Karst Soil Patch Heterogeneity with Gravels Promotes Plant Root Development and Nutrient Utilization Associated with Arbuscular Mycorrhizal Fungi
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Treatments
2.2. Measurements of Mycorrhizal Colonization Rate, Dry Weight, and Traits of Root
2.3. Statistical Analysis
3. Results
3.1. Mycorrhizal Colonization Rate for B. pilosa Seedling Roots in Different Heterogeneous Patches
3.2. The Dry Weight of B. pilosa Seedling Roots in Different Heterogeneous Patches
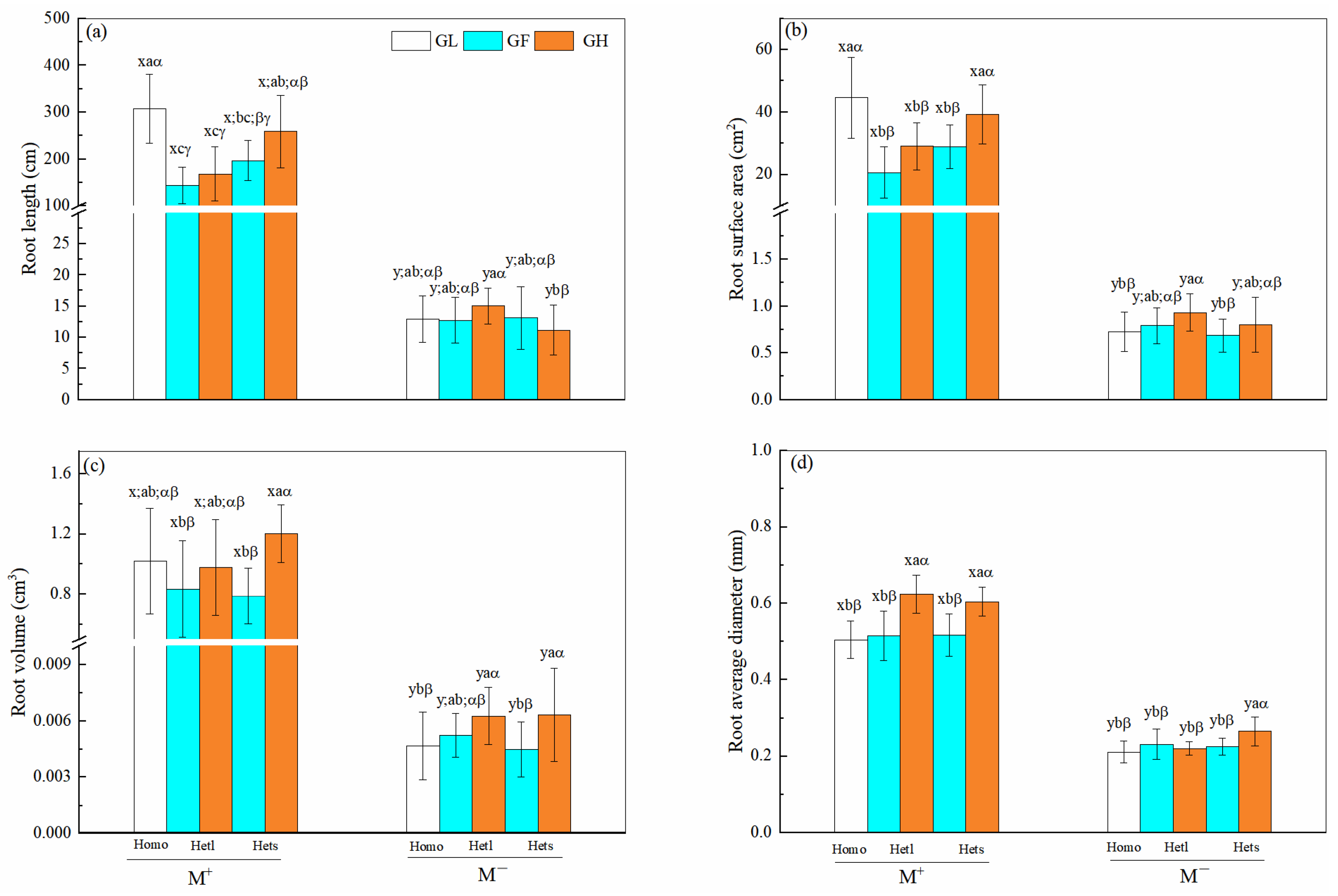
3.3. B. pilosa Roots Length, Surface Area, Average Diameter, and Volume in Different Heterogeneous Patches
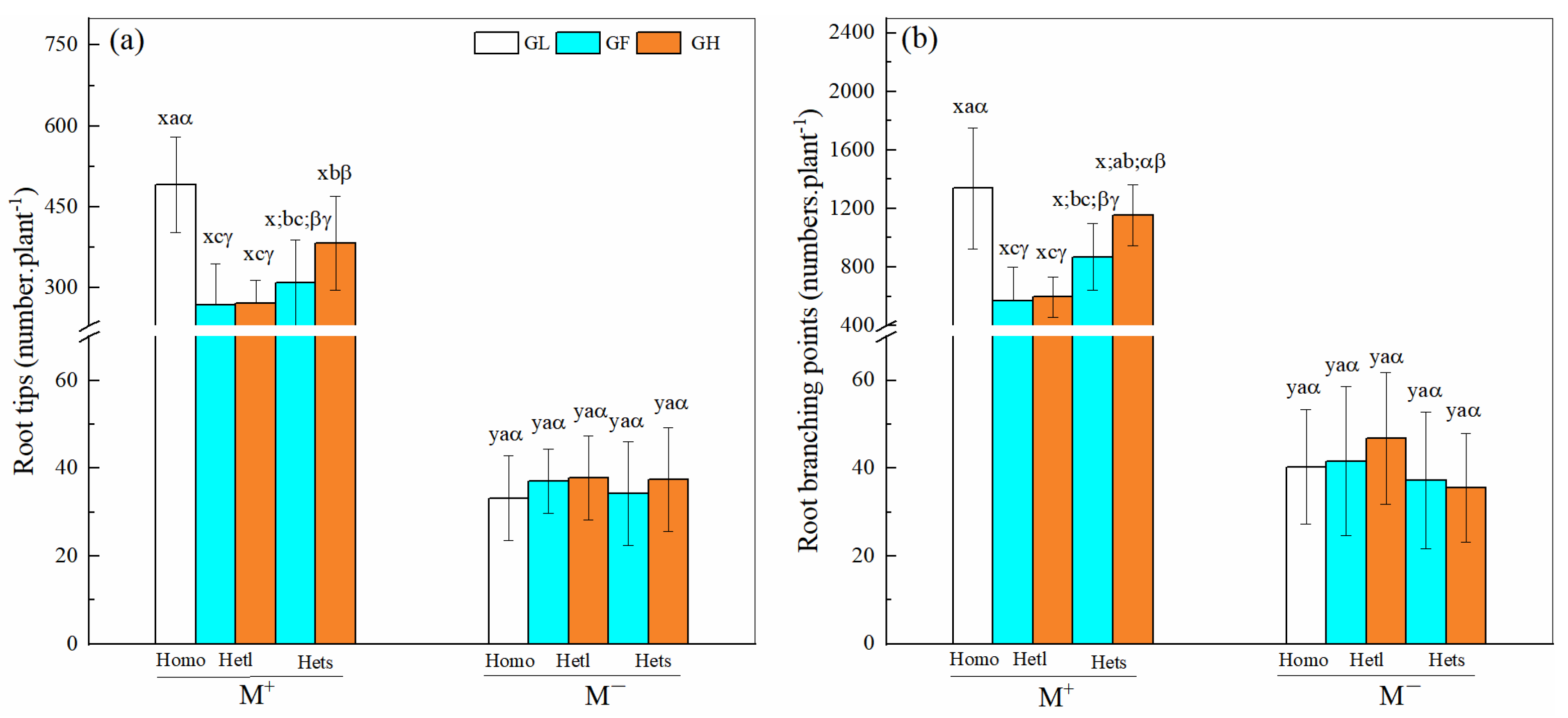
3.4. Root Tips and Branching Points of B. pilosa
3.5. B. pilosa Specific Root Traits in Different Heterogeneous Treatments
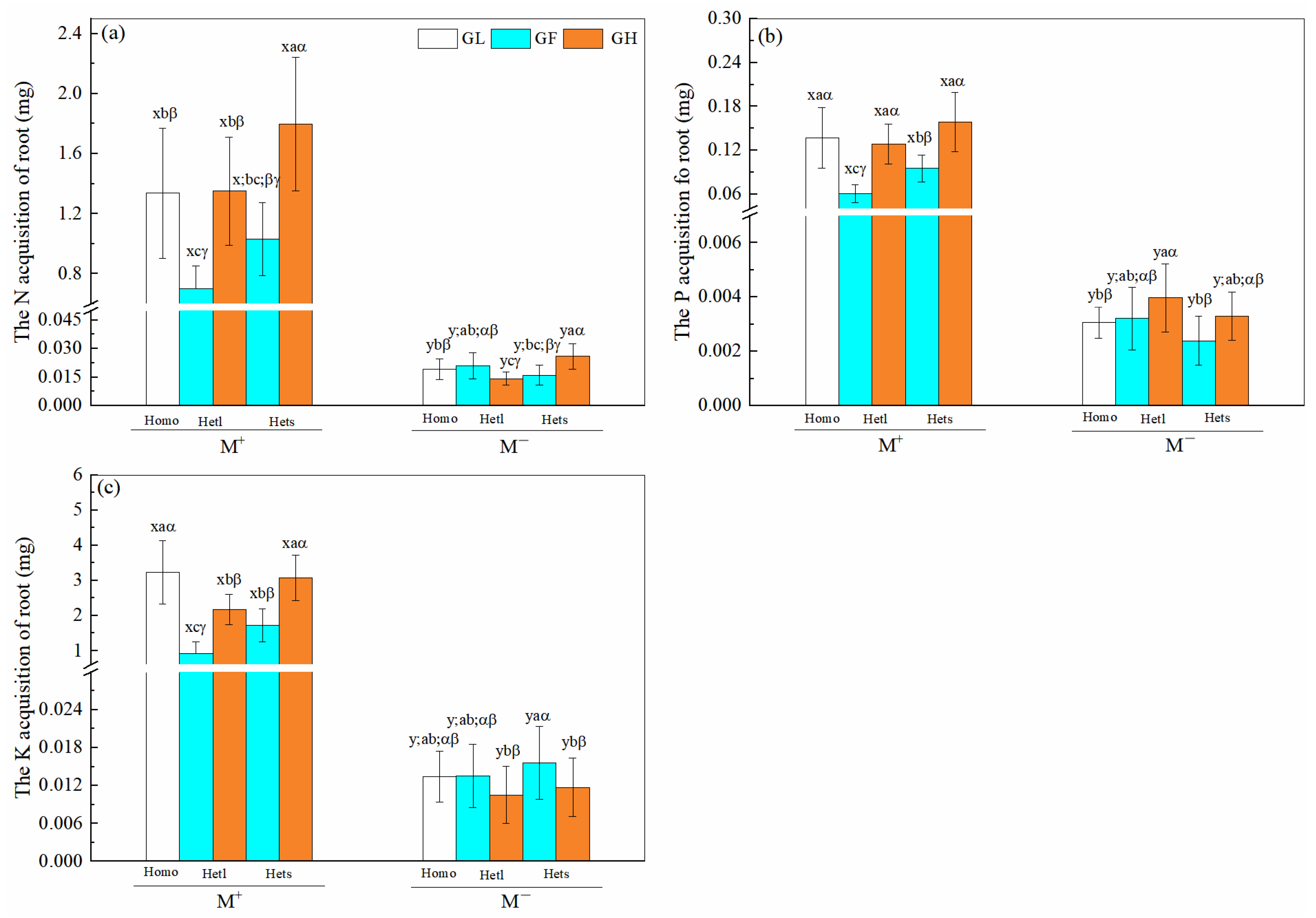
3.6. N, P and K Acquisitions of B. pilosa Roots
4. Discussion
4.1. Effect of AM Fungi on Root Dry Weight, Morphology, and Nutrients Uptakes of B. pilosa in Heterogeneous Soil
4.2. Effects of Heterogeneous Patch Size on Plant Root Traits and Nutrients Uptake Regulated by AM Fungi
4.3. Effects of Substrate Heterogeneity on Plant Root Morphology and Nutrients Uptake Regulated by AM Fungi
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, Q.H.; Cai, Y.L. Spatial pattern of Karst rock desertification in the Middle of Guizhou Province, Southwestern China. Environ. Geol. 2006, 52, 1325–1330. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, X.; Wang, Z.; Zhang, X.; Chen, C.; Liu, H. The challenge of soil loss control and vegetation restoration in the karst area of southwestern China. Int. Soil. Water. Conserv. Res. 2020, 8, 26–34. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, J.; Pan, F.; Li, D.; Chen, H.; Wang, K. Changes in nitrogen and phosphorus limitation during secondary succession in a karst region in southwest China. Plant Soil 2015, 391, 77–91. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Yue, Y.S.; Zhang, X.D.; Kai, M.; Herbert, S.J. Spatial variability of nutrient properties in black soil of northeast China. Pedosphere 2007, 17, 19–29. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Z.; Yan, L.; Li, B. Quantitative Evaluation of Ecosystem Health in a Karst Area of South China. Sustainability 2016, 8, 975. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; He, S.; Li, J. Weathering-pedogenesis of Carbonate Rocks and Its Environmental Effects in Subtropical Region. Acta Geol. Sin.-Engl. 2008, 82, 982–993. [Google Scholar] [CrossRef]
- Loreau, M.; Naeem, S.; Inchausti, P.; Bengtsson, J.; Grime, J.P.; Hector, A.; Hooper, D.U.; Huston, M.A.; Raffaelli, D.; Schmid, B.; et al. Biodiversity and ecosystem functioning: Current knowledge and future challenges. Science 2001, 284, 804–808. [Google Scholar] [CrossRef] [Green Version]
- Questad, E.J.; Foster, B.L. Coexistence through spatio-temporal heterogeneity and species sorting in grassland plant communities. Ecol. Lett. 2008, 11, 717–726. [Google Scholar] [CrossRef]
