Comparative Study between the Photosynthetic Parameters of Two Avocado (Persea americana) Cultivars Reveals Natural Variation in Light Reactions in Response to Frost Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Frost Stress Treatment
2.3. Chlorophyll a Fluorescence Measurement
2.4. Statistical Analysis
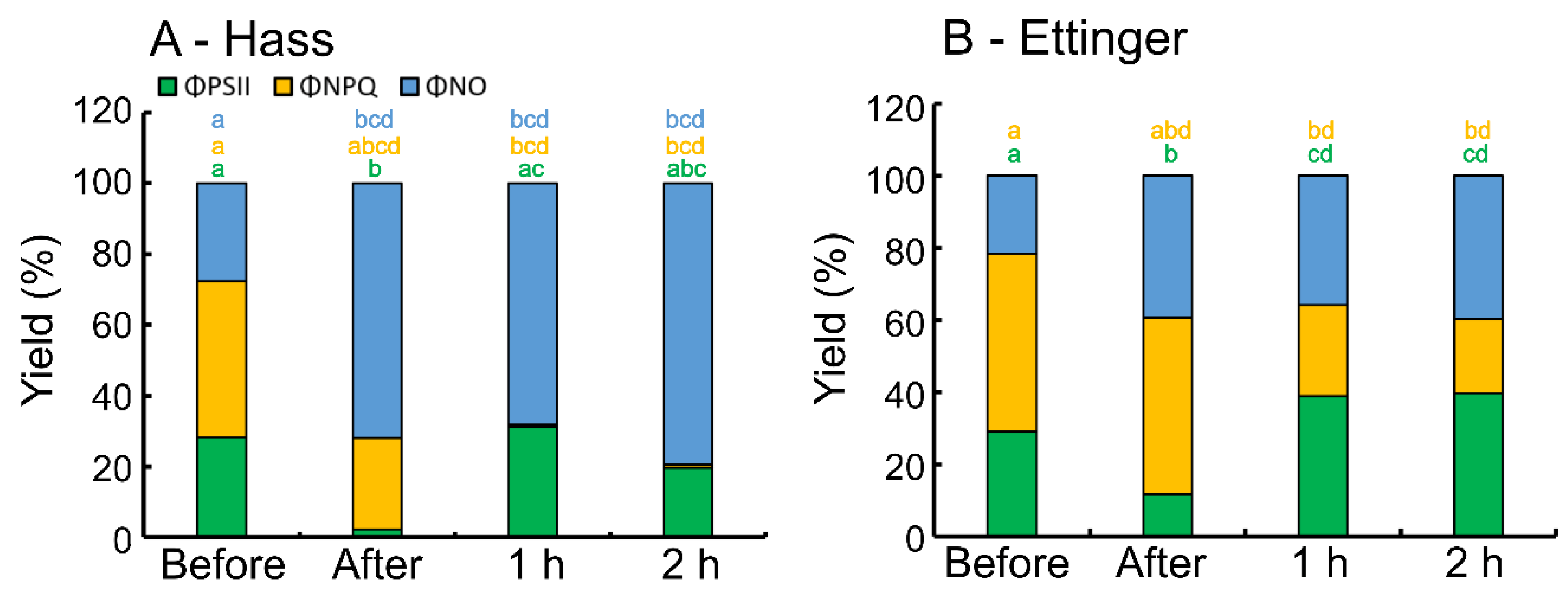
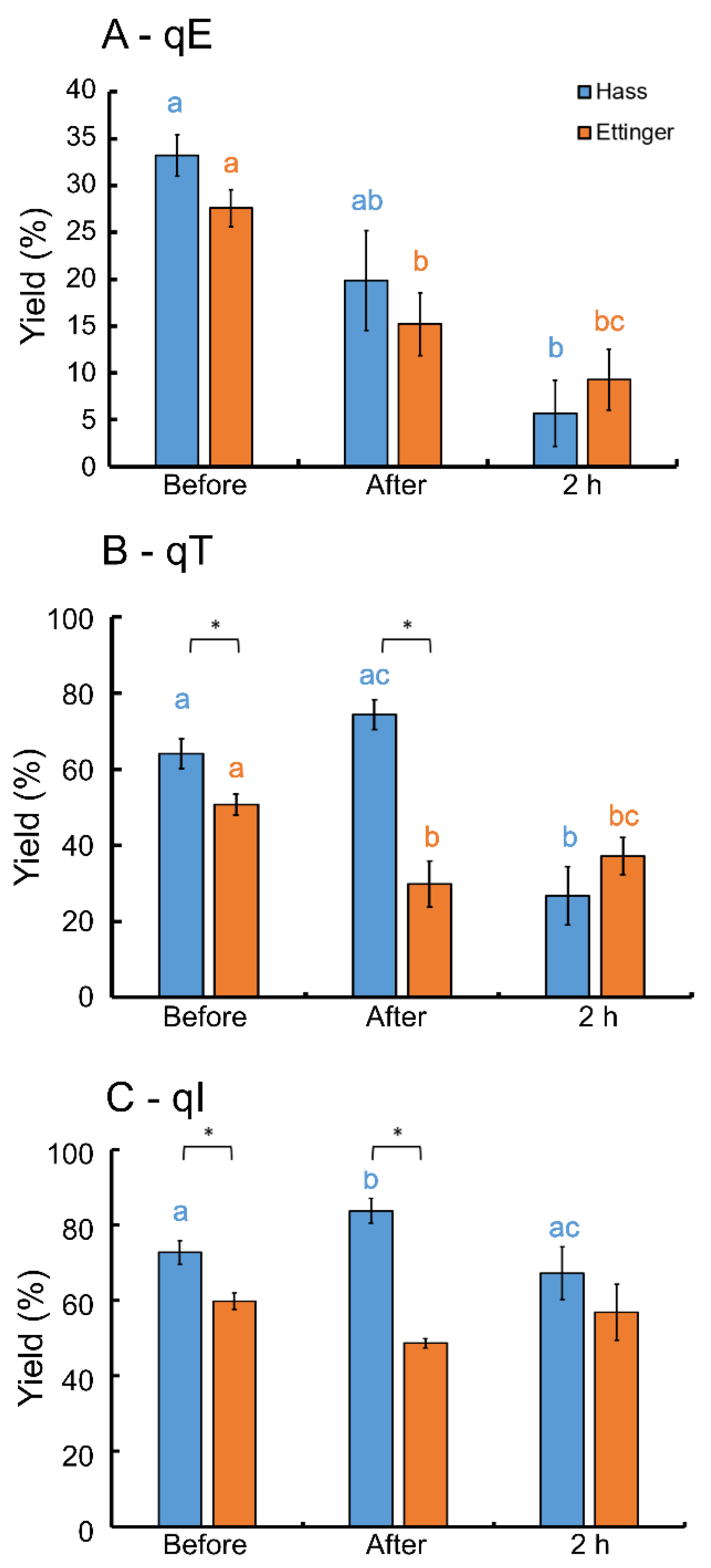
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rendón-Anaya, M.; Ibarra-Laclette, E.; Méndez-Bravo, A.; Lan, T.; Zheng, C.; Carretero-Paulet, L.; Perez-Torres, C.A.; Chacón-López, A.; Hernandez-Guzmán, G.; Chang, T.-H. The Avocado Genome Informs Deep Angiosperm Phylogeny, Highlights Introgressive Hybridization, and Reveals Pathogen-Influenced Gene Space Adaptation. Proc. Natl. Acad. Sci. USA 2019, 116, 17081–17089. [Google Scholar] [CrossRef] [Green Version]
- Joshi, N.C.; Yadav, D.; Ratner, K.; Kamara, I.; Aviv-Sharon, E.; Irihimovitch, V.; Charuvi, D. Sodium Hydrosulfide Priming Improves the Response of Photosynthesis to Overnight Frost and Day High Light in Avocado (Persea Americana Mill, Cv. ‘Hass’). Physiol. Plant. 2020, 168, 394–405. [Google Scholar] [CrossRef] [Green Version]
- Weil, A.; Sofer-Arad, C.; Bar-Noy, Y.; Liran, O.; Rubinovich, L. Comparative Study of Leaf Antioxidant Activity as a Possible Mechanism for Frost Tolerance in ‘Hass’ and ‘Ettinger’Avocado Cultivars. J. Agric. Sci. 2019, 157, 342–349. [Google Scholar] [CrossRef]
- Rivero, R.M.; Mittler, R.; Blumwald, E.; Zandalinas, S.I. Developing Climate-Resilient Crops: Improving Plant Tolerance to Stress Combination. Plant J. 2022, 109, 373–389. [Google Scholar] [CrossRef] [PubMed]
- Bar-Noy, Y.; Sofer-Arad, C.; Perel, M.; Cohen, H.; Senesh, N.; Noy, M.; Rubinovich, L. Frost protection efficiency evaluation in avocado with a horizontal wind machine. Fruits Int. J. Trop. Subtrop. Hortic. 2019, 74, 124–129. [Google Scholar] [CrossRef]
- Larcher, W. Climatic Constraints Drive the Evolution of Low Temperature Resistance in Woody Plants. J. Agric. Meteorol. 2005, 61, 189–202. [Google Scholar] [CrossRef] [Green Version]
