Genetic Association between Blast Resistance and Yield Traits in Rice Detected Using a High-Density Bin Map
Abstract
:1. Introduction
2. Materials and Methods
2.1. Rice Material
2.2. Map Construction
2.3. Phenotype Evaluation
- NP = number of panicles/5;
- NSP = (total number of filled grains + total number of unfilled grains)/number of panicles;
- NGP = total number of filled grains/number of panicles;
- GY = total weight of filled grains/5.
- RLW = grain length/grain width.
2.4. Data Analysis
3. Results
3.1. Phenotypic Performance of Leaf Blast Resistance and Yield Traits
3.2. QTL Detected in D71/ZH161 RIL Population
3.3. Phenotypic Performance of LBR and GY in Different Combinations of Four Rice Blast Resistance Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Asibi, A.E.; Chai, Q.; Coulter, J.A. Rice blast: A disease with implications for global food security. Agronomy 2019, 9, 451. [Google Scholar] [CrossRef] [Green Version]
- Skamnioti, P.; Gurr, S.J. Against the grain: Safeguarding rice from rice blast disease. Trends Biotechnol. 2009, 27, 141–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Chern, M.; Yin, J.; Wang, J.; Chen, X. Recent advances in broad-spectrum resistance to the rice blast disease. Curr. Opin. Plant Biol. 2019, 50, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Chen, S.; Que, Z.; Wang, L.; Liu, X.; Pan, Q. The blast resistance gene Pi37 encodes a nucleotide binding site-leucine-rich repeat protein and is a member of a resistance gene cluster on rice chromosome 1. Genetics 2007, 177, 1871–1880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, J.; Lei, C.; Xu, X.; Hao, K.; Wang, J.; Cheng, Z.; Ma, X.; Ma, J.; Zhou, K.; Zhang, X.; et al. Pi64, encoding a novel CC-NBS-LRR protein, confers resistance to leaf and neck blast in rice. Mol. Plant Microbe Interact. 2015, 28, 558–568. [Google Scholar] [CrossRef] [Green Version]
- Fukuoka, S.; Yamamoto, S.-L.; Mizobuchi, R.; Yamanouchi, U.; Ono, K.; Kiatazawa, N.; Yasuda, N.; Fujita, Y.; Nguyen, T.T.T.; Koizumi, S.; et al. Multiple functional polymorphisms in a single disease resistance gene in rice enhance durable resistance to blast. Sci. Rep. 2015, 4, 4550. [Google Scholar] [CrossRef]
- Deng, Y.; Zhai, K.; Xie, Z.; Yang, D.; Zhu, X.; Liu, J.; Wang, X.; Qin, P.; Yang, Y.; Zhang, G.; et al. Epigenetic regulatiuon of antagonistic receptors confers rice blast resistance with yield balance. Science 2017, 355, 962–965. [Google Scholar] [CrossRef]