- Price, J.N.; Gazol, A.; Tamme, R.; Hiiesalu, I.; Pärtel, M.; Brody, A. The functional assembly of experimental grasslands in relation to fertility and resource heterogeneity. Funct. Ecol. 2014, 28, 509–519. [Google Scholar] [CrossRef]
- Tilman, D.; Pacala, S. The Maintenance of Species Richness in Plant Communities; University of Chicago Press: Chicago, IL, USA, 1993; pp. 13–25. [Google Scholar]
- Fitter, A.; Hodge, A.; Robinson, D. Plant response to patchy soils. In Ecological Consequences of Environmental Heterogeneity; Blackwell Science: Oxford, UK, 2000; pp. 71–90. [Google Scholar]
- Eilts, J.A.; Mittelbach, G.G.; Reynolds, H.L.; Gross, K.L. Resource Heterogeneity, Soil Fertility, and Species Diversity: Effects of Clonal Species on Plant Communities. Am. Nat. 2011, 177, 574–588. [Google Scholar] [CrossRef]
- Xue, W.; Huang, L.; Yu, F.-H. Spatial heterogeneity in soil particle size: Does it affect the yield of plant communities with different species richness? J. Plant Ecol. 2016, 9, 608–615. [Google Scholar] [CrossRef] [Green Version]
- Xue, W.; Huang, L.; Yu, F.; Bezemer, T.M. Intraspecific aggregation and soil heterogeneity: Competitive interactions of two clonal plants with contrasting spatial architecture. Plant Soil 2018, 425, 231–240. [Google Scholar] [CrossRef]
- Liu, Y.; Qi, W.; He, D.; Xiang, Y.; Liu, J.; Huang, H.; Chen, M.; Tao, J. Soil resource availability is much more important than soil resource heterogeneity in determining the species diversity and abundance of karst plant communities. Ecol. Evol. 2021, 11, 16680–16692. [Google Scholar] [CrossRef]
- Désilets, P.; Houle, G. Effects of resource availability and heterogeneity on the slope of the species-area curve along a floodplain-upland gradient. J. Veg. Sci. 2005, 16, 487–496. [Google Scholar] [CrossRef]
- Fransen, B.; De, K.H. Long-term disadvantages of selective root placement: Root proliferation and shoot biomass of two perennial grass species in a 2-year experiment. J. Ecol. 2001, 89, 711–722. [Google Scholar] [CrossRef] [Green Version]
- Facelli, E.; Facelli, J.M. Soil phosphorus heterogeneity and mycorrhizal symbiosis regulate plant intra-specific competition and size distribution. Oecologia 2002, 133, 54–61. [Google Scholar] [CrossRef]
- Day, K.; Hutchings, M.; John, E. The effects of spatially heterogeneous nutrient supply on yield, intensity of competition and root placement patterns in Briza media and Festuca ovina. Funct. Ecol. 2003, 17, 454–463. [Google Scholar] [CrossRef]
- Tamme, R.; Gazol, A.; Price, J.N.; Hiiesalu, I.; Pärtel, M.; Roxburgh, S. Co-occurring grassland species vary in their responses to fine-scale soil heterogeneity. J. Veg. Sci. 2016, 27, 1012–1022. [Google Scholar] [CrossRef]
- Hutchings, M.J.; John, E.A. The effects of environmental heterogeneity on root growth and root/shoot partitioning. Ann. Bot. 2004, 94, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Qian, Y.; Luo, D.; Gong, G.; Han, L.; Ju, G.; Sun, Z. Effects of Spatial Scale of Soil Heterogeneity on the Growth of a Clonal Plant Producing Both Spreading and Clumping Ramets. J. Plant. Growth. Regul. 2013, 33, 214–221. [Google Scholar] [CrossRef]
