Citrus Canker—Distribution, Taxonomy, Epidemiology, Disease Cycle, Pathogen Biology, Detection, and Management: A Critical Review and Future Research Agenda
Abstract
:1. Introduction
2. Taxonomy of Citrus Canker Bacterium
3. Phylogenetically Distinct Groups of CC
3.1. Asiatic Citrus Canker Strains
3.2. South-American Canker Strains
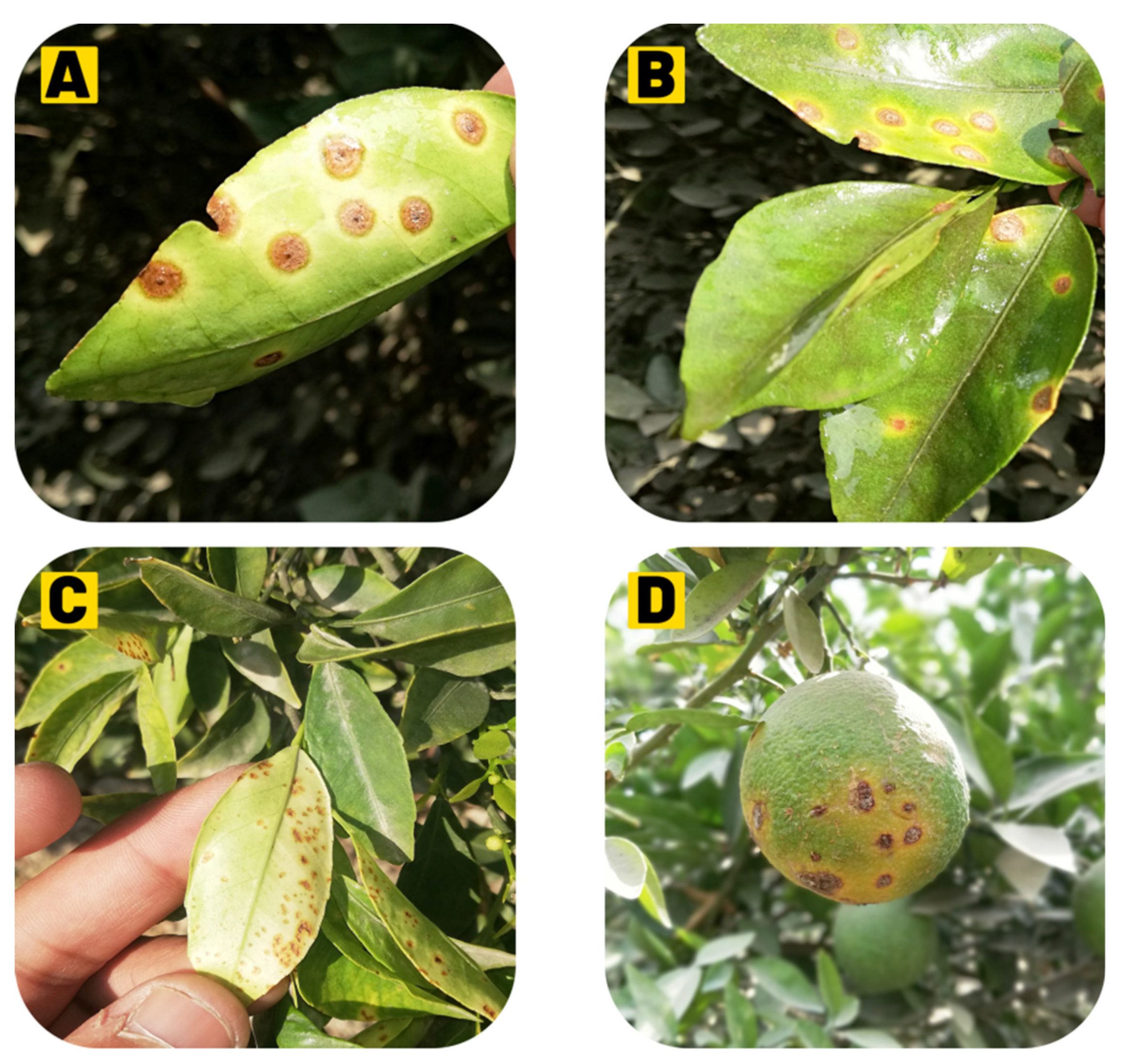
4. Symptomatology
4.1. Leaf Lesions
4.2. Fruit Lesions
4.3. Twig Lesions
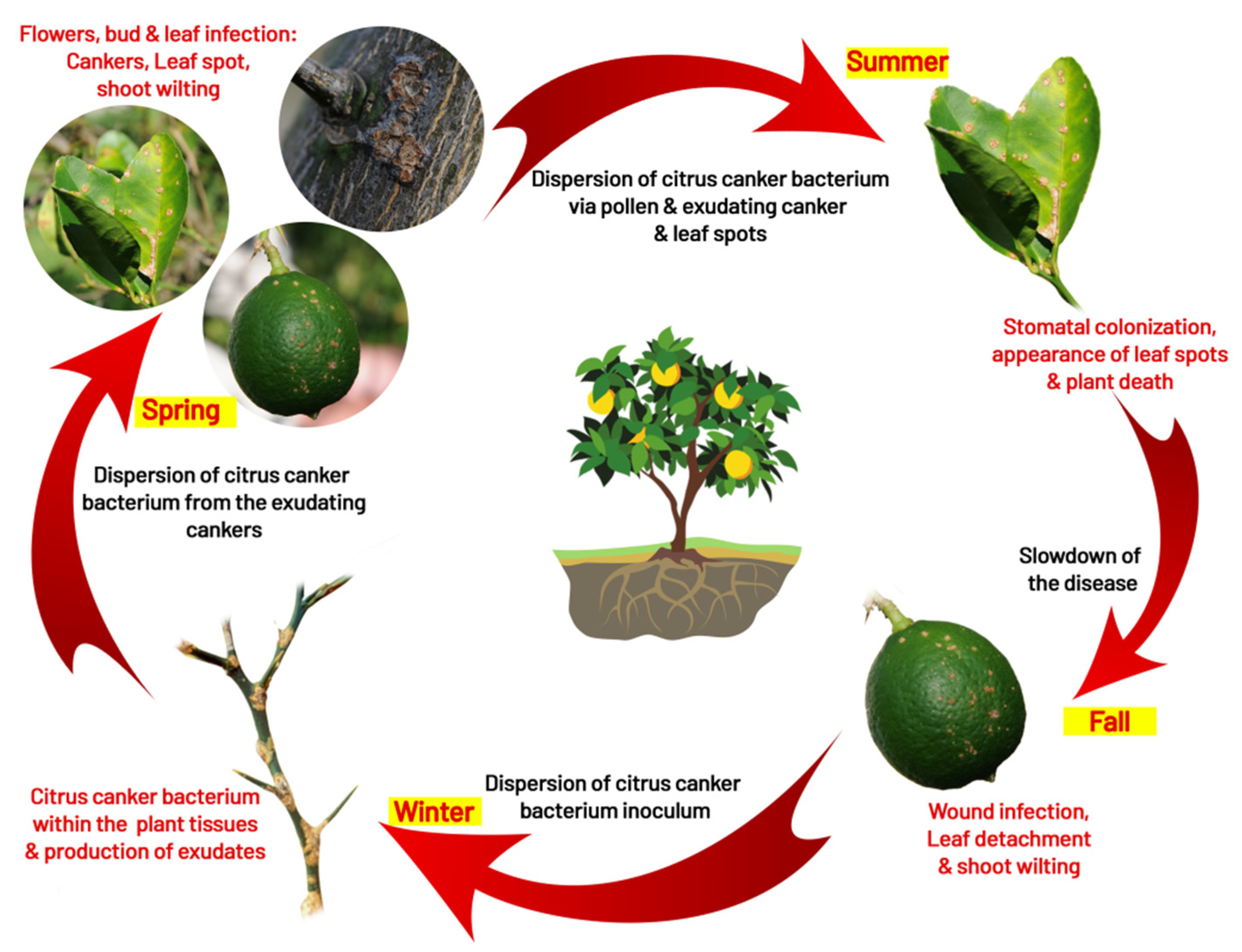
5. Disease Cycle and Epidemiology
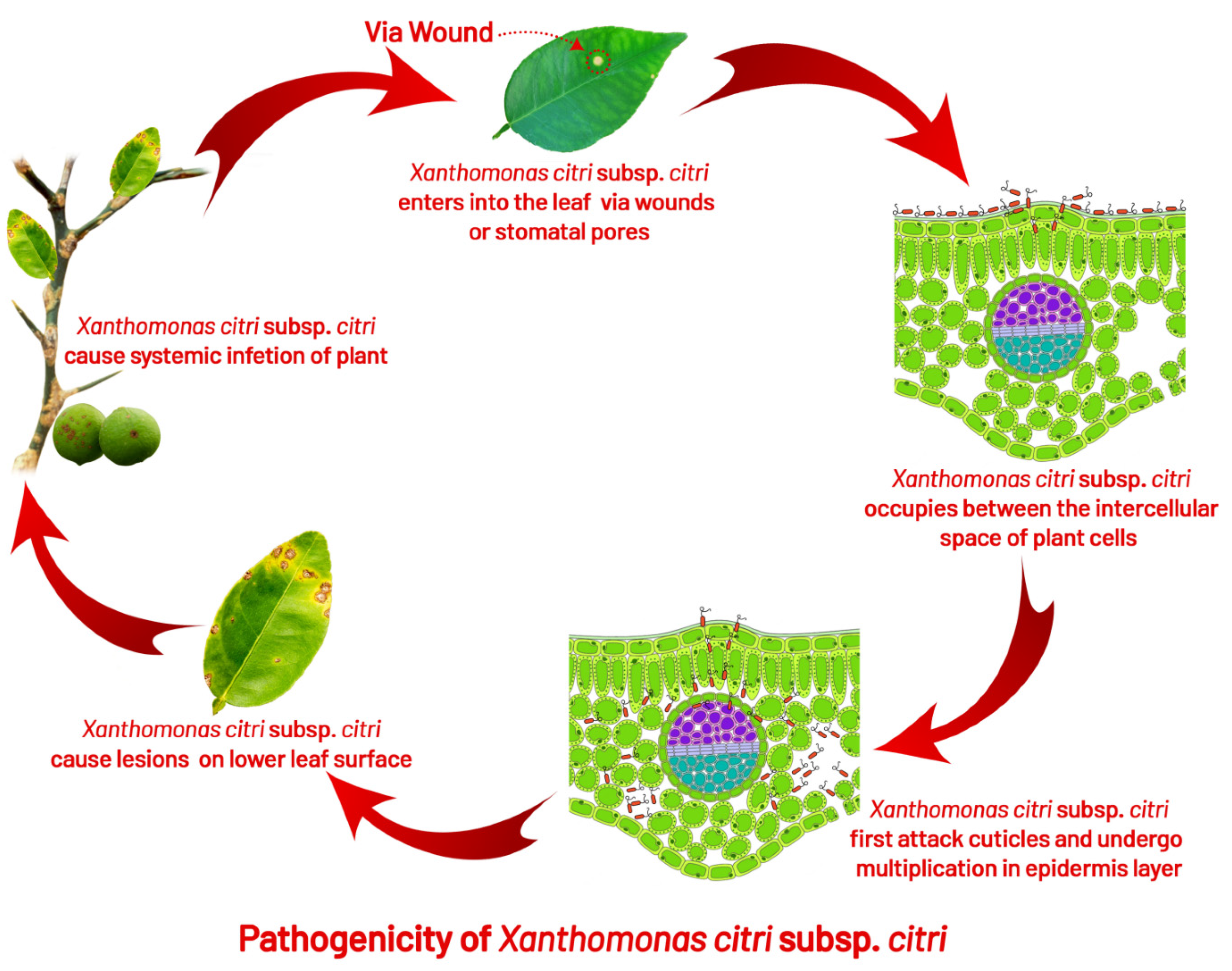
5.1. Infection
5.2. Survival
5.3. Dispersal
5.4. Role of Insect (Leaf Miner Interaction)
6. Detection and Identification of Citrus Bacterial Canker
7. Genome
8. Virulence
8.1. Type III Secretion System (T3SS)
8.2. Citrus Specific pthA and Its Requirement for Canker Development
8.3. Adhesion and Extracellular Polysaccharides (EPSs)
8.4. Lipopolysaccharides (LPSs)
8.5. Quorum Sensing
9. Nutrition
10. Integrated Management Programs
10.1. Quarantines
10.2. Cultural Control
10.3. Chemical Control
10.4. Biological Control
10.5. Field Screening
10.6. Induced Systemic Resistance
10.7. Leaf Miner Control
10.8. Control through Plant Extracts
10.9. Factors Affecting Successful Eradication of Citrus Canker
11. Conclusions and Future Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ministry of National Food Security and Research. Agricultural-Statistics-of-Pakistan-2019–2020; Ministry of National Food Security and Research: Islamabad, Pakistan, 2020. [Google Scholar]
- FAO STAT, Statistics Division, Food and Agriculture Organization of the United Nations. Agriculture Statistics 2019–2020; Food and Agriculture Organization of the United Nations: Rome, Italy, 2020. [Google Scholar]
- Siddique, M.I.; Garnevska, E. Citrus Value Chain(s): A Survey of Pakistan Citrus Industry. In Agricultural Value Chain; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef] [Green Version]
- Al-Snafi, A.E. Nutritional value and pharmacological importance of citrus species grown in Iraq. IOSR J. Pharm. 2016, 6, 76–108. [Google Scholar] [CrossRef]
- Pakistan Horticulture Development Export Company (PHDECo). Citrus Marketing Strategy; Pakistan Horticulture Development Export Company: Lahore, Pakistan, 2018. [Google Scholar]
- Agriculture Marketing Information Service of Pakistan. District Wise Data of Citrus; Agriculture Marketing Information Service of Pakistan: Lahore, Pakistan, 2018. [Google Scholar]
- Martins, P.M.M.; de Oliveira Andrade, M.; Benedetti, C.E.; de Souza, A.A. Xanthomonas citri subsp. citri: Host interaction and control strategies. Trop. Plant Pathol. 2020, 45, 213–236. [Google Scholar] [CrossRef]
- Ference, C.M.; Gochez, A.M.; Behlau, F.; Wang, N.; Graham, J.H.; Jones, J.B. Recent advances in the understanding of Xanthomonas citri ssp. citri pathogenesis and citrus canker disease management. Mol. Plant Pathol. 2018, 19, 1302–1318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Randsborg, K. Introduction. EPPO Bull. 1979, 9, 341–342. [Google Scholar] [CrossRef]
- da Gama, M.A.S.; de Lima Ramos Mariano, R.; da Silva J’unior, W.J.; de Farias, A.R.G.; Barbosa, M.A.G.; da Silva Velloso Ferreira, M.A.; J’unior, C.R.L.C.; Santos, L.A.; de Souza, E.B. Taxonomic Repositioning of Xanthomonas campestris pv. viticola (Nayudu 1972) Dye 1978 as Xanthomonas citri pv. viticola (Nayudu 1972) Dye 1978 comb. nov. and Emendation of the Description of Xanthomonas citri pv. anacardii to Include Pigmented Isolates Pathogenic to Cashew Plant. Phytopathology 2018, 108, 1143–1153. [Google Scholar] [PubMed] [Green Version]
- Mehrotra, R. Bacteria and Bacterial Diseases. In Plant Pathology; Tata McGraw-Hill Pub. Co. Ltd.: New Delhi, India, 1980; pp. 636–638. [Google Scholar]
- Whiteside, J.O.; Garnsey, S.M. Compendium of Citrus Diseases; APS Press: College Park, MD, USA, 1988. [Google Scholar]
- Li, L.; Li, J.; Zhang, Y.; Wang, N. Diffusible signal factor (DSF)-mediated quorum sensing modulates expression of diverse traits in Xanthomonas citri and responses of citrus plants to promote disease. BMC Genom. 2019, 20, 55. [Google Scholar] [CrossRef]
- Lee, H.A. Further data on the susceptibility of rutaceous plants to citrus-canker. J. Agric. Res. 1918, 15, 661–665. [Google Scholar]
- Fawcett, H.S.; Jenkins, A.E. Records of Citrus Canker from Herbarium Specimens of the Genus Citrus in England and the United States. Phytopathology 1933, 23, 820–824. [Google Scholar]
- Graham, J.; Hartung, J.; Stall, R.; Chase, A. Pathological, restriction-fragment length polymorphism, and fatty acid profile relationships between Xanthomonas campestris from citrus and noncitrus hosts. Phytopathology 1990, 80, 829–836. [Google Scholar] [CrossRef]
- Gottwald, T.R.; Hughes, G.; Graham, J.H.; Sun, X.; Riley, T. The citrus canker epidemic in Florida: The scientific basis of regulatory eradication policy for an invasive species. Phytopathology 2001, 91, 30–34. [Google Scholar] [CrossRef] [Green Version]
- Rossetti, V. Citrus canker in Latin America: A review. In Proceedings of the International Society of Citriculture, Orlando, FL, USA, 1–8 May 1977; pp. 918–924. [Google Scholar]
- Doidge, E.M. Citrus canker in South Africa. S. Afr. Fruit Grow. 1916, 3, 265–268. [Google Scholar]
- Garnsey, S.; Ducharme, E.; Lightfield, J.; Seymour, C.; Griffiths, J. Citrus canker. Citrus Ind. 1979, 60, 5. [Google Scholar]
- Bártová, V.; Bárta, J.; Jarošová, M. Antifungal and antimicrobial proteins and peptides of potato (Solanum tuberosum L.) tubers and their applications. Appl. Microbiol. Biotechnol. 2019, 103, 5533–5547. [Google Scholar] [CrossRef] [PubMed]
- Okabe, N. Bacterial Diseases of Plants Occurring in Formosa I. J. Soc. Trop. Agric. 1932, 4, 470–483. [Google Scholar]
- Del Campo, R.; Russi, P.; Mara, P.; Mara, H.; Peyrou, M.; De León, I.P.; Gaggero, C. Xanthomonas axonopodispv. citri enters the VBNC state after copper treatment and retains its virulence. FEMS Microbiol. Lett. 2009, 298, 143–148. [Google Scholar] [CrossRef] [Green Version]
- Schubert, T.S.; Rizvi, S.A.; Sun, X.; Gottwald, T.R.; Graham, J.H.; Dixon, W.N. Meeting the challenge of eradicating citrus canker in Florida—Again. Plant Dis. 2001, 85, 340–356. [Google Scholar] [CrossRef] [Green Version]
- Naseem, S.; Shah, H.A.; Ali, Z. First report on characterization of citrus disease causing bacteria and related phages isolated in Pakistan. Int. J. Agric. Biol. 2017, 19, 857–864. [Google Scholar] [CrossRef]
- Stevens, H.E. Citrus canker. A preliminary bulletin. Fla. Agric. Expt. Sta. Bull. 1914, 122, 113–118. [Google Scholar]
- Hasse, C.H. Pseudomonas citri, the cause of citrus canker—A preliminary report. J. Agric. Res. 1915, 4, 97–100. [Google Scholar]
- Doidge, E.M. The Origin and Cause of Citrus Canker in South Africa. Union So. Afr. Dept. Agric. Sei. Bul. 1916, 8, 20. [Google Scholar]
- Dowson, W.J. On the systematic position and generic names of the gram negative bacterial plant pathogens. Zentr. Bakteriol. Parasitenk. Abt. II 1939, 100, 177–193. [Google Scholar]
- Sena-Velez, M.; Redondo, C.; Graham, J.H.; Cubero, J. Presence of extracellular DNA during biofilm formation by Xanthomonas citri subsp citri strains with different host range. PLoS ONE 2016, 11, e0156695. [Google Scholar] [CrossRef] [PubMed]
- Bansal, K.; Kumar, S.; Patil, P.B. Phylogenomic insights into diversity and evolution of nonpathogenic Xanthomonas strains associated with citrus. mSphere 2020, 5, e00087-20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patané, J.S.L.; Martins, J.; Rangel, L.T.; Belasque, J.; Digiampietri, L.A.; Facincani, A.P.; Ferreira, R.M.; Jaciani, F.J.; Zhang, Y.; Varani, A.M.; et al. Origin and diversification of Xanthomonas citri subsp. citri pathotypes revealed by inclusive phylogenomic, dating, and biogeographic analyses. BMC Genom. 2019, 20, 700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, J.M.; Dye, D.W.; Bradbury, J.F.; Panagopoulos, C.G.; Robbs, C.F. Proposed nomenclature and classification for plant pathogenic bacteria. N. Z. J. Agric. Res. 1978, 21, 153–177. [Google Scholar] [CrossRef]
- Gabriel, D.W.; Kingsley, M.T.; Hunter, J.E.; Gottwald, T. Reinstatement of Xanthomonas citri (ex Hasse) and Xanthomonas phaseoli (ex Smith) to species and reclassification of all Xanthomonas campestris pv citri strains. Int. J. Syst. Bacteriol. 1989, 39, 14–22. [Google Scholar] [CrossRef] [Green Version]