- Asada, K. Production and Scavenging of Reactive Oxygen Species in Chloroplasts and Their Functions. Plant Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powles, S.B.; Berry, J.A.; Björkman, O. Interaction between Light and Chilling Temperature on the Inhibition of Photosynthesis in Chilling-Sensitive Plants*. Plant Cell Environ. 1983, 6, 117–123. [Google Scholar] [CrossRef]
- Allen, D.J.; Ratner, K.; Giller, Y.E.; Gussakovsky, E.E.; Shahak, Y.; Ort, D.R. An Overnight Chill Induces a Delayed Inhibition of Photosynthesis at Midday in Mango (Mangifera indica L.). J. Exp. Bot. 2000, 51, 1893–1902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauer, H.; Wierer, R.; Hatheway, W.H.; Larcher, W. Photosynthesis of Coffea arabica after chilling. Physiol. Plant. 1985, 64, 449–454. [Google Scholar] [CrossRef]
- Zaro, G.C.; Caramori, P.H.; Gaspar de Oliveira, C.M.; Nagashima, G.T.; Rosisca, J.R.; Cavenaghi Prete, C.E. Assessment of cold stress in avocado cultivars based on visual, physiological and biochemical criteria. Aust. J. Crop Sci. 2019, 13, 881–888. [Google Scholar] [CrossRef]
- Woolf, A.B.; Cox, K.A.; White, A.; Ferguson, I.B. Low Temperature Conditioning Treatments Reduce External Chilling Injury of ‘Hass’ Avocados. Postharvest Biol. Technol. 2003, 28, 113–122. [Google Scholar] [CrossRef] [Green Version]
- Tadmor, Y.; Raz, A.; Reikin-Barak, S.; Ambastha, V.; Shemesh, E.; Leshem, Y.; Crane, O.; Stern, R.A.; Goldway, M.; Tchernov, D.; et al. Metamitron, a Photosynthetic Electron Transport Chain Inhibitor, Modulates the Photoprotective Mechanism of Apple Trees. Plants 2021, 10, 2803. [Google Scholar] [CrossRef]
- Kramer, D.M.; Johnson, G.; Kiirats, O.; Edwards, G.E. New Fluorescence Parameters for the Determination of QA Redox State and Excitation Energy Fluxes. Photosynth. Res. 2004, 79, 209–218. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C.; Knapp, M. How to Correctly Determine the Different Chlorophyll Fluorescence Parameters and the Chlorophyll Fluorescence Decrease Ratio RFd of Leaves with the PAM Fluorometer. Photosynthetica 2005, 43, 379–393. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the Chlorophyll a Fluorescence Transient. In Chlorophyll a Fluorescence: A Signature of Photosynthesis; Papageorgiou, G.C., Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 321–362. ISBN 978-1-4020-3218-9. [Google Scholar]