- Mi, M.; Shao, M.; Liu, B. Effect of rock fragments content on water consumption, biomass and water-use efficiency of plants under different water conditions. Ecol. Eng. 2016, 94, 574–582. [Google Scholar] [CrossRef]
- Masoni, A.; Ercoli, L.; Mariotti, M.; Pampana, S. Nitrogen and phosphorus accumulation and remobilization of durum wheat as affected by soil gravel content. Cereal. Res. Commun. 2008, 36, 157–166. [Google Scholar] [CrossRef] [Green Version]
- David, R.; Angela, H.; Griffths, B.S.; Fitter, A.H. Plant root proliferation in nitrogen-rich patches confers competitive advantage. P. Roy. Soc. B-Biol. Sci. 1999, 266, 431–435. [Google Scholar] [CrossRef] [Green Version]
- Pan, F.; Liang, Y.; Wang, K.; Zhang, W. Responses of Fine Root Functional Traits to Soil Nutrient Limitations in a Karst Ecosystem of Southwest China. Forests 2018, 9, 743. [Google Scholar] [CrossRef] [Green Version]
- Wijesinghe, D.K.; Hutchings, M.J. The Effects of Spatial Scale of Environmental Heterogeneity on the Growth of a Clonal Plant: An Experimental Study with Glechoma Hederacea. J. Ecol. 1997, 85, 17–28. [Google Scholar] [CrossRef]
- Day, K.; John, E.; Hutchings, M. The effects of spatial pattern of nutrient supply on the early stages of growth in plant populations. J. Ecol. 2003, 91, 305–315. [Google Scholar] [CrossRef]
- Alagna, A.; Fernandez, T.V.; Anna, G.D.; Magliola, C.; Mazzola, S.; Badalamenti, F. Assessing Posidonia oceanica seedling substrate preference: An experimental determination of seedling anchorage success in rocky vs. sandy substrates. PLoS ONE 2015, 10, e0125321. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhang, M.; Niu, J.; Li, H.; Xiao, R.; Zheng, H.; Bech, J. Rock fragments and soil hydrological processes: Significance and progress. Catena 2016, 147, 153–166. [Google Scholar] [CrossRef]
- Shi, Z.; Xu, L.; Wang, Y.; Yang, X.; Jia, Z.; Guo, H.; Xiong, W.; Yu, P. Contribution of rock fragments on formation of forest soil macropores in the stoney mountains of the Loess Plateau, China. Afric. J. Biotechnol. 2012, 11, 9350–9361. [Google Scholar]
- Alameda, D.; Villar, R. Linking root traits to plant physiology and growth in Fraxinus angustifolia Vahl. seedlings under soil compaction conditions. Environ. Exp. Bot. 2012, 79, 49–57. [Google Scholar] [CrossRef]
- Bengough, A. Root growth and function in relation to soil structure, composition, and strength. In Root Ecology; Springer: Berlin/Heidelberg, Germany, 2003; pp. 151–171. [Google Scholar]
- Laliberté, E. Below-ground frontiers in trait-based plant ecology. New Phytol. 2016, 213, 1597–1603. [Google Scholar] [CrossRef]
- Van Der Heijden, M.G.A.; Bardgett, R.D.; Van Straalen, N.M. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef]
- Manuel, D.B.; Maestre, F.T.; Reich, P.B.; Jeffries, T.C.; Gaitan, J.J.; Encinar, D.; Berdugo, M.; Campbell, C.D.; Singh, B.K. Microbial diversity drives multifunctionality in terrestrial ecosystems. Nat. Commun. 2016, 7, 10541. [Google Scholar] [CrossRef] [Green Version]
- Manuel, D.B.; Reich, P.B.; Trivedi, C.; Eldridge, D.J.; Abades, S.; Alfaro, F.D.; Bastida, F.; Berhe, A.A.; Cutler, N.A.; Gallardo, A.; et al. Multiple elements of soil biodiversity drive ecosystem functions across biomes. Nat. Ecol. Evol. 2020, 4, 210–220. [Google Scholar] [CrossRef]