- Young, J.M.; Bradbury, J.F.; Gardan, L.; Gvozdyak, R.I.; Stead, D.E.; Takikawa, Y.; Vidaver, A.K. Comment on the reinstatement of Xanthomonas citri (Ex Hasse 1915) Gabriel et al. 1989 and X. phaseoli (Ex Smith 1897) Gabriel et al. 1989—Indication of the need for minimal standards for the genus Xanthomonas. Int. J. Syst. Bacteriol. 1991, 41, 172–177. [Google Scholar] [CrossRef]
- Vauterin, L.; Hoste, B.; Kersters, K.; Swings, J. Reclassification of Xanthomonas. Int. J. Syst. Bacteriol. 1995, 45, 472–489. [Google Scholar] [CrossRef]
- Schaad, N.W.; Postnikova, E.; Lacy, G.; Sechler, A.; Agarkova, I.; Stromberg, P.E.; Stromberg, V.K.; Vidaver, A.K. Reclassification of Xanthomonas campestris pv. citri (ex Hasse 1915) Dye 1978 forms A, B/C/D, and E as X. smithii subsp. citri (ex Hasse) sp. nov. nom. rev. comb. nov., X. fuscans subsp. aurantifolii (ex Gabriel 1989) sp. nov. nom. rev. comb. nov., and X. alfalfae subsp. citrumelo (ex Riker and Jones) Gabriel et al., 1989 sp. nov. nom. rev. comb. nov.; X. campestris pv. malvacearum (ex Smith 1901) Dye 1978 as X. smithii subsp. smithii nov. comb. nov. nom. nov.; X. campestris pv. alfalfae (ex Riker and Jones, 1935) Dye 1978 as X. alfalfae subsp. alfalfae (ex Riker et al., 1935) sp. nov. nom. rev.; and ‘‘var. fuscans’’ of X. campestris pv. phaseoli (ex Smith, 1987) Dye 1978 as X. fuscans subsp. fuscans sp. nov. Syst. Appl. Microbiol. 2005, 28, 494–518. [Google Scholar]
- Schaad, N.W.; Postnikova, E.; Lacy, G.; Sechler, A.; Agarkova, I.; Stromberg, P.E.; Stromberg, V.K.; Vidaver, A.K. Emended classification of Xanthomonad pathogens on citrus. Syst. Appl. Microbiol. 2006, 29, 690–695. [Google Scholar] [CrossRef] [Green Version]
- Euzeby, J. List of new names and new combinations previously effectively, but no validly, published, list. Int. J. Syst. Evol. Microbiol. 2007, 57, 893–897. [Google Scholar]
- Constantin, E.C.; Cleenwerck, I.; Maes, M.; Baeyen, S.; Van Malderghem, C.; De Vos, P.; Cottyn, B. Genetic characterization of strains named as Xanthomonas axonopodis pv. dieffenbachiae leads to a taxonomic revision of the X. axonopodis species complex. Plant Pathol. 2016, 65, 792–806. [Google Scholar] [CrossRef]
- Holland, D.F.V. The families and genera of the bacteria. V. Generic index of the commoner forms of bacteria. J. Bacteriol. 1920, 5, 191–229. [Google Scholar]
- Bergey, D.H.; Harrison, F.C.; Breed, R.S.; Hammer, B.W.; Huntoon, F.M. Bergey’s Manual of Determinative Bacteriology, 1st ed.; Williams Wilkins: Baltimore, MD, USA, 1923. [Google Scholar]
- Namekata, T.; Oliveira, A.D. Comparative serological studies between Xanthomonas citri and a bacterium causing canker on Mexican lime. In Proceedings of the Third International Conference on Plant Pathogenic Bacteria; Maas Geesteranus, H.P., Ed.; Centre of the Agricultural Publication and Documentation: Wageningen, The Netherlands, 1972; pp. 151–152. [Google Scholar]
- Dye, D.W.; Bradbury, J.F.; Goto, M.; Hayward, A.C.; Lelliott, R.A.; Schroth, M.N. International standards for naming pathovars of phytopathogenic bacteria and a list of pathovar names and pathotypes. Rev. Plant Pathol. 1980, 59, 153–168. [Google Scholar]
- Brunings, A.M.; Gabriel, D.W. Xanthomonas citri: Breaking the surface. Mol. Plant Pathol. 2003, 4, 141–157. [Google Scholar] [CrossRef]
- Maloy, O.; Baudoin, A. Disease control principles. In Enclyclopedia of Plant Pathology; Maloy, O.C., Murray, T.D., Eds.; Wiley: New York, NY, USA, 2001; pp. 330–332. [Google Scholar]
- Izadiyan, M.; Taghavi, S.M.; Farahbakhsh, F. Characterization of Xanthomonas citri subsp. CITRI isolated from grapefruit in Iran. J. Plant Pathol. 2018, 100, 257–267. [Google Scholar] [CrossRef]
- Civerolo, E. Bacterial canker disease of citrus [Xanthomonas campestris]. J. Rio Gd. Val. Hortic. Soc. 1984, 35, 811–818. [Google Scholar]
- Civerolo, E. Citrus bacterial canker disease in tropical regions. Colloques-Inra 1994, 66, 45. [Google Scholar]
- Stall, R.E.; Civerolo, E.L. Research relating to the recent outbreak of citrus canker in Florida. Annu. Rev. Phytopathol. 1991, 29, 399–420. [Google Scholar] [CrossRef]
- Humphries, J. Bacteriology; John Murray Albermack Street: London, UK, 1974; p. 452. [Google Scholar]
- Schaad, N.W.; Jones, J.B.; Chun, W. Laboratory Guide for the Identification of Plant Pathogenic Bacteria; American Phytopathological Society (APS Press): St. Paul, MN, USA, 2001. [Google Scholar]
- Vernière, C.; Hartung, J.S.; Pruvost, O.P.; Civerolo, E.L.; Alvarez, A.M.; Maestri, P.; Luisetti, J. Characterization of phenotypically distinct strains of Xanthomonas axonopodispv. citri from Southwest Asia. Eur. J. Plant Pathol. 1998, 104, 477–487. [Google Scholar] [CrossRef]
- Cubero, J.; Graham, J. Genetic relationship among worldwide strains of Xanthomonas causing canker in citrus species and design of new primers for their identification by PCR. Appl. Environ. Microbiol. 2002, 68, 1257–1264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leite, R.; Minsavage, G.V.; Bonas, U.; Stall, R.E. Detection and identification of phytopathogenic Xanthomonas strains by amplification of DNA sequences related to the hrp genes of Xanthomonas campestris pv. vesicatoria. Appl. Environ. Microbiol. 1994, 60, 1068–1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vauterin, L.; Yang, P.; Hoste, B.; Vancanneyt, M.; Civerolo, E.; Swings, J.; Kersters, K. Differentiation of Xanthomonas campestris pv. citri strains by sodium dodecyl sulfate-polyacrylamide gel electrophoresis of proteins, fatty acid analysis, and DNA-DNA hybridization. Int. J. Syst. Evol. Microbiol. 1991, 41, 535–542. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.K.; Gee, A.D.; Wesselink, P.; Moorer, W. Fluid transport and bacterial penetration along root canal fillings. Int. Endod. J. 1993, 26, 203–208. [Google Scholar] [CrossRef]
- Egel, D.; Graham, J.; Stall, R. Genomic relatedness of Xanthomonas campestris strains causing diseases of citrus. Appl. Environ. Microbiol. 1991, 57, 2724–2730. [Google Scholar] [CrossRef] [Green Version]
- Pruvost, O.; Hartung, J.; Civerolo, E.; Dubois, C.; Perrier, X. Plasmid DNA fingerprints distinguish pathotypes of Xanthomonas campestris pv. citri, the causal agent of citrus bacterial canker disease. Phytopathology 1992, 82, 485–490. [Google Scholar] [CrossRef]
- Hartung, J.; Daniel, J.-F.; Pruvost, O. Detection of Xanthomonas campestris pv. citri by the polymerase chain reaction method. Appl. Environ. Microbiol. 1993, 59, 1143–1148. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Meng, Q. Automatic citrus canker detection from leaf images captured in field. Pattern Recognit. Lett. 2011, 32, 2036–2046. [Google Scholar] [CrossRef] [Green Version]
- Swarup, S.; De Feyter, R.; Brlansky, R.H.; Gabriel, D.W. A pathogenicity locus from Xanthomonas citri enables strains from several pathovars of X. campestris to elicit cankerlike lesions on citrus. Phytopathology 1991, 81, 802–809. [Google Scholar] [CrossRef]
- Swarup, S.; Yang, Y.; Kingsley, M.T.; Gabriel, D.W. An Xanthomonas citri pathogenicity gene, pthA, pleiotropically encodes gratuitous avirulence on nonhosts. Mol. Plant Microbe. Interact. 1992, 5, 204–213. [Google Scholar] [CrossRef]
- Medina-Urrutia, V.M.; Stapleton, J.J. Control of Mexican lime bacteriosis with copper-based products. Proc. Fla. State Hortic. Soc. 1987, 99, 22–25. [Google Scholar]
- Stapleton, J.; Garza-Lopez, J. Epidemiology of a citrus leaf-spot disease in Colima, Mexico. Phytopathology 1988, 78, 440–443. [Google Scholar] [CrossRef]
- Urrutia, M. Isolation, pathogenicity, and partial host range of Alternaria limicola, causal agent of mancha foliar de los citricos in Mexico. Plant Dis. 1994, 78, 879. [Google Scholar]
- Graham, J.H.; Gottwald, T. Research perspectives on eradication of citrus bacterial diseases in Florida. Plant Dis. 1991, 75, 1193–1200. [Google Scholar] [CrossRef]
- International Standards for Phytosanitary Measures (ISPM) ISPM 27 Diagnostic Protocols, DP 6: Xanthomonas citri subsp. citri; IPPC, FAO: Rome, Italy, 2014.
- Timmer, L. Anthracnose diseases. In Compendium of Citrus Diseases, 2nd ed.; Timmer, L.W., Garnsey, S.M., Graham, J.H., Eds.; APS Press: St. Paul, MN, USA, 2000; pp. 21–22. [Google Scholar]
- Cernadas, R.A.; Benedetti, C.E. Role of auxin and gibberellin in citrus canker development and in the transcriptional control of cell-wall remodeling genes modulated by Xanthomonas axonopodis pv. citri. Plant Sci. 2009, 177, 190–195. [Google Scholar] [CrossRef]