- Eilers, P.H.C.; Peeters, J.C.H. A Model for the Relationship between Light Intensity and the Rate of Photosynthesis in Phytoplankton. Ecol. Model. 1988, 42, 199–215. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- RStudio Team. RStudio: Integrated Development Environment for R; RStudio, PBC: Boston, MA, USA, 2022. [Google Scholar]
- Govindjee. Sixty-Three Years since Kautsky: Chlorophyll a Fluorescence. Aust. J. Plant Physiol. 1995, 22, 131–160. [Google Scholar]
- Allen, D.J.; Ort, D.R. Impacts of Chilling Temperatures on Photosynthesis in Warm-Climate Plants. Trends Plant Sci. 2001, 6, 36–42. [Google Scholar] [CrossRef]
- Havaux, M.; Kloppstech, K. The Protective Functions of Carotenoid and Flavonoid Pigments against Excess Visible Radiation at Chilling Temperature Investigated in Arabidopsis Npq and Tt Mutants. Planta 2001, 213, 953–966. [Google Scholar] [CrossRef]
- Li, X.-G.; Duan, W.; Meng, Q.-W.; Zou, Q.; Zhao, S.-J. The Function of Chloroplastic NAD (P) H Dehydrogenase in Tobacco during Chilling Stress under Low Irradiance. Plant Cell Physiol. 2004, 45, 103–108. [Google Scholar] [CrossRef] [Green Version]
- DeTar, R.A.; Höhner, R.; Manavski, N.; Blackholm, M.; Meurer, J.; Kunz, H.H. Loss of Salt Overly Sensitive 1 prevents virescence in chloroplast K+/H+ EFFLUX ANTIPORTER-deficient mutants. Plant Physiol. 2022, kiac142. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, Z.; Han, S.; Jiang, H.; Xu, S.; Zhao, H.; Ren, S. Using the diurnal variation characteristics of effective quantum yield of PSII photochemistry for drought stress detection in maize. Ecol. Indic. 2022, 138, 108842. [Google Scholar] [CrossRef]
- Sperdouli, I.; Andreadis, S.S.; Adamakis, I.D.S.; Moustaka, J.; Koutsogeorgiou, E.I.; Moustakas, M. Reactive Oxygen Species Initiate Defence Responses of Potato Photosystem II to Sap-Sucking Insect Feeding. Insects 2022, 13, 409. [Google Scholar] [CrossRef]