- Qu, S.; Liu, G.; Zhou, B.; Bellizzi, M.; Zeng, L.; Dai, L.; Han, B.; Wang, G.-L. The broad-spectrum blast resistance gene Pi9 encodes a nucleotide-binding site-leucine-rich repeat protein and is a member of a multigene family in rice. Genetics 2006, 172, 1901–1914. [Google Scholar] [CrossRef] [Green Version]
- Su, J.; Wang, W.; Han, J.; Chen, S.; Wang, C.; Zeng, L.; Feng, A.; Yang, J.; Zhou, B.; Zhu, X. Functional divergence of duplicated genes results in a novel blast resistance gene Pi50 at the Pi2/9 locus. Theor. Appl. Genet. 2015, 128, 2213–2225. [Google Scholar] [CrossRef]
- Zhou, B.; Qu, S.; Liu, G.; Dolan, M.; Sakai, H.; Lu, G.; Bellizzi, M.; Wang, G. The eight amino-acid differences within three leucine-rich repeats between Pi2 and Piz-t resistance proteins determine the resistance specificity to Magnaporthe grisea. Mol. Plant Microbe Interact. 2006, 19, 1216–1228. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Zhao, W.; Zhu, X.; Zou, C.; Yin, J.; Chern, M.; Zhou, X.; Ying, H.; Jiang, X.; Li, Y.; et al. Identification and characterization of rice blast resistance gene Pid4 by a combination of transcriptomic profiling and genome analysis. J. Genet. Genom. 2018, 45, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Tao, Y.; Chen, X.; Zou, Y.; Lei, C.; Wang, J.; Li, X.; Zhao, X.; Zhang, M.; Lu, Z.; et al. Identification of a new rice blast resistance gene, Pid3, by genomewide comparison of paired nucleotide-binding site-leucine-rice repeat genes and their pseudogene alleles between the two sequenced rice genomes. Genetics 2009, 182, 1303–1311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Shi, Y.; Liu, W.; Chai, R.; Fu, Y.; Zhuang, J.; Wu, J. A Pid3 allele from rice cultivar Gumei2 confers resistance to Magnaporthe oryzae. J. Genet. Genom. 2011, 38, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Bryan, G.T.; Wu, K.-S.; Farrall, L.; Jia, Y.; Hershey, H.P.; McAdams, S.A.; Faulk, K.N.; Donaldson, G.K.; Tarchini, R.; Valent, B. A single amino acid difference distinguishes resistant and susceptible alleles of the rice blast resistance gene Pi-ta. Plant Cell 2000, 12, 2033–2045. [Google Scholar] [CrossRef]
- Dong, L.; Liu, S.; Xu, P.; Deng, W.; Li, X.; Tharreau, D.; Li, J.; Zhou, J.; Wang, Q.; Tao, D.; et al. Fine mapping of Pi57(t) conferring broad spectrum resistance against Magnaporthe oryzae in introgression line IL-E1454 derived from Oryza longistaminata. PLoS ONE 2017, 12, e0186201. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Wang, X.; Jia, Y.; Minkenberg, B.; Wheatley, M.; Fan, J.; Jia, M.H.; Famoso, A.; Edwards, J.D.; Wamishe, Y.; et al. The rice blast resistance gene Ptr encodes an atypical protein required for broad-spectrum disease resistance. Nat. Commun. 2018, 9, 2039. [Google Scholar] [CrossRef]