- Swings, J.; Van den Mooter, M.; Vauterin, L.; Hoste, B.; Gillis, M.; Mew, T.; Kersters, K. Reclassification of the Causal Agents of Bacterial Blight (Xanthomonas campestris pv. oryzae) and Bacterial Leaf Streak (Xanthomonas campestris pv. oryzicola) of Rice as Pathovars of Xanthomonas oryzae (ex Ishiyama 1922) sp. nov., nom. rev. Int. J. Syst. Evol. Microbiol. 1990, 40, 309–311. [Google Scholar] [CrossRef] [Green Version]
- Mahaffee, W.F.; Kloepper, J.W. Temporal changes in the bacterial communities of soil, rhizosphere and endorhiza associated with field-grown cucumber (Cucumis sativus L.). Microb. Ecol. 1997, 34, 210–223. [Google Scholar] [CrossRef]
- Holt, J.G.; Krieg, N.R.; Sneath, P.H.A.; Staley, J.T.; Williams, S.T. Bergey’s Manual of Determinative Bacteriology, 9th ed.; Williams and Wilkins: Baltimore, MD, USA, 1994. [Google Scholar]
- Gottwald, T.R.; Sun, X.; Riley, T.; Graham, J.H.; Ferrandino, F.; Taylor, E.L. Geo-referenced spatiotemporal analysis of the urban citrus canker epidemic in Florida. Phytopathology 2002, 92, 361–377. [Google Scholar] [CrossRef] [Green Version]
- Graham, J.H.; Gottwald, T.R.; Cubero, J.; Achor, D.S. Xanthomonas axonopodispv. citri: Factors affecting successful eradication of citrus canker. Mol. Plant Pathol. 2004, 5, 1–15. [Google Scholar] [CrossRef]
- Daungfu, O.; Youpensuk, S.; Lumyong, S. Endophytic Bacteria Isolated from Citrus Plants for Biological Control of Citrus Canker in Lime Plants. Trop. Life Sci. Res. 2019, 30, 73–88. [Google Scholar] [CrossRef]
- Graham, J.; Gottwald, T.; Riley, T.; Achor, D. Penetration through leaf stomata and growth of strains of Xanthomonas campestris in citrus cultivars varying in susceptibility to bacterial diseases. Phytopathology 1992, 82, 1319–1325. [Google Scholar] [CrossRef]
- Koizumi, M. Citrus Canker: The World Situation. Citrus Canker: An International Perspective; Timmer, L.W., Ed.; University of Florida: Lake Alfred, FL, USA, 1985; pp. 2–7. [Google Scholar]
- Loucks, K.W. Citrus Canker and Its Eradication in Florida; Department of Agriculture, Division of Plant Industry: St. Gainesville, FL, USA, 1934. [Google Scholar]
- Goto, M. Citrus canker. Plant Dis. Int. Importance 1992, 3, 170–208. [Google Scholar]
- Chagas, M.; Parra, J.R.; Namekata, T.; Hartung, J.S.; Yamamoto, P.T. Phyllocnistiscitrella Stainton (Lepidoptera: Gracillariidae) and its relationship with the citrus canker bacterium Xanthomonas axonopodispvcitri in Brazil. Neotrop. Entomol. 2001, 30, 55–59. [Google Scholar] [CrossRef]
- Christiano, R.; Dalla Pria, M.; Jesus Junior, W.C.; Parra, J.R.P.; Amorim, L.; Bergamin Filho, A. Effect of citrus leaf-miner damage, mechanical damage and inoculum concentration on severity of symptoms of Asiatic citrus canker in Tahiti lime. Crop Prot. 2007, 26, 59–65. [Google Scholar] [CrossRef]
- Hall, D.G.; Gottwald, T.R.; Bock, C.H. Exacerbation of citrus canker by citrus leafminerPhyllocnistiscitrella in Florida. Fla. Entomol. 2010, 93, 558–566. [Google Scholar] [CrossRef]
- Das, A. Citrus canker—A review. J. Appl. Hortic. 2003, 5, 52–60. [Google Scholar] [CrossRef]
- Stall, R. Xanthomonas campestris pv. citri detection and identification by enzyme-linked immunosorbent assay. Plant Dis. 1982, 231, 231–236. [Google Scholar]
- Bergamin Filho, A.; Hughes, G. Citrus Canker Epidemiology-Methodologies and Approaches: A Moderated Discussion Session. In Proceedings of the International Citrus Canker Research Workshop, Ft. Pierce, FL, USA, 20–22 June 2022; pp. 24–25. [Google Scholar]
- Francis, M.; Redondo, A.; Burns, J.; Graham, J. Soil application of imidacloprid and related SAR-inducing compounds produces effective and persistent control of citrus canker. Eur. J. Plant Pathol. 2009, 124, 283–292. [Google Scholar] [CrossRef]
- Dewdney, M.; Graham, J. Florida Citrus Pest Management Guide: Citrus Canker; Institute of Food and Agricultural Sciences, University of Florida: Gainesville, FL, USA, 2012; p. 4. [Google Scholar]
- Duan, Y.P.; Castaneda, A.; Zhao, G.; Erdos, G.; Gabriel, D. Expression of a single, host-specific, bacterial pathogenicity gene in plant cells elicits division, enlargement, and cell death. Mol. Plant-Microbe Interact. 1999, 12, 556–560. [Google Scholar] [CrossRef] [Green Version]
- Gottwald, T.; Graham, J.; Schubert, T. An epidemiological analysis of the spread of citrus canker in urban Miami, Florida, and synergistic interaction with the Asian citrus leafminer. Fruits 1997, 6, 383–390. [Google Scholar]
- Graham, J.; Gottwald, T.; Riley, T.; Cubero, J.; Drouillard, D. Survival of Xanthomonas campestris pv. citri (Xcc) on various surfaces and chemical control of Asiatic Citrus Canker (ACC). In Proceedings of the International Citrus Canker Research Workshop, Ft. Pierce, FL, USA, 20–22 June 2000; p. 7. [Google Scholar]
- Rao, Y.; Hingorani, M. Survival of Xanthomonas citri (Hasse) Dowson in leaves and soil. Indian Phytopath 1963, 16, 362–364. [Google Scholar]
- Verniere, C.; Gottwald, T.; Pruvost, O. Disease development and symptom expression of Xanthomonas axonopodispv. citri in various citrus plant tissues. Phytopathology 2003, 93, 832–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goto, M.; Serizawa, S.; Morita, M. Studies on Citrus Canker Disease. III. Survival of Xanthomonas Citri (Hasse) Dowson in Soils and on the Surface of Weeds; Bulletin of the Faculty of Agriculture, Shizuoka University: Shizuoka, Japan, 1970; Volume 20, pp. 21–29. [Google Scholar]
- Goto, M. Survival of Xanthomonas citri in the bark tissues of citrus trees. Can. J. Bot. 1972, 50, 2629–2635. [Google Scholar] [CrossRef]
- Leite, R., Jr.; Mohan, S. Evaluation of citrus cultivars for resistance to canker caused by Xanthomonas campestris pv. citri (Hasse) Dye in the State of Paraná, Brazil. Proc. Int. Soc. Citric. 1984, 1, 385–389. [Google Scholar]
- Graham, J.; Gottwald, T.; Civerolo, E.; McGuire, R. Population dynamics and survival of Xanthomonas campestris in soil in citrus nurseries in Maryland and Argentina. Plant Dis. 1989, 43, 423–427. [Google Scholar] [CrossRef]
- Teper, D.; Pandey, S.S.; Wang, N. The HrpG/HrpX Regulon of Xanthomonads—An Insight to the Complexity of Regulation of Virulence Traits in Phytopathogenic Bacteria. Microorganisms 2021, 9, 187. [Google Scholar] [CrossRef]
- Luthra, J.C.; Sattar, A. Citrus canker and its control in Punjab. Punjab Fruit J. 1942, 6, 179–182. [Google Scholar]
- Stall, R.E.; Miller, J.; Marco, G.M.; de Echenique, B.C. Population dynamics of Xanthomonas citri causing cancrosis of citrus in Argentina. In Proceedings of the Florida State Horticultural Society; Florida State Horticultural Society: Alexandria, VA, USA, 1980; pp. 10–14. [Google Scholar]
- Traoré, Y.N.; Ngoc, L.B.T.; Vernière, C.; Pruvost, O. First report of Xanthomonas citripv. citri causing citrus canker in Mali. Plant Dis. 2008, 92, 977. [Google Scholar] [CrossRef]
- Heppner, J.B. Citrus leafminer, Phyllocnistiscitrella, in Florida (Lepidoptera: Gracillariidae: Phyllocnistinae). Trop. Lepid. Res. 1993, 1, 49–64. [Google Scholar]
- Parsai, P.S. Citrus canker. In Proceedings of the Seminar on Diseases of Horticultural Plants, Simla, India, 10–15 June 1959; pp. 91–95. [Google Scholar]
- Bacon, C.W.; Hinton, D.M. Endophytic and biological control potential of Bacillus mojavensis and related species. Biol. Control 2002, 23, 274–284. [Google Scholar] [CrossRef] [Green Version]
- Knapp, J. Citrus Leafminer, Phyllocnistiscitrella Stainton: Current Status in Florida-1995; University of Florida: Gainesville, FL, USA, 1995. [Google Scholar]
- Peña, J.E.; Hunsberger, A.; Schaffer, B. Citrus leafminer (Lepidoptera: Gracillariidae) density: Effect on yield of ‘Tahiti’ lime. J. Econ. Entomol. 2000, 93, 374–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behlau, F.; Belasque, J. Cancro Citrico—A Doenca e Seu Controle; Fundecitrus: Araraquara, Brazil, 2014. [Google Scholar]
- Coletta-Filho, H.D.; Takita, M.A.; Souza, A.A.; Neto, J.R.; Destefano, S.A.L.; Hartung, J.S.; Machado, M.A. Primers based on the rpf gene region provide improved detection of Xanthomonas axonopodis pv. citri in naturally and artificially infected citrus plants. J. Appl. Microbiol. 2006, 100, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Hartung, J.S. Plasmid-based hybridization probes for detection and identification of Xanthomonas campestris pv citri. Plant Dis. 1992, 76, 889–893. [Google Scholar] [CrossRef]