- Klughammer, C.; Schreiber, U. Complementary PS II Quantum Yields Calculated from Simple Fluorescence Parameters Measured by PAM Fluorometry and the Saturation Pulse Method. PAM Appl. Notes 2008, 1, 27–35. [Google Scholar]
- Krüger, G.H.; Tsimilli-Michael, M.; Strasser, R.J. Light stress provokes plastic and elastic modifications in structure and function of photosystem II in camellia leaves. Physiol. Plant. 1997, 101, 265–277. [Google Scholar] [CrossRef]
- Lootens, P.; Devacht, S.; Baert, J.; Van Waes, J.; Van Bockstaele, E.; Roldàn-Ruiz, I. Evaluation of cold stress of young industrial chicory (Cichorium intybus L.) by chlorophyll a fluorescence imaging. II. Dark relaxation kinetics. Photosynthetica 2011, 49, 185–194. [Google Scholar] [CrossRef]
- Frankart, C.; Eullaffroy, P.; Vernet, G. Comparative effects of four herbicides on non-photochemical fluorescence quenching in Lemna minor. Environ. Exp. Bot. 2003, 49, 159–168. [Google Scholar] [CrossRef]
- Jedmowski, C.; Brüggemann, W. Imaging of fast chlorophyll fluorescence induction curve (OJIP) parameters, applied in a screening study with wild barley (Hordeum spontaneum) genotypes under heat stress. J. Photochem. Photobiol. B Biol. 2015, 151, 153–160. [Google Scholar] [CrossRef]
- Srivastava, A.; Guissé, B.; Greppin, H.; Strasser, R.J. Regulation of antenna structure and electron transport in photosystem II of Pisum sativum under elevated temperature probed by the fast polyphasic chlorophyll a fluorescence transient: OKJIP. Biochim. Et Biophys. Acta BBA-Bioenerg. 1997, 1320, 95–106. [Google Scholar] [CrossRef] [Green Version]
- Baker, N.R. Chlorophyll Fluorescence: A Probe of Photosynthesis in Vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef] [Green Version]
- Kalaji, H.M.; Schansker, G.; Ladle, R.J.; Goltsev, V.; Bosa, K.; Allakhverdiev, S.I.; Brestic, M.; Bussotti, F.; Calatayud, A.; Dąbrowski, P. Frequently Asked Questions about in Vivo Chlorophyll Fluorescence: Practical Issues. Photosynth. Res. 2014, 122, 121–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Stress | Apparent Antenna Size | Density of Reducing PSIIs | Density of Energy Dissipating PSIIs | Electron Transfer Qa− to Qb | Electron Transfer to PSI Acceptor Side | |