- He, Y.; Zhong, Z. Effects of Water Stress and AM Inoculation on Root Morphological Characteristics in Cinnamomum camphora Seedlings. J. Southwest. Univ. 2012, 34, 33–39. [Google Scholar] [CrossRef]
- Ryan, M.H.; Tibbett, M.; Edmonds-Tibbett, T.; Suriyagoda, L.D.B.; Lambers, H.; Cawthray, G.R.; Pang, J. Carbon trading for phosphorus gain: The balance between rhizosphere carboxylates and arbuscular mycorrhizal symbiosis in plant phosphorus acquisition. Plant Cell Environ. 2012, 35, 2170–2180. [Google Scholar] [CrossRef]
- Mei, L.; Yang, X.; Zhang, S.; Zhang, T.; Guo, J. Arbuscular mycorrhizal fungi alleviate phosphorus limitation by reducing plant N:P ratios under warming and nitrogen addition in a temperate meadow ecosystem. Sci. Total Environ. 2019, 686, 1129–1139. [Google Scholar] [CrossRef]
- Shen, K.; Cornelissen, J.H.C.; Wang, Y.; Wu, C.; He, Y.; Ou, J.; Tan, Q.; Xia, T.; Kang, L.; Guo, Y.; et al. AM Fungi Alleviate Phosphorus Limitation and Enhance Nutrient Competitiveness of Invasive Plants via Mycorrhizal Networks in Karst Areas. Front. Ecol. Evol. 2020, 8, 125. [Google Scholar] [CrossRef]
- Chen, W.; Koide, R.T.; Eissenstat, D.M. Nutrient foraging by mycorrhizas: From species functional traits to ecosystem processes. Funct. Ecol. 2018, 32, 858–869. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: New York, NY, USA, 2008. [Google Scholar]
- Shi, Z.; Wang, F.; Zhang, C.; Yang, Z. Exploitation of phosphorus patches with different phosphorus enrichment by three arbuscular mycorrhizal fungi. J. Plant Nutr. 2011, 34, 1096–1106. [Google Scholar] [CrossRef]
- Yang, Z.; Midmore, D.J. Modelling plant resource allocation and growth partitioning in response to environmental heterogeneity. Ecol. Model. 2005, 181, 59–77. [Google Scholar] [CrossRef]
- Croft, S.A.; Hodge, A.; Pitchford, J.W. Optimal root proliferation strategies: The roles of nutrient heterogeneity, competition and mycorrhizal networks. Plant Soil 2012, 351, 191–206. [Google Scholar] [CrossRef]
- Simard, S.W.; Beiler, K.J.; Bingham, M.A.; Deslippe, J.R.; Philip, L.J.; Teste, F.P. Mycorrhizal networks: Mechanisms, ecology and modelling. Fungal. Biol. Rev. 2012, 26, 39–60. [Google Scholar] [CrossRef]
- Liang, Y.; He, X.; Chen, C.; Feng, S.; Liu, L.; Chen, X.; Zhao, Z.; Su, Y. Influence of plant communities and soil properties during natural vegetation restoration on arbuscular mycorrhizal fungal communities in a karst region. Ecol. Eng. 2015, 82, 57–65. [Google Scholar] [CrossRef]
- He, Y.; Cornelissen, J.H.C.; Wang, P.; Dong, M.; Ou, J. Nitrogen transfer from one plant to another depends on plant biomass production between conspecific and heterospecific species via a common arbuscular mycorrhizal network. Environ. Sci. Pollut. Res. Int. 2019, 26, 8828–8837. [Google Scholar] [CrossRef]