- Mavrodieva, V.; Levy, L.; Gabriel, D.W. Improved sampling methods for real-time polymerase chain reaction diagnosis of citrus canker from field samples. Phytopathology 2004, 94, 61–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Stall, R.E.; Jones, J.B.; Cubero, J.; Gottwald, T.R.; Graham, J.H.; Dixon, W.N.; Schubert, T.S.; Chaloux, P.H.; Stromberg, V.K.; et al. Detection and characterization of a new strain of citrus canker bacteria from key Mexican lime and alemow in South Florida. Plant Dis. 2004, 88, 1179–1188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cubero, J.; Graham, J.H. Quantitative real-time polymerase chain reaction for bacterial enumeration and allelic discrimination to differentiate Xanthomonas strains on citrus. Phytopathology 2005, 95, 1333–1340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golmohammadi, M.; Cubero, J.; Penalver, J.; Quesada, J.M.; Lopez, M.M.; Llop, P. Diagnosis of Xanthomonas axonopodis pv. citri, causal agent of citrus canker, in commercial fruits by isolation and PCR-based methods. J. Appl. Microbiol. 2007, 103, 2309–2315. [Google Scholar] [CrossRef]
- Park, D.S.; Wook Hyun, J.; Jin Park, Y.; Sun Kim, J.; Wan Kang, H.; Ho Hahn, J.; Joo Go, S. Sensitive and specific detection of Xanthomonas axonopodis pv. citri by PCR using pathovar specific primers based on hrpW gene sequences. Microbiol. Res. 2006, 161, 145–149. [Google Scholar] [CrossRef]
- Rigano, L.A.; Siciliano, F.; Enrique, R.; Sendín, L.; Filippone, P.; Torres, P.S.; Qüesta, J.; Dow, J.M.; Castagnaro, A.P.; Vojnov, A.A. Biofilm formation, epiphytic fitness, and canker development in Xant Hartung homonas axonopodispv. citri. Mol. Plant-Microbe Interact. 2007, 20, 1222–1230. [Google Scholar] [CrossRef] [Green Version]
- Hartung, J.S.; Pruvost, O.P.; Villemot, I.; Alvarez, A. Rapid and sensitive colorimetric detection of Xanthomonas axonopodispv. citri by immunocapture and a nested-polymerase chain reaction assay. Pathology 1996, 8695, 101. [Google Scholar]
- Gabriel, D.; Gottwald, T.R.; Lopes, S.A.; Wulff, N.A. Bacterial pathogens of citrus: Citrus canker, citrus variegated chlorosis and Huanglongbing. In The Genus Citrus; Woodhead Publishing: Sawston, UK, 2020; pp. 371–389. [Google Scholar]
- Cubero, J.; Graham, J.; Gottwald, T. Quantitative PCR method for diagnosis of citrus bacterial canker. Appl. Environ. Microbiol. 2001, 67, 2849–2852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, M.; Yang, Y. Quantification of Magnaporthegrisea during infection of rice plants using real-time polymerase chain reaction and northern blot/phosphoimaging analyses. Phytopathology 2002, 92, 870–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winton, L.; Stone, J.; Watrud, L.; Hansen, E. Simultaneous one-tube quantification of host and pathogen DNA with real-time polymerase chain reaction. Phytopathology 2002, 92, 112–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandemark, G.; Barker, B.; Gritsenko, M. Quantifying Aphanomyces euteiches in alfalfa with a fluorescent polymerase chain reaction assay. Phytopathology 2002, 92, 265–272. [Google Scholar] [CrossRef] [Green Version]
- Schaad, N.; Berthier-Schaad, Y.; Sechler, A.; Knorr, D. Detection of Clavibactermichiganensis subsp. sepedonicus in potato tubers by BIO-PCR and an automated real-time fluorescence detection system. Plant Dis. 1999, 83, 1095–1100. [Google Scholar] [CrossRef] [Green Version]
- Schaad, N.W.; Frederick, R.D. Real-time PCR and its application for rapid plant disease diagnostics. Can. J. Plant Pathol. 2002, 24, 250–258. [Google Scholar] [CrossRef]
- Weller, S.; Elphinstone, J.; Smith, N.; Stead, D. Detection of Ralstonia solanacearum from potato tissue by post-enrichment TaqMan PCR. EPPO Bull. 2000, 30, 381–383. [Google Scholar] [CrossRef]
- Roberts, C.A.; Dietzgen, R.G.; Heelan, L.A.; Maclean, D.J. Real-time RT-PCR fluorescent detection of tomato spotted wilt virus. J. Virol. Methods 2000, 88, 1–8. [Google Scholar] [CrossRef]
- Mumford, R.; Walsh, K.; Boonham, N. A comparison of molecular methods for the routine detection of viroids. EPPO Bull. 2000, 30, 431–435. [Google Scholar] [CrossRef]
- Mackay, I.M.; Arden, K.E.; Nitsche, A. Real-time PCR in virology. Nucleic Acids Res. 2002, 30, 1292–1305. [Google Scholar] [CrossRef] [Green Version]
- Al-Saleh, M.A.; Widyawan, A.; Saleh, A.A.; Ibrahim, Y.E. Distribution and pathotype identification of Xanthomonas citri subsp. citri recovered from southwestern region of Saudi Arabia. Afr. J. Microbiol. Res. 2014, 8, 673–679. [Google Scholar]
- Adriko, J.; Aritua, V.; Mortensen, C.N.; Tushemereirwe, W.K.; Kubiriba, J.; Lund, O.S. Multiplex PCR for specific and robust detection of Xanthomonas campestris pv. musacearum in pure culture and infected plant material. Plant Pathol. 2012, 61, 489–497. [Google Scholar] [CrossRef]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adriko, J.; Mbega, E.R.; Mortensen, C.N.; Wulff, E.G.; Tushemereirwe, W.K.; Kubiriba, J.; Lund, O.S. Improved PCR for identification of members of the genus Xanthomonas. Eur. J. Plant Pathol. 2014, 138, 293–306. [Google Scholar] [CrossRef]
- da Silva, A.R.; Ferro, J.A.; Reinach, F.d.C.; Farah, C.S.; Furlan, L.R.; Quaggio, R.B.; Monteiro-Vitorello, C.B.; Van Sluys, M.-A.; Almeida, N.A.; Alves, L. Comparison of the genomes of two Xanthomonas pathogens with differing host specificities. Nature 2002, 417, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Kalita, P.; Bora, L.C.; Bhagabati, K.N. Phylloplane microflora of citrus and their role in management of citrus canker. Indian Phytopathol. 1996, 49, 234–237. [Google Scholar]
- Jalan, N.; Kumar, D.; Andrade, M.O.; Yu, F.; Jones, F.B.; Graham, J.H.; White, F.F.; Setubal, J.C.; Wang, N. Comparative genomic and transcriptome analyses of pathotypes of Xanthomonas citri subsp. citri provide insights into mechanisms of bacterial virulence and host range. BMC Genom. 2013, 14, 551. [Google Scholar] [CrossRef] [Green Version]
- Hartung, J.S.; Civerolo, E. Genomic Fingerprints of Xanthomonas campestris pv. citri Strains. Phytopathology 1987, 77, 282–285. [Google Scholar] [CrossRef]
- Lazo, G.R.; Roffey, R.; Gabriel, D.W. Pathovars of Xanthomonas campestris are distinguishable by restriction fragment-length polymorphism. Int. J. Syst. Evol. Microbiol. 1987, 37, 214–221. [Google Scholar] [CrossRef] [Green Version]
- Cooksey, D.A.; Graham, J.H. Genomic fingerprinting of two pathovars of phytopathogenic bacteria by rare-cutting restriction enzymes and field inversion gel electrophoresis. Phytopathology 1989, 79, 745–750. [Google Scholar] [CrossRef]
- Leach, J.; White, F.; Rhoads, M.; Leung, H. A repetitive DNA sequence differentiates Xanthomonas campestris pv. oryzae from other pathovars of X. campestris. Mol. Plant-Microbe Interact. 1990, 3, 238–246. [Google Scholar] [CrossRef]
- Pooler, M.R.; Ritchie, D.F.; Hartung, J.S. Genetic relationships among strains of Xanthomonas fragariae based on random amplified polymorphic DNA PCR, repetitive extragenic palindromic PCR, and enterobacterial repetitive intergenic consensus PCR data and generation of multiplexed PCR primers useful for the identification of this phytopathogen. Appl. Environ. Microbiol. 1996, 62, 3121–3127. [Google Scholar] [PubMed]
- Hauben, L.; Vauterin, L.; Swings, J.; Moore, E. Comparison of 16S ribosomal DNA sequences of all Xanthomonas species. Int. J. Syst. Evol. Microbiol. 1997, 47, 328–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Sluys, M.A.; Monteiro-Vitorello, C.B.; Camargo, L.E.A.; Menck, C.F.M.; Da Silva, A.C.R.; Ferro, J.A.; Simpson, A.J. Comparative genomic analysis of plant-associated bacteria. Annu. Rev. Phytopathol. 2002, 40, 169–189. [Google Scholar] [CrossRef] [PubMed]
- Dunger, G.; Relling, V.M.; Tondo, M.L.; Barreras, M.; Ielpi, L.; Orellano, E.G.; Ottado, J. Xanthan is not essential for pathogenicity in citrus canker but contributes to Xanthomonas epiphytic survival. Arch. Microbiol. 2007, 188, 127–135. [Google Scholar] [CrossRef]
- Rossier, O.; Van den Ackerveken, G.; Bonas, U. HrpB2 and HrpF from Xanthomonas are type III-secreted proteins and essential for pathogenicity and recognition by the host plant. Mol. Microbiol. 2000, 38, 828–838. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Figueiredo, F.; Jones, J.; Wang, N. HrpG and HrpX play global roles in coordinating different virulence traits of Xanthomonas axonopodispv. citri. Mol. Plant-Microbe Interact. 2011, 24, 649–661. [Google Scholar] [CrossRef] [Green Version]