- Xiao, N.; Wu, Y.; Pan, C.; Yu, L.; Chen, Y.; Liu, G.; Li, Y.; Zhang, X.; Wang, Z.; Dai, Z.; et al. Improving of rice blast resistances in Japonica by pyramiding major R genes. Front. Plant Sci. 2017, 7, 1918. [Google Scholar] [CrossRef] [Green Version]
- Tao, H.; Shi, X.; He, F.; Wang, D.; Xiao, N.; Fang, H.; Wang, R.; Zhang, F.; Wang, M.; Li, A.; et al. Engineering broad-spectrum disease-resistant rice by editing multiple susceptibility genes. J. Integr. Plant Biol. 2021, 63, 1639–1648. [Google Scholar] [CrossRef]
- Wu, Y.; Xiao, N.; Chen, Y.; Yu, L.; Pan, C.; Li, Y.; Zhang, X.; Huang, N.; Ji, H.; Dai, Z.; et al. Comprehensive evaluation of resistance effects of pyramiding lines with different broad-spectrum resistance genes against Magnaporthe oryzae in rice (Oryza sativa L.). Rice 2019, 12, 11. [Google Scholar] [CrossRef]
- Fukuoka, S.; Saka, N.; Koga, H.; Ono, K.; Shimizu, T.; Ebana, K.; Hayashi, N.; Takahashi, A.; Hirochika, H.; Okuno, K.; et al. Loss of function of a proline-containing protein confers durable disease resistance in rice. Science 2009, 325, 998–1001. [Google Scholar] [CrossRef]
- Wu, J.L.; Fan, Y.Y.; Li, D.B.; Zheng, K.L.; Leung, H.; Zhuang, J.Y. Genetic control of rice blast resistance in the durably resistant cultivar Gumei 2 against multiple isolates. Theor. Appl. Genet. 2005, 111, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.-L.; Zhuang, J.-Y.; Wu, J.-L.; Chai, R.-Y.; Cao, L.-Y.; Leung, H.; Fan, Y.-Y.; Jin, M.-Z.; Rao, Z.-M. Marker-based analysis of genetic drag between blast-resistance and yield-trait genes in rice. In Proceedings of the International Rice Research Conference, Beijing, China, 16–19 September 2002. [Google Scholar]
- Liu, W.Q.; Fan, Y.Y.; Chen, J.; Shi, Y.; Wu, J.L. Breakdown or avoidance of genetic drag between blast resistance and spikelet fertility based on genotype selection against heading date in rice. Chin. J. Rice Sci. 2008, 22, 359–364, (In Chinese with English Abstract). [Google Scholar]
- Cao, L.Y.; Wu, J.-L.; Fan, Y.-Y.; Cheng, S.-H.; Zhuang, J.-Y. QTL analysis for heading date and yield traits using recombinant inbred lines of indica rice grown in different cropping seasons. Plant Breed. 2010, 6, 676–682. [Google Scholar] [CrossRef]
- Xue, W.; Xing, Y.; Weng, X.; Zhao, Y.; Tang, W.; Wang, L.; Zhou, H.; Yu, S.; Xu, C.; Li, X.; et al. Natural variation in Ghd7 is an important regulator of heading date and yield potential in rice. Nat. Genet. 2008, 40, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zheng, K.L. A simple method for isolation of rice DNA. Chin. J. Rice Sci. 1992, 6, 47–48. (In Chinese) [Google Scholar]