- Xia, T.; Wang, Y.; He, Y.; Wu, C.; Shen, K.; Tan, Q.; Kang, L.; Guo, Y.; Wu, B.; Han, X. An invasive plant experiences greater benefits of root morphology from enhancing nutrient competition associated with arbuscular mycorrhizae in karst soil than a native plant. PLoS ONE 2020, 15, e0234410. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, J.; Xu, G.; Zhou, L.; Li, Y. Arbuscular mycorrhizal fungi improve the growth and drought tolerance of Zenia insignis seedlings under drought stress. New For. 2019, 50, 593–604. [Google Scholar] [CrossRef]
- Bao, S.D. Soil and Agricultural Chemistry Analysis; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Wang, Y.; Zhong, Q.; Bin, X.; Zhang, Z.; Cheng, D. Effect of adding a combination of nitrogen and phosphorus on fine root morphology and soil microbes of Machilus pauhoi seedling. Acta Ecol. Sin. 2018, 38, 8–2271. [Google Scholar] [CrossRef]
- Nottingham, A.T.; Turner, B.L.; Winter, K.; Chamberlain, P.M.; Stott, A.; Tanner, E.V. Root and arbuscular mycorrhizal mycelial interactions with soil microorganisms in lowland tropical forest. FEMS Microbiol. Ecol. 2013, 85, 37–50. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Wang, Y.; Sun, S.; Mu, C.; Yan, X. Effects of arbuscular mycorrhizal fungi on the growth, photosynthesis and photosynthetic pigments of Leymus chinensis seedlings under salt-alkali stress and nitrogen deposition. Sci. Total Environ. 2017, 576, 234–241. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, D.; Yan, R.; Yu, F.; Van Kleunen, M. Invasive alien clonal plants are competitively superior over co-occurring native clonal plants. Perspect. Plant. Ecol. 2019, 40, 125484. [Google Scholar] [CrossRef]
- Zhang, H.; Wei, S.; Hu, W.; Xiao, L.; Tang, M. Arbuscular mycorrhizal fungus Rhizophagus irregularis increased potassium content and expression of genes encoding potassium channels in Lycium barbarum. Front. Plant Sci. 2017, 8, 440. [Google Scholar] [CrossRef] [Green Version]
- He, Y.J.; Zhong, Z.C.; Liu, J.M.; Liu, J.C.; Jin, J.; Song, H.X. Response of n and p absorption on Broussonetia papyrifera seedlings to inoculate vesicular-arbuscular mycorrhizal fungus. Acta Ecol. Sin. 2007, 27, 4840–4847. [Google Scholar] [CrossRef]
- Hafiz, A.; Zhang, Q.; Saddam, H.; Li, H.; Ahmed, W.; Zhang, L. Effects of Arbuscular Mycorrhizal Fungi on Maize Growth, Root Colonization, and Root Exudates Varied with Inoculum and Application Method. J. Soil. Sci. Plant Nut. 2021, 21, 1577–1590. [Google Scholar] [CrossRef]
- Ostonen, I.; Rosenvald, K.; Helmisaari, H.S.; Godbold, D.; Parts, K.; Uri, V.; Lõhmus, K. Morphological plasticity of ectomycorrhizal short roots in Betula sp and Picea abies forests across climate and forest succession gradients: Its role in changing environments. Front. Plant Sci. 2013, 4, 335. [Google Scholar] [CrossRef] [Green Version]
- He, Y.M.; Fan, X.M.; Zhang, G.Q.; Li, B.; Li, T.G.; Zu, Y.Q.; Zhan, F. Effects of arbuscular mycorrhizal fungi and dark septate endophytes on maize performance and root traits under a high cadmium stress. S. Afr. J. Bot. 2020, 134, 415–423. [Google Scholar] [CrossRef]
- Hodge, A. The plastic plant: Root responses to heterogeneous supplies of nutrients. New Phytol. 2004, 162, 9–24. [Google Scholar] [CrossRef]