- Yamazaki, A.; Hirata, H.; Tsuyumu, S. HrpG regulates type II secretory proteins in Xanthomonas axonopodis pv. citri. J. Gen. Plant Pathol. 2008, 74, 138–150. [Google Scholar] [CrossRef]
- Yamazaki, A.; Hirata, H.; Tsuyumu, S. Type III regulators hrpG and hrpXct control synthesis of alpha-amylase, which is involved in in planta multiplication of Xanthomonas axonopodis pv. citri. J. Gen. Plant Pathol. 2008, 74, 254–257. [Google Scholar] [CrossRef]
- Sturz, A.V.; Christie, B.R.; Nowak, J. Bacterial endophytes: Potential role in developing sustainable systems of crop production. Crit. Rev. Plant Sci. 2000, 19, 1–30. [Google Scholar] [CrossRef]
- Jaciani, F.J.; Ferro, J.A.; Ferro, M.I.T.; Vernière, C.; Pruvost, O.; Belasque, J., Jr. Genetic diversity of a Brazilian strain collection of Xanthomonas citri subsp. citri based on the type III effector protein genes. Plant Dis. 2012, 96, 193–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mysore, K.S.; Ryu, C.M. Nonhost resistance: How much do we know? Trends Plant Sci. 2004, 9, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Weber, E.; Berger, C.; Bonas, U.; Koebnik, R. Refinement of the Xanthomonas campestris pv. vesicatoriahrpD and hrpE operon structure. Mol. Plant-Microbe Interact. 2007, 20, 559–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunger, G.; Arabolaza, A.; Gottig, N.; Orellano, E.; Ottado, J. Participation of Xanthomonas axonopodispv. citrihrp cluster in citrus canker and nonhost plant responses. Plant Pathol. 2005, 54, 781–788. [Google Scholar] [CrossRef]
- Dunger, G.; Garofalo, C.G.; Gottig, N.; Garavaglia, B.S.; Rosa, M.C.P.; Farah, C.S.; Orellano, E.G.; Ottado, J. Analysis of three Xanthomonas axonopodispv. citri effector proteins in pathogenicity and their interactions with host plant proteins. Mol. Plant Pathol. 2012, 13, 865–876. [Google Scholar] [CrossRef]
- Sgro, G.G.; Ficarra, F.A.; Dunger, G.; Scarpeci, T.E.; Valle, E.M.; Cortadi, A.; Orellano, E.G.; Gottig, N.; Ottado, J. Contribution of a harpin protein from X anthomonasaxonopodispv. citri to pathogen virulence. Mol. Plant Pathol. 2012, 13, 1047–1059. [Google Scholar] [CrossRef]
- Al-Saadi, A.; Reddy, J.D.; Duan, Y.P.; Brunings, A.M.; Yuan, Q.; Gabriel, D.W. All five host-range variants of Xanthomonas citri carry one pthA homolog with 17.5 repeats that determines pathogenicity on citrus, but none determine host-range variation. Mol. Plant-Microbe Interact. 2007, 20, 934–943. [Google Scholar] [CrossRef] [Green Version]
- Alegria, M.C.; Docena, C.; Khater, L.; Ramos, C.H.; Da Silva, A.C.; Farah, C.S. New protein-protein interactions identified for the regulatory and structural components and substrates of the type III Secretion system of the phytopathogen Xanthomonas axonopodis Pathovar citri. J. Bacteriol. 2004, 186, 6186–6197. [Google Scholar] [CrossRef] [Green Version]
- Büttner, D.; Bonas, U. Regulation and secretion of Xanthomonas virulence factors. FEMS Microbiol. Rev. 2010, 34, 107–133. [Google Scholar] [CrossRef] [Green Version]
- Boch, J.; Bonas, U. Xanthomonas AvrBs3 family-type III effectors: Discovery and function. Annu. Rev. Phytopathol. 2010, 48, 419–436. [Google Scholar] [CrossRef]
- Leach, J.E.; White, F.F. Bacterial avirulence genes. Annu. Rev. Phytopathol. 1996, 34, 153–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakrabarty, P.; Duan, Y.; Gabriel, D. Cloning and characterization of a member of the Xanthomonas avr/pth gene family that evades all commercially utilized cotton R genes in the United States. Phytopathology 1997, 87, 1160–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreira, L.M.; Almeida, N.F.; Potnis, N.; Digiampietri, L.A.; Adi, S.S.; Bortolossi, J.C.; da Silva, A.C.; da Silva, A.M.; de Moraes, F.E.; de Oliveira, J.C. Novel insights into the genomic basis of citrus canker based on the genome sequences of two strains of Xanthomonas fuscans subsp. aurantifolii. BMC Genom. 2010, 11, 238. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, D. Why do pathogens carry avirulence genes? Physiol. Mol. Plant Pathol. 1999, 55, 205–214. [Google Scholar] [CrossRef] [Green Version]
- Koizumi, M.; Kochinotsu, B. Relation of temperature to the development of citrus canker lesions in the spring. Proc. Int. Soc. Citric. 1977, 3, 924–928. [Google Scholar]
- Kanamori, H.; Tsuyumu, S. Comparison of nucleotide sequences of canker-forming and non-canker-forming pthA homologues in Xanthomonas campestris pv. citri. Jpn. J. Phytopathol. 1998, 64, 462–470. [Google Scholar] [CrossRef]
- Yang, B.; Zhu, W.; Johnson, L.B.; White, F.F. The virulence factor AvrXa7 of Xanthomonas oryzaepv. oryzae is a type III secretion pathway-dependent nuclear-localized double-stranded DNA-binding protein. Proc. Natl. Acad. Sci. USA 2000, 97, 9807–9812. [Google Scholar] [CrossRef] [Green Version]
- Wengelnik, K.; Bonas, U. HrpXv, an AraC-type regulator, activates expression of five of the six loci in the hrp cluster of Xanthomonas campestris pv. vesicatoria. J. Bacteriol. 1996, 178, 3462–3469. [Google Scholar] [CrossRef] [Green Version]
- Wengelnik, K.; Van den Ackerveken, G.; Bonas, U. HrpG, a key hrp regulatory protein of Xanthomonas campestris pv. vesicatoria ls homologous to two-component response regulators. Mol. Plant-Microbe Interact. 1996, 9, 704–712. [Google Scholar] [CrossRef]
- Laia, M.L.; Moreira, L.M.; Dezajacomo, J.; Brigati, J.B.; Ferreira, C.B.; Ferro, M.I.; Silva, A.C.; Ferro, J.A.; Oliveira, J.C. New genes of Xanthomonas citri subsp. citri involved in pathogenesis and adaptation revealed by a transposon-based mutant library. BMC Microbiol. 2009, 9, 12. [Google Scholar] [CrossRef]
- Kingsley, M.T.; Gabriel, D.W.; Marlow, G.C.; Roberts, P.D. The opsX locus of Xanthomonas campestris affects host range and biosynthesis of lipopolysaccharide and extracellular polysaccharide. J. Bacteriol. 1993, 175, 5839–5850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casabuono, A.; Petrocelli, S.; Ottado, J.; Orellano, E.G.; Couto, A.S. Structural analysis and involvement in plant innate immunity of Xanthomonas axonopodispv. citri lipopolysaccharide. J. Biol. Chem. 2011, 286, 25628–25643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimaro, T.; Thomas, L.; Marondedze, C.; Sgro, G.G.; Garofalo, C.G.; Ficarra, F.A.; Gottig, N. The type III protein secretion system contributes to Xanthomonas citri subsp. citri biofilm formation. BMC Microbiol. 2014, 14, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Jansson, P.-E.; Kenne, L.; Lindberg, B. Structure of the extracellular polysaccharide from Xanthomonas campestris. Carbohydr. Res. 1975, 45, 275–282. [Google Scholar] [CrossRef]
- Becker, A.; Katzen, F.; Pühler, A.; Ielpi, L. Xanthan gum biosynthesis and application: A biochemical/genetic perspective. Appl. Microbiol. Biotechnol. 1998, 50, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Vojnov, A.A.; Zorreguieta, A.; Dow, J.M.; Daniels, M.J.; Dankert, M.A. Evidence for a role for the gumB and gumC gene products in the formation of xanthan from its pentasaccharide repeating unit by Xanthomonas campestris. Microbiology 1998, 144, 1487–1493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, J.-L.; Liu, Y.-N.; Barber, C.; Dow, J.; Wootton, J.; Daniels, M. Genetic and molecular analysis of a cluster of rpf genes involved in positive regulation of synthesis of extracellular enzymes and polysaccharide in Xanthomonas campestris pathovar campestris. Mol. Gen. Genet. MGG 1991, 226, 409–417. [Google Scholar] [CrossRef]
- Denny, T. Involvement of bacterial polysaccharides in plant pathogenesis. Annu. Rev. Phytopathol. 1995, 33, 173–197. [Google Scholar] [CrossRef]
- Chan, J.W.; Goodwin, P.H. The molecular genetics of virulence of Xanthomonas campestris. Biotechnol. Adv. 1999, 17, 489–508. [Google Scholar] [CrossRef]
- Hao, G.; Stover, E.; Gupta, G. Overexpression of a modified plant thionin enhances disease resistance to citrus canker and huanglongbing (HLB). Front. Plant Sci. 2016, 7, 1078. [Google Scholar] [CrossRef] [Green Version]