- Zhang, Z.; Zhu, Y.; Wang, S.; Fan, Y.; Zhuang, J. Genetic interaction of Hd1 with Ghd7, DTH8 and Hd2 largely determines eco-geographical adaption of rice varieties in southern China. Rice Sci. 2021, 28, 114–118. [Google Scholar]
- Yin, D.-S.; Xia, M.-Y.; Li, J.-B.; Wan, B.-L.; Zha, Z.-P.; Du, X.-S.; Qi, H.-X. Development of STS marker linked to rice blast resistance gene Pi9 in marker-assisted selection breeding. Chin. J. Rice Sci. 2011, 25, 25–30, (In Chinese with English Abstract). [Google Scholar]
- Zhu, Y.; Huang, D.; Fan, Y.; Zhuang, J.; Shen, B. Marker-assisted selection for hybrid rice restorer line R153 conferring resistance to blast. China Rice 2021, 27, 95–97, (In Chinese with English Abstract). [Google Scholar]
- Meng, L.; Li, H.; Zhang, L.; Wang, J. QTL IciMapping: Integrated software for genetic linkage map construction and quantitative trait locus mapping in biparental populations. Crop. J. 2015, 3, 269–283. [Google Scholar] [CrossRef] [Green Version]
- McCouch, S.R. CGSNL (Committee on Gene Symbolization, Nomenclature and Linkage, Rice Genetics Cooperative). Gene nomenclature system for rice. Rice 2008, 1, 72–84. [Google Scholar] [CrossRef] [Green Version]
- Peng, M.; Lin, X.; Xiang, X.; Ren, H.; Fan, X.; Chen, K. Characterization and evaluation of transgenic rice pyramided with the Pi genes Pib, Pi25 and Pi54. Rice 2021, 14, 78. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Galindo, J.C.; Ordas, B.; Butron, A.; Samayoa, L.F.; Malvar, R.A. QTL mapping for yield and resistance against Mediterranean corn borer in Maize. Front. Plant Sci. 2017, 8, 698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zetzsche, H.; Friedt, W.; Ordon, F. Breeding progress for pathogen resistance is a second major driver for yield increase in German winter wheat at contrasting N levels. Sci. Rep. 2020, 10, 20374. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liao, H.; Chern, M.; Yin, J.; Chen, Y.; Wang, J.; Zhu, X.; Chen, Z.; Yuan, C.; Zhao, W.; et al. Loss of function of a rice TPR-domain RNA-binding protein confers broad-spectrum disease resistance. Proc. Natl. Acad. Sci. USA 2018, 115, 3174–3179. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Zhu, Z.; Chern, M.; Yin, J.; Yang, C.; Ran, L.; Cheng, M.; He, M.; Wang, K.; Wang, J.; et al. A natural allele of a transcription factor in rice confers broad-spectrum blast resistance. Cell 2017, 170, 114–126. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zheng, Y.-P.; Zhou, X.-H.; Yang, X.-M.; He, X.-R.; Feng, Q.; Zhu, Y.; Li, G.-B.; Wang, H.; Zhao, J.-H.; et al. Rice miR1432 fine-tune the balance of yield and blast disease resistance via different modules. Rice 2021, 14, 87. [Google Scholar] [CrossRef]