- Farley, R.; Fitter, A. The responses of seven co-occurring woodland herbaceous perennials to localized nutrient-rich patches. J. Ecol. 1999, 87, 849–859. [Google Scholar] [CrossRef]
- Hutchings, M.J.; Wijesinghe, D.K. Performance of a clonal species in patchy environments: Effects of environmental context on yield at local and whole-plant scales. Evol. Ecol. 2007, 22, 313–324. [Google Scholar] [CrossRef]
- Cui, M.; Caldwell, M.M. Nitrate and phosphate uptake by Agropyron desertorum and Artemisia tridentata from soil patches with balanced and unbalanced nitrate and phosphate supply. New Phytol. 1998, 139, 267–272. [Google Scholar] [CrossRef]
- Hodge, A.; Robinson, D.; Griffiths, B.; Fitter, A. Nitrogen capture by plants grown in N-rich organic patches of contrasting size and strength. J. Exp. Bot. 1999, 50, 1243–1252. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, J.; Huang, Y. Effects of arbuscular mycorrhizal fungi on the drought tolerance of Cyclobalanopsis glauca seedlings under greenhouse conditions. New For. 2014, 45, 545–556. [Google Scholar] [CrossRef]
- Hodge, A.; Berta, G.; Doussan, C.; Merchan, F.; Crespi, M. Plant root growth, architecture and function. Plant Soil 2009, 321, 153–187. [Google Scholar] [CrossRef]
- Lin, S.; Sun, X.; Wang, X.; Dou, C.; Li, Y.; Luo, Q.; Sun, L.; Jin, L. Mycorrhizal studies and their application prospects in China. Acta Prata. Sin. 2013, 22, 310. [Google Scholar]
- Mommer, L.; Visser, E.J.W.; Van Ruijven, J.; De Caluwe, H.; Pierik, R.; De Kroon, H. Contrasting root behaviour in two grass species: A test of functionality in dynamic heterogeneous conditions. Plant Soil 2011, 344, 347–360. [Google Scholar] [CrossRef] [Green Version]
- Shao, Y.; Zhang, D.; Hu, X.; Wu, Q.; Jiang, C.; Xia, T.; Gao, X.B.; Kuča, K. Mycorrhiza-induced changes in root growth and nutrient absorption of tea plants. Plant Soil. Environ. 2018, 64, 283–289. [Google Scholar] [CrossRef] [Green Version]
- Campbell, B.; Grime, J.; Mackey, J.A. trade-off between scale and precision in resource foraging. Oecologia 1991, 87, 532–538. [Google Scholar] [CrossRef]
- Hodge, A. Plastic plants and patchy soils. J. Exp. Bot. 2006, 57, 401–411. [Google Scholar] [CrossRef]
- Rajaniemi, T.K.; Reynolds, H.L. Root foraging for patchy resources in eight herbaceous plant species. Oecologia 2004, 141, 519–525. [Google Scholar] [CrossRef]
- Rytter, R. Stone and gravel contents of arable soils influence estimates of C and N stocks. Catena 2012, 95, 153–159. [Google Scholar] [CrossRef]
- Suo, G.; Xie, Y.; Zhang, Y.; Luo, H. Long-term effects of different surface mulching techniques on soil water and fruit yield in an apple orchard on the Loess Plateau of China. Sci. Hortic. 2019, 246, 643–651. [Google Scholar] [CrossRef]
- Hanson, C.T.; Blevins, R. Soil water in coarse fragments. Soil Sci. Soc. Am. J. 1979, 43, 819–820. [Google Scholar] [CrossRef]
- Clark, L.; Whalley, W.; Barraclough, P. How do roots penetrate strong soil. In Roots: The Dynamic Interface between Plants and the Earth; Springer: Berlin/Heidelberg, Germany, 2003; pp. 93–104. [Google Scholar]
