1057. https://doi.org/10.3390/agronomy12051057
APA StyleWang, S., Yi, Z., Iqbal, Y., Chen, Z., Xue, S., Fu, T., & Li, M. (2022). Polyploid Miscanthus Lutarioriparius: A Sustainable and Scalable Biomass Feedstock for Cellulose Nanocrystal Preparation in Biorefinery. Agronomy, 12(5), 1057. https://doi.org/10.3390/agronomy12051057