- Zhou, S.-X.; Zhu, Y.; Wang, L.-F.; Zheng, Y.-P.; Chen, J.-F.; Li, T.-T.; Yang, X.-M.; He, W.; Li, X.-P.; Ma, X.-C.; et al. Osa-miR1873 fine-tunes rice immunity against Magnaporthe oryzae and yield traits. J. Integr. Plant Biol. 2020, 62, 1213–1226. [Google Scholar] [CrossRef] [Green Version]
- Xiao, N.; Pan, C.; Li, Y.; Wu, Y.; Cai, Y.; Lu, Y.; Wang, R.; Yu, R.; Yu, L.; Shi, W.; et al. Genomic insight into balancing high yield, good quality, and blast resistance of japonica rice. Genome Biol. 2021, 22, 283. [Google Scholar] [CrossRef]
- Nicol, H.; Thornton, H.G. Competition between related strains of nodule bacteria and its influence on infection of the legume host. Proc. R. Soc. Lond. Ser. B-Biol. Sci. 1941, 130, 32–59. [Google Scholar]
- Chen, X.; Shang, J.; Chen, D.; Lei, C.; Zou, Y.; Zhai, W.; Liu, G.; Xu, J.; Ling, Z.; Cao, G.; et al. A B-lectin receptor kinase gene conferring rice blast resistance. Plant J. 2006, 46, 794–804. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, L.; Wu, W.; He, L.; Yang, X.; Pan, Q. Function and evolution of Magnaporthe oryzae avirulence gene AvrPib responding to the rice blast resistance gene Pib. Sci. Rep. 2015, 5, 11642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, Y.; McAdams, S.A.; Bryan, G.T.; Hershey, H.P.; Valent, B. Direct interaction of resistance gene and avirulence gene products confers rice blast resistance. EMBO J. 2000, 19, 4004–4014. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Feng, Q.; Qian, Q.; Zhao, Q.; Wang, L.; Wang, A.; Guan, J.; Fan, D.; Weng, Q.; Huang, T.; et al. High-throughput genotyping by whole-genome resequencing. Genome Res. 2009, 19, 1068–1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, H.; Xie, W.; Wang, J.; Xing, Y.; Xu, C.; Li, X.; Xiao, J.; Zhang, Q. Gains in QTL detection using an ultra-high density SNP map based on population sequencing relative to traditional RFLP/SSR markers. PLoS ONE 2011, 6, e17595. [Google Scholar]
- Jiang, S.; Wang, L.; Yang, X.; Zhang, X.; Meng, Y.; Li, M.; Chi, L.; Li, Z.; Zhao, Q.; Liu, Y.; et al. Detection of quantitative trait loci for heading date and temperature responsiveness in a re-sequenced, recombinant inbred line of Japonica rice from Heilongjiang province, China. Plant Breed. 2021, 140, 1011–1022. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, Y.; Ma, Q.; Mu, Y.; Huang, Z.; Xia, Q.; Zhang, G.; Nian, H. QTL fine-mapping of soybean (Glycine max L.) leaf type associated traits in two RILs populations. BMC Genom. 2019, 20, 260. [Google Scholar] [CrossRef]