- Singh, S.; Kapoor, K. Inoculation with phosphate-solubilizing microorganisms and a vesicular-arbuscular mycorrhizal fungus improves dry matter yield and nutrient uptake by wheat grown in a sandy soil. Biol. Fert. Soils 1999, 28, 139–144. [Google Scholar] [CrossRef]
- Lambers, H.; Teste, F.P. Interactions between arbuscular mycorrhizal and non-mycorrhizal plants: Do non-mycorrhizal species at both extremes of nutrient availability play the same game. Plant Cell Environ. 2013, 36, 1911–1915. [Google Scholar] [CrossRef] [PubMed]
- Fitter, A. Costs and benefits of mycorrhizas: Implications for functioning under natural conditions. Experientia 1991, 47, 350–355. [Google Scholar] [CrossRef]
- Barber, S.; Silberbush, M. Plant Root Morphology and Nutrient Uptake; American Society of Agronomy: Madison, WI, USA, 1984; Volume 49, pp. 65–87. [Google Scholar] [CrossRef]
- Hutchings, M.; De Kroon, H. Heterogeneous Soil-Resource Distribution and Plant Responses from Individual-Plant Growth to Ecosystem Functioning; Springer: Berlin/Heidelberg, Germany, 1994; pp. 451–476. [Google Scholar]
- Luo, Y.; Zhou, J.; Li, Y.; Li, Q.; Li, D.; Xiao, Z.; Zhang, F.; Hu, Z. Effects of tephra gravel content on growth of flue-cured tobacco at seedling stage. J. South. Agric. 2014, 45, 570–574. [Google Scholar]
- Laliberté, E.; Lambers, H.; Burgess, T.I.; Wright, S.J. Phosphorus limitation, soil-borne pathogens and the coexistence of plant species in hyperdiverse forests and shrublands. New Phytol. 2015, 206, 507–521. [Google Scholar] [CrossRef] [Green Version]
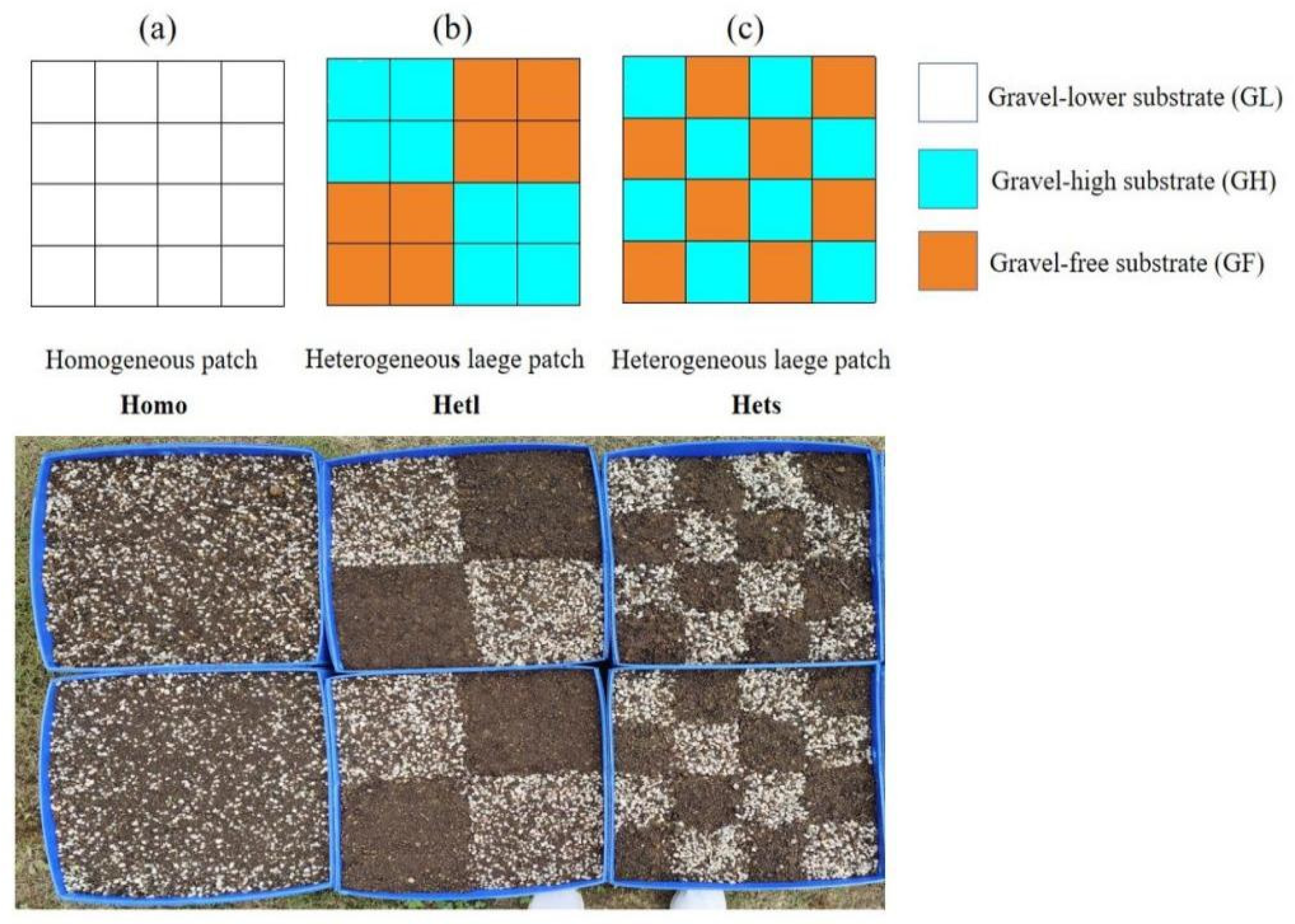


| Treatments | Homo | Hetl | Hets | ||
|---|---|---|---|---|---|
| GL | GF | GF | GF | GH | |
| M+ | 58.75 ± 0.66xbcβγ | 48.13 ± 0.97xdΔ | 48.13 ± 0.97xdΔ | 58.21 ± 0.67xcγ | 63.79 ± 0.49xaα |
| M− | 0 yaα | 0 yaα | 0 yaα | 0 yaα | 0 yaα |
| Treatments | Df | Root Dry Weight | |
|---|---|---|---|
| F | p | ||
| Mycorrhizal fungi (M) | 1 | 760.150 | <0.001 *** |
| Patch heterogeneity (P) | 2 | 4.469 | 0.037 * |
| Substrate heterogeneity (S) | 2 | 45.832 | <0.001 *** |
| M × P | 2 | 4.658 | 0.034 * |
| M × S | 2 | 44.903 | <0.001 *** |
| P × S | 4 | 1.206 | 0.275 |
| M × P × S | 4 | 1.269 | 0.263 |
| Treatments | df | Root Length (cm) | Root Surface Area (cm2) | Root Volume (cm3) | Average Diameter (mm) | ||||
|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | ||
| Mycorrhizal fungi (M) | 1 | 545.125 | <0.001 *** | 518.377 | <0.001 *** | 501.696 | <0.001 *** | 1346.734 | <0.001 *** |
| Patch heterogeneity (P) | 2 | 9.943 | 0.002 ** | 6.612 | 0.012 * | 0.703 | 0.404 | 0.243 | 0.623 |
| Substrate heterogeneity (S) | 2 | 3.805 | 0.054 | 7.061 | 0.009 ** | 7.126 | <0.001 *** | 28.376 | <0.001 *** |
| M × P | 2 | 10.970 | <0.001 ** | 6.955 | 0.010 ** | 0.714 | 0.401 | 1.755 | 0.189 |
| M × S | 2 | 3.747 | 0.056 | 6.683 | 0.011 * | 6.981 | 0.010 ** | 15.542 | <0.001 *** |
| P × S | 4 | 0.550 | 0.461 | 0.067 | 0.796 | 1.721 | 0.193 | 0.470 | 0.495 |
| M × P × S | 4 | 0.872 | 0.353 | 0.072 | 0.789 | 1.701 | 0.196 | 2.992 | 0.087 |
| Treatments | df | Root Tips | Root Branching Points | ||
|---|---|---|---|---|---|
| F | p | F | p | ||
| Mycorrhizal fungi (M) | 1 | 800.275 | <0.001 *** | 459.121 | <0.001 *** |