- Duan, S.; Jia, H.; Pang, Z.; Teper, D.; White, F.; Jones, J.; Zhou, C.; Wang, N. Functional characterization of the citrus canker susceptibility gene CsLOB1. Mol. Plant Pathol. 2018, 19, 1908–1916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chou, F.-L.; Chou, H.-C.; Lin, Y.-S.; Yang, B.-Y.; Lin, N.-T.; Weng, S.-F.; Tseng, Y.-H. TheXanthomonas campestris gumDGene Required for Synthesis of Xanthan Gum Is Involved in Normal Pigmentation and Virulence in Causing Black Rot. Biochem. Biophys. Res. Commun. 1997, 233, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Dharmapuri, S.; Sonti, R.V. A transposon insertion in the gumG homologue of Xanthomonas oryzaepv. oryzae causes loss of extracellular polysaccharide production and virulence. FEMS Microbiol. Lett. 1999, 179, 53–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kemp, B.P.; Horne, J.; Bryant, A.; Cooper, R.M. Xanthomonas axonopodispv. manihotisgumD gene is essential for EPS production and pathogenicity and enhances epiphytic survival on cassava (Manihot esculenta). Physiol. Mol. Plant Pathol. 2004, 64, 209–218. [Google Scholar] [CrossRef]
- Kim, J.-G.; Li, X.; Roden, J.A.; Taylor, K.W.; Aakre, C.D.; Su, B.; Lalonde, S.; Kirik, A.; Chen, Y.; Baranage, G. Xanthomonas T3S effector XopN suppresses PAMP-triggered immunity and interacts with a tomato atypical receptor-like kinase and TFT1. Plant Cell 2009, 21, 1305–1323. [Google Scholar] [CrossRef] [Green Version]
- Siciliano, F.; Torres, P.; Sendín, L.; Bermejo, C.; Filippone, P.; Vellice, G.; Ramallo, J.; Castagnaro, A.; Vojnov, A.; Marano, M.R. Analysis of the molecular basis of Xanthomonas axonopodispv. citri pathogenesis in Citrus limon. Electron. J. Biotechnol. 2006, 9, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Huang, Q.; Wu, J.; Li, X.; Liu, M.; Kong, Y. Toxicity and biochemical action of amicarthiazol on citrus canker pathogen, Xanthomonas citri ex Hasse. Pesticide Biochem. Physiol. 2006, 84, 188–195. [Google Scholar] [CrossRef]
- Goto, M.; Hyodo, H. Role of extracellular polysaccharides of Xanthomonas campestris pv. citri in the early stage of infection. Jpn. J. Phytopathol. 1985, 51, 22–31. [Google Scholar] [CrossRef]
- Takahashi, T.; Doke, N. A role of extracellular polysaccharides of Xanthomonas campestris pv. citri in bacterial adhesion to citrus leaf tissues in preinfectious stage. Jpn. J. Phytopathol. 1984, 50, 565–573. [Google Scholar] [CrossRef]
- Yun, M.H.; Torres, P.S.; El Oirdi, M.; Rigano, L.A.; Gonzalez-Lamothe, R.; Marano, M.R.; Castagnaro, A.P.; Dankert, M.A.; Bouarab, K.; Vojnov, A.A. Xanthan induces plant susceptibility by suppressing callose deposition. Plant Physiol. 2006, 141, 178–187. [Google Scholar] [CrossRef] [Green Version]
- Aslam, S.N.; Newman, M.-A.; Erbs, G.; Morrissey, K.L.; Chinchilla, D.; Boller, T.; Jensen, T.T.; De Castro, C.; Ierano, T.; Molinaro, A. Bacterial polysaccharides suppress induced innate immunity by calcium chelation. Curr. Biol. 2008, 18, 1078–1083. [Google Scholar] [CrossRef] [PubMed]
- Dow, J.M.; Crossman, L.; Findlay, K.; He, Y.-Q.; Feng, J.-X.; Tang, J.-L. Biofilm dispersal in Xanthomonas campestris is controlled by cell–cell signaling and is required for full virulence to plants. Proc. Natl. Acad. Sci. USA 2003, 100, 10995–11000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Limoli, D.H.; Jones, C.J.; Wozniak, D.J. Bacterial Extracellular Polysaccharides in Biofilm Formation and Function. Microbiol. Spectr. 2015, 26, 3. [Google Scholar] [CrossRef] [Green Version]
- Branda, S.S.; Vik, Å.; Friedman, L.; Kolter, R. Biofilms: The matrix revisited. Trends Microbiol. 2005, 13, 20–26. [Google Scholar] [CrossRef]
- Sutherland, I.W. Microbial polysaccharides from Gram-negative bacteria. Int. Dairy J. 2001, 11, 663–674. [Google Scholar] [CrossRef]
- Stoodley, P.; Sauer, K.; Davies, D.G.; Costerton, J.W. Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 2002, 56, 187–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hilario, E.; De Keyser, S.; Fan, L. Structural and biochemical characterization of a glutathione transferase from the citrus canker pathogen Xanthomonas. Acta Crystallogr. Sect. D Struct. Biol. 2020, 76, 778–789. [Google Scholar] [CrossRef]
- Omar, A.A.; Murata, M.M.; El-Shamy, H.A.; Graham, J.H.; Grosser, J.W. Enhanced resistance to citrus canker in transgenic mandarin expressing Xa21 from rice. Transgenic Res. 2018, 27, 179–191. [Google Scholar] [CrossRef]
- Vorhölter, F.-J.; Niehaus, K.; Pühler, A. Lipopolysaccharide biosynthesis in Xanthomonas campestris pv. campestris: A cluster of 15 genes is involved in the biosynthesis of the LPS O-antigen and the LPS core. Mol. Genet. Genom. 2001, 266, 79–95. [Google Scholar] [CrossRef]
- Dow, J.M.; Osbourn, A.E.; Wilson, T.G.; Daniels, M.J. A locus determining pathogenicity of Xanthomonas campestris is involved in lipopolysaccharide biosynthesis. MPMI-Mol. Plant Microbe Interact. 1995, 8, 768–777. [Google Scholar] [CrossRef]
- Petrocelli, S.; Tondo, M.L.; Daurelio, L.D.; Orellano, E.G. Modifications of Xanthomonas axonopodispv. citri lipopolysaccharide affect the basal response and the virulence process during citrus canker. PLoS ONE 2012, 7, e40051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patil, P.B.; Bogdanove, A.J.; Sonti, R.V. The role of horizontal transfer in the evolution of a highly variable lipopolysaccharide biosynthesis locus in xanthomonads that infect rice, citrus and crucifers. BMC Evol. Biol. 2007, 7, 243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Wang, N. Genome-wide mutagenesis of Xanthomonas axonopodispv. citri reveals novel genetic determinants and regulation mechanisms of biofilm formation. PLoS ONE 2011, 6, e21804. [Google Scholar]
- Waters, C.M.; Bassler, B.L. Quorum sensing: Cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol. 2005, 21, 319–346. [Google Scholar] [CrossRef] [Green Version]
- Papenfort, K.; Bassler, B.L. Quorum sensing signal–response systems in Gram-negative bacteria. Nat. Rev. Microbiol. 2016, 14, 576. [Google Scholar] [CrossRef]
- Ryan, R.P.; Vorhölter, F.-J.; Potnis, N.; Jones, J.B.; Van Sluys, M.-A.; Bogdanove, A.J.; Dow, J.M. Pathogenomics of Xanthomonas: Understanding bacterium–plant interactions. Nat. Rev. Microbiol. 2011, 9, 344–355. [Google Scholar] [CrossRef]
- Ryan, R.P.; An, S.-Q.; Allan, J.H.; McCarthy, Y.; Dow, J.M. The DSF family of cell–cell signals: An expanding class of bacterial virulence regulators. PLoS Pathog. 2015, 11, e1004986. [Google Scholar] [CrossRef]
- Dow, J.M. Diffusible signal factor-dependent quorum sensing in pathogenic bacteria and its exploitation for disease control. J. Appl. Microbiol. 2017, 122, 2–11. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.; Li, J.-L.; Wang, N. Diffusible signal factor-mediated quorum sensing plays a central role in coordinating gene expression of Xanthomonas citri subsp. citri. Mol. Plant-Microbe Interact. 2012, 25, 165–179. [Google Scholar] [CrossRef] [Green Version]
- Tan, X.; Huang, S.; Ren, J.; Yan, W.; Cen, Z. Study on a bacterial strain Bt8 for biocontrol against citrus bacterial canker. Wei Sheng Wuxue Bao Acta Microbiol. 2006, 46, 292–296. [Google Scholar]
- Balogh, B.; Canteros, B.I.; Stall, R.E.; Jones, J.B. Control of citrus canker and citrus bacterial spot with bacteriophages. Plant Dis. 2008, 92, 1048–1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCollum, G.; Bowman, K.; Gottwald, T. (262) Screening Citrus Germplasm for Resistance to Xanthomonas axonopodispv. Citri. HortScience 2006, 41, 1048E–1049E. [Google Scholar] [CrossRef] [Green Version]
- Lahaye, T.; Bonas, U. Molecular secrets of bacterial type III effector proteins. Trends Plant Sci. 2001, 6, 479–485. [Google Scholar] [CrossRef]
- Cernadas, R.A.; Camillo, L.R.; Benedetti, C.E. Transcriptional analysis of the sweet orange interaction with the citrus canker pathogens Xanthomonas axonopodispv. citri and Xanthomonas axonopodispv. aurantifolii. Mol. Plant Pathol. 2008, 9, 609–631. [Google Scholar] [CrossRef] [PubMed]
- Leite, R., Jr.; Mohan, S. Integrated management of the citrus bacterial canker disease caused by Xanthomonas campestris pv. citri in the State of Paraná, Brazil. Crop Prot. 1990, 9, 3–7. [Google Scholar] [CrossRef]
- Nazaré, A.C.; Polaquini, C.R.; Cavalca, L.B.; Anselmo, D.B.; Saiki MD, F.C.; Monteiro, D.A.; Zielinska, A.; Rahal, P.; Gomes, E.; Scheffers, D.; et al. Design of antibacterial agents: Alkyl dihydroxybenzoates against Xanthomonas citri subsp. citri. Int. J. Mol. Sci. 2018, 19, 3050. [Google Scholar] [CrossRef] [Green Version]
- Stall, R.E.; Seymour, C.P. Canker, a threat to citrus in the Gulf-Coast states. Plant Dis. 1983, 67, 581–585. [Google Scholar] [CrossRef]
- Behlau, F.; Barelli, N.; Belasque, J., Jr. Lessons from a case of successful eradication of citrus canker in a citrus-producing farm in São Paulo State, Brazil. J. Plant Pathol. 2014, 96, 561–568. [Google Scholar]