- Hu, J.; Wang, Y.; Fang, Y.; Zeng, L.; Xu, J.; Yu, H.; Shi, Z.; Pan, J.; Zhang, D.; Kang, S.; et al. A rare allele of GS2 enhances grain size and grain yield in Rice. Mol. Plant 2015, 8, 1455–1465. [Google Scholar] [CrossRef] [Green Version]
- Che, R.; Tong, H.; Shi, B.; Liu, Y.; Fang, S.; Liu, D.; Xiao, Y.; Hu, B.; Liu, L.; Wang, H.; et al. Control of grain size and rice yield by GL2-mediated brassinosteroid responses. Nat. Plants 2016, 2, 15195. [Google Scholar] [CrossRef]
- Ruan, B.; Shang, L.; Zhang, B.; Hu, J.; Wang, Y.; Lin, H.; Zhang, A.; Liu, C.; Peng, Y.; Zhu, L.; et al. Natural variation in the promoter of TGW2 determines grain width and weight in rice. New Phytol. 2020, 227, 629–640. [Google Scholar] [CrossRef]
- Yu, J.; Xiong, H.; Zhu, X.; Zhang, H.; Li, H.; Miao, J.; Wang, W.; Tang, Z.; Zhang, Z.; Yao, G.; et al. OsLG3 contributing to rice grain length and yield was mined by Ho-LAMap. BMC Biol. 2017, 15, 28. [Google Scholar] [CrossRef] [Green Version]
- Fan, C.; Xing, Y.; Mao, H.; Lu, T.; Han, B.; Xu, C.; Li, X.; Zhang, Q. GS3, a major QTL for grain length and weight and minor QTL for grain width and thickness in rice, encodes a putative transmembrane protein. Theor. Appl. Genet. 2006, 112, 1164–1171. [Google Scholar] [CrossRef] [PubMed]
- Qi, P.; Lin, Y.S.; Song, X.J.; Shen, J.B.; Huang, W.; Shan, J.X.; Zhu, M.Z.; Jiang, L.; Gao, J.P.; Lin, H.X. The novel quantitative trait locus GL3.1 controls rice grain size and yield by regulating Cyclin-T1;3. Cell Res. 2012, 22, 1666–1680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Wang, J.; Huang, J.; Lan, H.; Wang, C.; Yin, C.; Wu, Y.; Tang, H.; Qian, Q.; Li, J.; et al. Rare allele of OsPPKL1 associated with grain length causes extra-large grain and a significant yield increase in rice. Proc. Natl. Acad. Sci. USA 2012, 109, 21534–21539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, N.Q.; Sun, Y.; Guo, T.; Shi, C.L.; Zhang, Y.M.; Kan, Y.; Xiang, Y.H.; Zhang, H.; Yang, Y.B.; Li, Y.C.; et al. UDP-glucosyltransferase regulates grain size and abiotic stress tolerance associated with metabolic flux redirection in rice. Nat. Commun. 2020, 11, 2629. [Google Scholar] [CrossRef]