| Patch heterogeneity (P) | 2 | 7.012 | 0.010 ** | 16.386 | <0.001 *** |
| Substrate heterogeneity (S) | 2 | 1.980 | 0.163 | 2.308 | 0.132 |
| M × P | 2 | 7.661 | 0.007 ** | 17.632 | <0.001 *** |
| M × S | 2 | 1.604 | 0.209 | 2.209 | 0.141 |
| P × S | 4 | 1.748 | 0.190 | 1.437 | 0.234 |
| M × P × S | 4 | 1.525 | 0.220 | 1.602 | 0.209 |
| Treatments | df | Specific Root Length (cm/g) | Specific Root Area (cm2/g) | Specific Root Volume (cm3/g) | |||
|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | ||
| Mycorrhizal fungi (M) | 1 | 231.635 | <0.001 *** | 11.610 | <0.001 *** | 163.225 | <0.001 *** |
| Patch heterogeneity (P) | 2 | 0.017 | 0.898 | 0.002 | 0.968 | 3.425 | 0.068 |
| Substrate heterogeneity (S) | 2 | 7.143 | 0.009 ** | 4.341 | 0.040 * | 7.568 | 0.007 ** |
| M × P | 2 | 0.180 | 0.673 | 0.613 | 0.436 | 3.646 | 0.060 |
| M × S | 2 | 0.126 | 0.724 | 2.788 | 0.099 | 9.035 | 0.003 ** |
| P × S | 4 | 2.440 | 0.122 | 0.041 | 0.840 | 8.273 | 0.005 ** |
| M × P × S | 4 | 7.533 | 0.007 ** | 2.319 | 0.131 | 7.897 | 0.006 ** |
| Treatments | df | Root N Acquisition | Root P Acquisition | Root K Acquisition | |||
|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | ||
| Mycorrhizal fungi (M) | 1 | 549.296 | <0.001 *** | 594.266 | <0.001 *** | 594.687 | <0.001 *** |
| Patch heterogeneity (P) | 2 | 9.226 | 0.003 ** | 7.540 | 0.007 ** | 13.492 | <0.001 *** |
| Substrate heterogeneity (S) | 2 | 30.304 | <0.001 *** | 33.139 | <0.001 *** | 31.153 | <0.001 *** |
| M × P | 2 | 8.905 | 0.004 ** | 8.270 | 0.005 ** | 13.390 | <0.001 *** |
| M × S | 2 | 30.042 | <0.001 *** | 31.502 | <0.001 *** | 31.481 | <0.001 *** |
| P × S | 4 | 0.279 | 0.599 | 0.028 | 0.868 | 0.050 | 0.824 |
| M × P × S | 4 | 0.160 | 0.690 | 0.032 | 0.858 | 0.051 | 0.821 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Umer, M.; Guo, Y.; Shen, K.; Xia, T.; Xu, X.; Han, X.; Ren, W.; Sun, Y.; Wu, B.; et al. Karst Soil Patch Heterogeneity with Gravels Promotes Plant Root Development and Nutrient Utilization Associated with Arbuscular Mycorrhizal Fungi. Agronomy 2022, 12, 1063. https://doi.org/10.3390/agronomy12051063
Li Q, Umer M, Guo Y, Shen K, Xia T, Xu X, Han X, Ren W, Sun Y, Wu B, et al. Karst Soil Patch Heterogeneity with Gravels Promotes Plant Root Development and Nutrient Utilization Associated with Arbuscular Mycorrhizal Fungi. Agronomy. 2022; 12(5):1063. https://doi.org/10.3390/agronomy12051063
Chicago/Turabian StyleLi, Qing, Muhammad Umer, Yun Guo, Kaiping Shen, Tingting Xia, Xinyang Xu, Xu Han, Wenda Ren, Yan Sun, Bangli Wu, and et al. 2022. "Karst Soil Patch Heterogeneity with Gravels Promotes Plant Root Development and Nutrient Utilization Associated with Arbuscular Mycorrhizal Fungi" Agronomy 12, no. 5: 1063. https://doi.org/10.3390/agronomy12051063
APA StyleLi, Q., Umer, M., Guo, Y., Shen, K., Xia, T., Xu, X., Han, X., Ren, W., Sun, Y., Wu, B., Liu, X., & He, Y. (2022). Karst Soil Patch Heterogeneity with Gravels Promotes Plant Root Development and Nutrient Utilization Associated with Arbuscular Mycorrhizal Fungi. Agronomy, 12(5), 1063. https://doi.org/10.3390/agronomy12051063