- Stein, B.; Ramallo, J.; Foguet, L.; Graham, J.H. Citrus leafminer control and copper sprays for management of citrus canker on lemon in Tucuman, Argentina. Florida State Hortic. Soc. 2007, 120, 127–131. [Google Scholar]
- McGuire, R.G. Evaluation of bactericidal chemicals for control of Xanthomonas on citrus. Plant Dis. 1988, 72, 1016–1020. [Google Scholar] [CrossRef]
- Bock, C.H.; Graham, J.H.; Gottwald, T.R.; Cook, A.Z.; Parker, P.E. Wind speed effects on the quantity of Xanthomonas citri subsp. citri dispersed downwind from canopies of grapefruit trees infected with citrus canker. Plant Dis. 2010, 94, 725–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talibi, I.; Boubaker, H.; Boudyach, E.H.; Ait Ben Aoumar, A. Alternative methods for the control of postharvest citrus diseases. J. Appl. Microbiol. 2014, 117, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Cacciola, S.O.; Lio, G.M.D.S. Management of citrus diseases caused by Phytophthora spp. In Integrated Management of Diseases Caused by Fungi, Phytoplasma and Bacteria; Springer: Dordrecht, The Netherlands, 2008; pp. 61–84. [Google Scholar]
- Luiz, M.H.R.; Takahashi, L.T.; Bassanezi, R.C. Optimal control in citrus diseases. Comput. Appl. Math. 2021, 40, 1–13. [Google Scholar] [CrossRef]
- Govinda Rao, P. Citrus diseases and their control in Andhra State. Andhra Agric. J. 1954, 1, 187–192. [Google Scholar]
- Paracer, C. Some important diseases of fruit trees. Punjab Hort. J. 1961, 1, 45–47. [Google Scholar]
- Graham, J.H.; Johnson, E.G.; Myers, M.E.; Young, M.; Rajasekaran, P.; Das, S.; Santra, S. Potential of nano-formulated zinc oxide for control of citrus canker on grapefruit trees. Plant Dis. 2016, 100, 2442–2447. [Google Scholar] [CrossRef]
- Patel, R.; Desai, M. Control of Citrus Canker1. Indian J. Hortic. 1970, 27, 93–98. [Google Scholar]
- Kishun, R.; Chand, R. Studies on germplasm resistance and chemical control of citrus canker. Indian J. Hortic. 1987, 44, 126–132. [Google Scholar]
- Chowdhury, S. Citrus canker in Assam. Pl. Prot. Bull 1951, 3, 78–79. [Google Scholar]
- Nirvan, R. Citrus canker and its control. Hort. Adv. 1961, 5, 171–175. [Google Scholar]
- Patel, M.; Padhya, A. Sodium arsenite-Copper sulphate spray for the control of citrus canker. Curr. Sci. 1964, 33, 87–88. [Google Scholar]
- Ram, G.; Nirvan, R.; Saxena, M. Control of citrus canker. Prog. Hort. 1972, 12, 240–243. [Google Scholar]
- Rangaswami, G.; Rao, R.R.; Lakshaman, A. Studies on the control of citrus canker with Streptomycin. Phytopathology 1959, 49, 224–226. [Google Scholar]
- Balaraman, K.; Purushotman, R. Control of citrus canker on acid lime. South Indian Hortic. 1981, 29, 175–177. [Google Scholar]
- El-Goorani, M.A. The occurrence of citrus canker disease in United Arab Emirates (UAE). J. Phytopathol. 1989, 125, 257–264. [Google Scholar] [CrossRef]
- Kale, K.; Raut, J.; Ohekar, G. Efficacy of fungicides and antibiotics against acidlime (Citrus aurantifolia (Christm.) swingle) canker. Pesticides 1988, 22, 26–27. [Google Scholar]
- Reddy, G.; Rao, A. Control of canker in citrus nurseries. Agric. J. 1960, 7, 1–13. [Google Scholar]
- Dakshinamurthi, V.; Rao, D. Preliminary studies on the control of citrus Canker on acid lime. Andhra Agric. J. 1959, 6, 145–148. [Google Scholar]
- Kale, K.; Kolte, S.; Peshney, N. Economics of chemical control of citrus canker caused by Xanthomonas campestris pv. citri under field conditions. Indian Phytopathol. 1994, 47, 253–255. [Google Scholar]
- Kumar, A.; Sharma, N.; Ahmad, M.; Siddiqui, M.W. Climate change, food security, and livelihood opportunities in mountain agriculture. Clim. Dyn. Hortic. Sci. 2015, 28, 349–360. [Google Scholar]
- Timmer, L. Evaluation of bactericides for control of citrus canker in Argentina. In Proceedings of the Florida State Horticultural Society; Florida State Horticultural Society: Alexandria, VA, USA, 1988; pp. 6–9. [Google Scholar]
- Canteros, B.I.; Gochez, A.M.; Moschini, R.C. Management of citrus canker in Argentina, a success story. Plant Pathol. J. 2017, 33, 441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, K.; Datta, K.; Karmakar, S.; Datta, S.K. Antimicrobial Peptides—Small but Mighty Weapons for Plants to Fight Phytopathogens. Protein Pept. Lett. 2019, 26, 720–742. [Google Scholar] [CrossRef] [PubMed]
- Das, R.M.B.; Mondal, P.; Khatua, D.; Mukherjee, N. Biological management of citrus canker on acid lime through Bacillus subtilis (S-12) in West Bengal, India. J. Biopestic. 2013, 7, 38–41. [Google Scholar]
- Ota, T. Interactions in vitro and in vivo between Xanthomonas campestris pv. citri and Antagonistic pseudomonas sp. Jpn. J. Phytopathol. 1983, 49, 308–315. [Google Scholar] [CrossRef]
- Goto, M.; Yaguchi, Y. Relationship between defoliation and disease severity in citrus canker. Jpn. J. Phytopathol. 1979, 45, 689–694. [Google Scholar] [CrossRef]
- Unnamalai, N.; Gnanamanickam, S. Pseudomonas fluorescens is an antagonist to Xanthomonas citri (Hasse) Dye, the incitant of citrus canker. Curr. Sci. 1984, 53, 703–704. [Google Scholar]
- Marco, G.M.; Stall, R.E. Control of bacterial spot of pepper initiated by strains of Xanthomonas campestris pv. vesicatoria that differ in sensitivity to copper. Plant Dis. 1983, 67, 779–781. [Google Scholar] [CrossRef] [Green Version]
- de Oliveira, A.G.; Spago, F.R.; Simionato, A.S.; Navarro, M.O.; da Silva, C.S.; Barazetti, A.R.; Andrade, G. Bioactive organocopper compound from Pseudomonas aeruginosa inhibits the growth of Xanthomonas citri subsp. citri. Front. Microbiol. 2016, 7, 113. [Google Scholar] [CrossRef]
- Murate, L.S.; de Oliveira, A.G.; Higashi, A.Y.; Barazetti, A.R.; Simionato, A.S.; da Silva, C.S.; Simões, G.C.; Santos, I.M.O.D.; Ferreira, M.R.; Cely, M.V.T.; et al. Activity of secondary bacterial metabolites in the control of citrus canker. In Embrapa Soja-Artigo em anais de congresso (ALICE). Agric. Sci. 2015, 6, 295–303. [Google Scholar]
- Spago, F.R.; Mauro, C.I.; Oliveira, A.G.; Beranger JP, O.; Cely MV, T.; Stanganelli, M.M.; Andrade, G. Pseudomonas aeruginosa produces secondary metabolites that have biological activity against plant pathogenic Xanthomonas species. Crop Prot. 2014, 62, 46–54. [Google Scholar] [CrossRef]
- Huang, T.P.; Tzeng, D.D.S.; Wong, A.C.L.; Chen, C.H.; Lu, K.M.; Lee, Y.H.; Huang, W.D.; Hwang, B.F.; Tzeng, K.C. DNA polymorphisms and biocontrol of Bacillus antagonistic to citrus bacterial canker with indication of the interference of phyllosphere biofilms. PLoS ONE 2012, 7, e42124. [Google Scholar] [CrossRef] [PubMed]
- Rabbee, M.F.; Ali, M.S.; Baek, K.-H. Endophyte Bacillus velezensis isolated from Citrus spp. Controls streptomycin-resistant Xanthomonas citri subsp. citri that causes citrus bacterial canker. Agronomy 2019, 9, 470. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.; Moon, E. Identification of novel bioactive hexapeptides against phytopathogenic bacteria through rapid screening of a synthetic combinatorial library. J. Microbiol. Biotechnol. 2009, 19, 792–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gholami, D.; Goodarzi, T.; Aminzadeh, S.; Alavi, S.M.; Kazemipour, N.; Farrokhi, N. Bacterial secretome analysis in hunt for novel bacteriocins with ability to control Xanthomonas citri subsp. citri. Iran J. Biotechnol. 2015, 13, 10–19. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Askora, A.; Kawasaki, T.; Fujie, M.; Yamada, T. The filamentous phage XacF1 causes loss of virulence in Xanthomonas axonopodis pv. citri, the causative agent of citrus canker disease. Front. Microbiol. 2014, 5, 321. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, A.S. Phage therapy-constraints and possibilities. Upsala J. Med. Sci. 2014, 119, 192–198. [Google Scholar] [CrossRef]
- Kering, K.K.; Kibii, B.J.; Wei, H. Biocontrol of phytobacteria with bacteriophage cocktails. Pest. Manag. Sci. 2019, 75, 1775–1781. [Google Scholar] [CrossRef]
- Yoshikawa, G.; Askora, A.; Blanc-Mathieu, R.; Kawasaki, T.; Li, Y.; Nakano, M.; Ogata, H.; Yamada, T. Xanthomonas citri jumbo phage XacN1 exhibits a wide host range and high complement of tRNA genes. Sci. Rep. 2018, 8, 4486. [Google Scholar] [CrossRef]
- Ibrahim, Y.E.; Saleh, A.A.; Al-Saleh, M.A. Management of Asiatic citrus canker under field conditions in Saudi Arabia using bacteriophages and acibenzolar-s-methyl. Plant Dis. 2017, 101, 761–765. [Google Scholar] [CrossRef] [Green Version]