- Hu, Z.; Lu, S.J.; Wang, M.J.; He, H.; Sun, L.; Wang, H.; Liu, X.H.; Jiang, L.; Sun, J.L.; Xin, X.; et al. A novel QTL qTGW3 encodes the GSK3/SHAGGY-Like kinase OsGSK5/OsSK41 that interacts with OsARF4 to negatively regulate grain size and weight in rice. Mol. Plant 2018, 11, 736–749. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Liu, X.; Wang, M.; Meyer, R.S.; Luo, X.; Ndjiondjop, M.-N.; Tan, L.; Zhang, J.; Wu, J.; Cai, H.; et al. A single-nucleotide polymorphism causes smaller grain size and loss of seed shattering during African rice domestication. Nat. Plants 2017, 3, 17064. [Google Scholar] [CrossRef]
- Duan, P.; Xu, J.; Zeng, D.; Zhang, B.; Geng, M.; Zhang, G.; Huang, K.; Huang, L.; Xu, R.; Ge, S.; et al. Natural variation in the promoter of GSE5 contributes to grain size diversity in rice. Mol. Plant 2017, 10, 685–694. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Xiong, G.; Hu, J.; Jiang, L.; Yu, H.; Xu, J.; Fang, Y.; Zeng, L.; Xu, E.; Xu, J.; et al. Copy number variation at the GL7 locus contributes to grain size diversity in rice. Nat. Genet. 2015, 47, 944–948. [Google Scholar] [CrossRef]
- Wang, S.; Wu, K.; Yuan, Q.; Liu, X.; Liu, Z.; Lin, X.; Zeng, R.; Zhu, H.; Dong, G.; Qian, Q.; et al. Control of grain size, shape and quality by OsSPL16 in rice. Nat. Genet. 2012, 44, 950–954. [Google Scholar] [CrossRef]
- Zuo, Z.-W.; Zhang, Z.-H.; Huang, D.-R.; Fan, Y.-Y.; Yu, S.-B.; Zhuang, J.-Y.; Zhu, Y.-J. Control of thousand-grain weight by OsMADS56 in rice. Int. J. Mol. Sci. 2022, 23, 125. [Google Scholar] [CrossRef]
- Wei, X.; Xu, J.; Guo, H.; Jiang, L.; Chen, S.; Yu, C.; Zhou, Z.; Hu, P.; Zhai, H.; Wan, J. DTH8 suppresses flowering in rice, influencing plant height and yield potential simultaneously. Plant Physiol. 2010, 153, 1747–1758. [Google Scholar] [CrossRef] [PubMed] [Green Version]

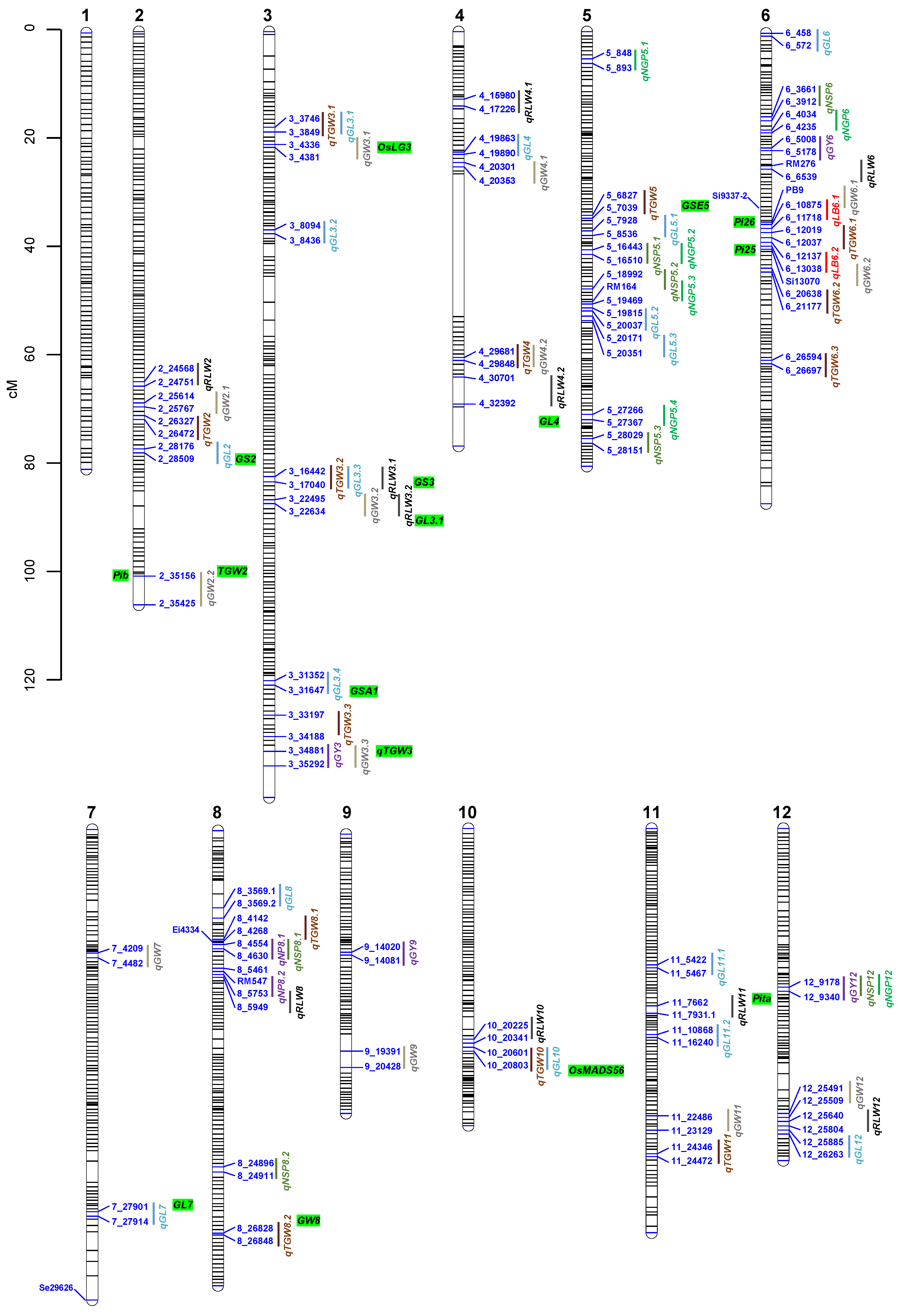
| Traits a | Year | Range | Mean | SD | CV | Skewness | Kurtosis | D71 b | ZH161 |
|---|---|---|---|---|---|---|---|---|---|
| LBR | 2020 | 0.0–9.0 | 3.0 | 3.1 | 105.0 | 0.27 | −1.66 | 1.0 ± 1.7 | 7.3 ± 0.6 |
| GY | 2019 | 16.83–47.47 | 32.17 | 5.55 | 17.3 | 0.16 | 0.24 | 43.16 ± 7.70 | 27.86 ± 0.16 |
| 2020 | 19.71–59.49 | 35.96 | 7.52 | 20.9 | 0.45 | −0.16 | 30.20 ± 3.76 | 22.57 ± 1.50 | |
| NP | 2019 | 5.5–14.7 | 8.9 | 1.3 | 15.0 | 0.50 | 0.96 | 9.8 ± 0.2 | 9.1 ± 0.1 |
| 2020 | 4.9–11.6 | 8.2 | 1.3 | 15.7 | 0.09 | −0.41 | 6.7 ± 0.4 | 8.0 ± 1.4 | |
| NSP | 2019 | 156.1–303.2 | 217.9 | 28.5 | 13.1 | −0.02 | −0.47 | 254.8 ± 3.1 | 178.3 ± 10.9 |
| 2020 | 137.3–356.9 | 266.1 | 50.1 | 18.8 | 0.37 | −0.35 | 308.5 ± 19.0 | 170.2 ± 16.9 | |
| NGP | 2019 | 122.0–260.5 | 183.3 | 26.2 | 14.3 | 0.17 | −0.19 | 194.3 ± 22.9 | 163.4 ± 6.4 |