|---|---|---|---|---|---|---|
| Hass | Before | 1.68 ± 0.08 * ’ | 0.60 ± 0.02 * | -- | 0.90 ± 0.02 ’ | 0.22 ± 0.02 * ’ |
| After | 1.89 ± 0.08 * ’ | 0.52 + 0.02 | 0.16 ± 0.03 | 1.03 ± 0.08 ’ | 0.29 ± 0.01 ’ | |
| Ettinger | Before | 1.99 ± 0.08 * ’ | 0.51 ± 0.02 * | -- | 0.84 ± 0.06 | 0.25 ± 0.01 * |
| After | 1.86 ± 0.03 * ’ | 0.54 ± 0.01 | 0.78 ± 0.31 | 1.2 ± 0.01 | 0.28 ± 0.01 |
| Cultivar | Slope (e− Photons−1) | Im (µmol Photons m−2 s−1) | Ik (µmol Photons m−2 s−1) | Pm (µmol e− m−2 s−1) | w (R.U.) |
|---|---|---|---|---|---|
| Hass | 0.22 ± 0.02 | 295.99 ± 42.28 ** | 74.23 ± 11.17 ** | 21.62 ± 6.15 ** | 2.02 ± 0.40 |
| Ettinger | 0.27 ± 0.00 | 705.09 ± 68.67 ** | 169.33 ± 30.37 ** | 45.93 ± 7.95 ** | 2.59 ± 0.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weil, A.; Rubinovich, L.; Tchernov, D.; Liran, O. Comparative Study between the Photosynthetic Parameters of Two Avocado (Persea americana) Cultivars Reveals Natural Variation in Light Reactions in Response to Frost Stress. Agronomy 2022, 12, 1129. https://doi.org/10.3390/agronomy12051129
Weil A, Rubinovich L, Tchernov D, Liran O. Comparative Study between the Photosynthetic Parameters of Two Avocado (Persea americana) Cultivars Reveals Natural Variation in Light Reactions in Response to Frost Stress. Agronomy. 2022; 12(5):1129. https://doi.org/10.3390/agronomy12051129
Chicago/Turabian StyleWeil, Amir, Lior Rubinovich, Dan Tchernov, and Oded Liran. 2022. "Comparative Study between the Photosynthetic Parameters of Two Avocado (Persea americana) Cultivars Reveals Natural Variation in Light Reactions in Response to Frost Stress" Agronomy 12, no. 5: 1129. https://doi.org/10.3390/agronomy12051129
APA StyleWeil, A., Rubinovich, L., Tchernov, D., & Liran, O. (2022). Comparative Study between the Photosynthetic Parameters of Two Avocado (Persea americana) Cultivars Reveals Natural Variation in Light Reactions in Response to Frost Stress. Agronomy, 12(5), 1129. https://doi.org/10.3390/agronomy12051129