- Buttimer, C.; McAuliffe, O.; Ross, R.P.; Hill, C.; O’Mahony, J.; Coffey, A. Bacteriophages and bacterial plant diseases. Front. Microbiol. 2017, 8, 34–41. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; He, Z.; Rosskopf, E.N.; Conn, K.L.; Powell, C.A.; Lazarovits, G. A nylon membrane bag assay for determination of the effect of chemicals on soilborne plant pathogens in soil. Plant Dis. 2010, 94, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Kessmann, H.; Staub, T.; Hofmann, C.; Maetzke, T.; Herzog, J.; Ward, E.; Uknes, S.; Ryals, J. Induction of systemic acquired disease resistance in plants by chemicals. Annu. Rev. Phytopathol. 1994, 32, 439–459. [Google Scholar] [CrossRef]
- Romero, A.; Kousik, C.; Ritchie, D. Resistance to bacterial spot in bell pepper induced by acibenzolar-S-methyl. Plant Dis. 2001, 85, 189–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Z.-M.; Laby, R.J.; Zumoff, C.H.; Bauer, D.W.; He, S.Y.; Collmer, A.; Beer, S.V. Harpin, elicitor of the hypersensitive response produced by the plant pathogen Erwinia amylovora. Science 1992, 257, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Oostendorp, M.; Kunz, W.; Dietrich, B.; Staub, T. Induced disease resistance in plants by chemicals. Eur. J. Plant Pathol. 2001, 107, 19–28. [Google Scholar] [CrossRef]
- Hostettmann, K.; Wolfender, J.L. The search for biologically active secondary metabolites. Pestic. Sci. 1997, 51, 471–482. [Google Scholar] [CrossRef]
- Mahajan, A.; Das, S. Plants and microbes-Potential source of pesticide for future use. Pestic. Inf. 2003, 28, 33–38. [Google Scholar]
- Balandrin, M.F.; Klocke, J.A.; Wurtele, E.S.; Bollinger, W.H. Natural plant chemicals: Sources of industrial and medicinal materials. Science 1985, 228, 1154–1160. [Google Scholar] [CrossRef]
- Dorman, H.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef]
- Parameswari, C.; Tulasi Latha, A. Antibacterial activity of Ricinus communis leaf extract. Indian Drugs 2001, 38, 587–588. [Google Scholar]
- Rath, C.; Dash, S.; Mishra, R. In vitro susceptibility of Japanese mint (Mentha arvensis L.) essential oil against five human pathogens. Indian Perfum. 2001, 45, 57–62. [Google Scholar]
- Britto, S.J.; Senthilkumar, S. Antibacterial activity of Solanum incanum L. leaf extracts. Asian J. Microbiol. Biotechnol. Environ. Sci. 2001, 3, 65–66. [Google Scholar]
- Bylka, W.; Szaufer-Hajdrych, M.; Matławska, I.; Goślińska, O. Antimicrobial activity of isocytisoside and extracts of Aquilegia vulgaris L. Lett. Appl. Microbiol. 2004, 39, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Shimpi, S.; Bendre, R. Stability and antibacterial activity of aqueous extracts of Ocimumcanum leaves. Indian Perfum. 2005, 49, 225. [Google Scholar]
- Tahir, H.A.; Sahi, S.T.; Habib, A.; Haq, I.U.; Ahmad, A.; Ashraf, W. Evaluation of plant extracts as biocontrol agents against Xanthomonas axonopodis pv citri the cause of citrus canker. Pak. J. Phytopathol. 2016, 28, 35–43. [Google Scholar]
- Rios, J.L. Essential oils: What they are and how the terms are used and defined. In Essential Oils in Food Preservation, Flavor and Safety; Academic Press: London, UK, 2016; pp. 3–10. [Google Scholar]
- Pandey, A.K.; Kumar, P.; Singh, P.; Tripathi, N.N.; Bajpai, V.K. Essential oils: Sources of antimicrobials and food preservatives. Front. Microbiol. 2017, 7, 2161. [Google Scholar] [CrossRef] [Green Version]
- Mirzaei-Najafgholi, H.; Tarighi, S.; Golmohammadi, M.; Taheri, P. The effect of citrus essential oils and their constituents on growth of Xanthomonas citri subsp. citri. Molecules 2017, 22, 591. [Google Scholar] [CrossRef] [Green Version]
- Feng, C.T.; Su, H.J.; Chen, C.T.; Ho, W.C.; Tsou, Y.R.; Chern, L.L. Inhibitory effects of Chinese medicinal herbs on plant-pathogenic bacteria and identification of the active components from gallnuts of Chinese sumac. Plant Dis. 2012, 96, 1193–1197. [Google Scholar] [CrossRef] [Green Version]
- Silva, I.C.; Regasini, L.O.; Petrãnio, M.S.; Silva, D.H.S.; Bolzani, B.S.; Belasque, J., Jr.; Sacramento, L.V.S.; Ferreira, H. Antibacterial activity of alkyl gallates against Xanthomonas citri subsp. citri. J. Bacteriol. 2013, 195, 85–94. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.Y.; Fang, H.S.; Shao, W.B.; Zhou, J.; Chen, Z.; Song, B.A.; Yang, S. Synthesis and biological evaluation of pyridinium-functionalized carbazole derivatives as promising antibacterial agents. Bioorg Med. Chem. Lett. 2017, 27, 4294–4297. [Google Scholar] [CrossRef]
- Król, E.; de Sousa Borges, A.; da Silva, I.L.; Polaquini, C.R.; Regasini, L.O.; Ferreira, H.; Scheffers, D.J. Antibacterial activity of alkyl gallates is a combination of direct targeting of FtsZ and permeabilization of bacterial membranes. Front. Microbiol. 2015, 6, 390. [Google Scholar] [CrossRef] [PubMed]
- Mohana, D.; Raveesha, K. Anti-bacterial activity of Caesalpiniacoriaria (Jacq.) Willd. against plant pathogenic Xanthomonas pathovars: An eco-friendly approach. J. Agric. Technol. 2006, 2, 317–327. [Google Scholar]
- Akhtar, M.A.; Rahber-Bhatti, M.; Aslam, M. Antibacterial activity of plant diffusate against Xanthomonas campestris pv. citri. Int. J. Pest Manag. 1997, 43, 149–153. [Google Scholar] [CrossRef]


| Sr. No. | Genus | Specie | *f.sp./*pv/subsp. | Year | Reference |
|---|---|---|---|---|---|
| 1. | Pseudomonas | citri | not reported | 1915 | [27] |
| 2. | Xanthomonas | citri | not reported | 1915 | [27] |
| 3. | Bacterium | citri | not reported | 1916 | [28] |
| 4. | Bacillus | citri | not reported | 1920 | [41] |
| 5. | Phytomonas | citri | not reported | 1923 | [42] |
| 6. | Xanthomonas | citri | not reported | 1939 | [29] |
| 7. | Xanthomonas | citri | aurantifolia | 1972 | [43] |
| 8. | Xanthomonas | campestris | aurantifolia | 1978 | [33] |
| 9. | Xanthomonas | campestris | citri | 1980 | [44] |
| 10. | Xanthomonas | citri | aurantifolia | 1989 | [34] |
| 11. | Xanthomonas | axonopodis | citri | 1995 | [36] |
| 12. | Xanthomonas | smithii | citri | 2005 | [37] |
| 13. | Xanthomonas | citri | citri | 2006 | [38] |
| 14. | Xanthomonas | citri | subsp. citri | 2007 | [39] |
| 15. | Xanthomonas | citri | subsp. citri | 2016 | [40] |
| Sr. No. | Primer | Target Region | Sequence | Reference |
|---|---|---|---|---|
| 1. | P16SF1/P16SR2 | 16S rDNA | 5-AGAGTTTGATCCTGGCTCAG-3 5-ACGGCTACCTTGTTACGACTT-3 | [129] |
| 2. | FD1/RP2 | 16S rDNA | 5-AGAGTTTGATCCTGGCTCAG-3 5-ACGGCTACCTTGTTACGACTT-3 | [130] |
| 3. | X-ITS, F3/X-ITS R2 | Internal transcribed spacer | 5-GGCGGGGACTTCGAGTCCCTAA-3 5-CTGCAGGATACTGCCGAAGCA-3 | [131] |
| 4. | X-fyuaF/X-fyuaR | FyuA | 5-GCCGGTGGACTACGATTGGAATTA-3 5-GTCGCGGCGCCACTTCA-3 | [131] |
| 5. | J-pth 1/J-pth 2 | Pathogenicity | 5-CTTCAACTCAAACGCCGGAC-3 5-CATCGCGCGCTGTTCGGGAG-3 | [54] |
| 6. | DLH 1/DLH 2 | Pathogenicity | 5-TTGGTGTCGTCGCTTGTAT-3 5-CACGGGTGCAAAAAATCT-3 | [60] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naqvi, S.A.H.; Wang, J.; Malik, M.T.; Umar, U.-U.-D.; Ateeq-Ur-Rehman; Hasnain, A.; Sohail, M.A.; Shakeel, M.T.; Nauman, M.; Hafeez-ur-Rehman; et al. Citrus Canker—Distribution, Taxonomy, Epidemiology, Disease Cycle, Pathogen Biology, Detection, and Management: A Critical Review and Future Research Agenda. Agronomy 2022, 12, 1075. https://doi.org/10.3390/agronomy12051075
Naqvi SAH, Wang J, Malik MT, Umar U-U-D, Ateeq-Ur-Rehman, Hasnain A, Sohail MA, Shakeel MT, Nauman M, Hafeez-ur-Rehman, et al. Citrus Canker—Distribution, Taxonomy, Epidemiology, Disease Cycle, Pathogen Biology, Detection, and Management: A Critical Review and Future Research Agenda. Agronomy. 2022; 12(5):1075. https://doi.org/10.3390/agronomy12051075
Chicago/Turabian StyleNaqvi, Syed Atif Hasan, Jie Wang, Muhammad Tariq Malik, Ummad-Ud-Din Umar, Ateeq-Ur-Rehman, Ammarah Hasnain, Muhammad Aamir Sohail, Muhammad Taimoor Shakeel, Muhammad Nauman, Hafeez-ur-Rehman, and et al. 2022. "Citrus Canker—Distribution, Taxonomy, Epidemiology, Disease Cycle, Pathogen Biology, Detection, and Management: A Critical Review and Future Research Agenda" Agronomy 12, no. 5: 1075. https://doi.org/10.3390/agronomy12051075
APA StyleNaqvi, S. A. H., Wang, J., Malik, M. T., Umar, U.-U.-D., Ateeq-Ur-Rehman, Hasnain, A., Sohail, M. A., Shakeel, M. T., Nauman, M., Hafeez-ur-Rehman, Hassan, M. Z., Fatima, M., & Datta, R. (2022). Citrus Canker—Distribution, Taxonomy, Epidemiology, Disease Cycle, Pathogen Biology, Detection, and Management: A Critical Review and Future Research Agenda. Agronomy, 12(5), 1075. https://doi.org/10.3390/agronomy12051075