| 2020 | 169.6–432.2 | 230.4 | 43.5 | 18.9 | 0.34 | −0.37 | 207.4 ± 7.8 | 161.9 ± 15.6 | |
| TGW | 2019 | 16.71–28.57 | 22.01 | 2.30 | 10.5 | 0.22 | −0.19 | 24.41 ± 0.04 | 19.80 ± 0.14 |
| 2020 | 16.30–26.36 | 20.68 | 1.97 | 9.5 | 0.29 | −0.32 | 23.29 ± 0.34 | 17.86 ± 0.56 | |
| GL | 2019 | 7.299–9.555 | 8.386 | 0.535 | 6.4 | 0.19 | −1.00 | 8.961 ± 0.005 | 7.869 ± 0.005 |
| 2020 | 7.273–9.517 | 8.301 | 0.526 | 6.3 | 0.23 | −1.00 | 8.786 ± 0.004 | 7.640 ± 0.164 | |
| GW | 2019 | 2.315–2.831 | 2.551 | 0.099 | 3.9 | 0.13 | −0.24 | 2.589 ± 0.006 | 2.538 ± 0.005 |
| 2020 | 2.270–2.734 | 2.503 | 0.090 | 3.6 | 0.19 | 0.01 | 2.587 ± 0.011 | 2.400 ± 0.047 | |
| RLW | 2019 | 2.769–3.826 | 3.306 | 0.251 | 7.6 | 0.14 | −0.92 | 3.468 ± 0.007 | 3.115 ± 0.004 |
| 2020 | 2.842–3.969 | 3.338 | 0.262 | 7.8 | 0.27 | −1.11 | 3.406 ± 0.016 | 3.205 ± 0.008 |
| Rice Material | Source of Variation | GY | NP | NSP | NGP | TGW | GL | GW | RLW |
|---|---|---|---|---|---|---|---|---|---|
| Parents | Between years | 0.0412 | 0.0160 | 0.0808 | 0.6074 | 0.0029 | 0.0254 | 0.0150 | 0.1211 |
| Between parents | 0.0202 | 0.6311 | 0.0004 | 0.0214 | <0.0001 | <0.0001 | 0.0023 | <0.0001 | |
| Line × year | 0.2806 | 0.1251 | 0.0349 | 0.5194 | 0.1566 | 0.6605 | 0.0161 | 0.0004 | |
| RIL | Between years | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Among lines | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| Line × year | <0.0001 | 0.0016 | <0.0001 | <0.0001 | <0.0001 | 0.4993 | <0.0001 | 0.0238 |
| Traits | LBR | GY | NP | NSP | NGP | TGW | GL | GW |
|---|---|---|---|---|---|---|---|---|
| GY | −0.121 * | |||||||
| NP | 0.080 | 0.395 *** | ||||||
| NSP | −0.050 | 0.474 *** | −0.409 *** | |||||
| NGP | −0.084 | 0.491 *** | −0.416 *** | 0.967 *** | ||||
| TGW | −0.183 ** | 0.321 *** | −0.078 | −0.138 * | −0.067 | |||
| GL | 0.018 | 0.216 *** | 0.111 | −0.268 *** | −0.222 *** | 0.814 *** | ||
| GW | −0.337 *** | 0.235 *** | −0.210 *** | 0.122 | 0.165 ** | 0.410 *** | −0.113 | |
| RLW | 0.179 ** | 0.065 | 0.193 ** | −0.283 *** | −0.266 *** | 0.477 *** | 0.881 *** | −0.569 *** |
| QTLa | Interval | LOD | Ab | R2 (%) c |
|---|---|---|---|---|
| qLBR6.1 | 6_10875–6_11718 | 69.91 | 2.13 | 69.06 |
| qLBR6.2 | 6_12137–6_13038 | 21.28 | 0.91 | 12.73 |
| QTL a | Chr | Interval | LOD (A) | LOD (ge) | Ab | gec | R2 (A) d | R2 (ae) e |
|---|---|---|---|---|---|---|---|---|
| qGY3 | 3 | 3_34881–3_35292 | 5.17 | - | 1.22 | - | 5.58 | - |
| qGY6 | 6 | 6_5008–6_5178 | 4.82 | - | −1.18 | - | 5.22 | - |
| qGY9 | 9 | 9_14020–9_14081 | 6.21 | - | −1.34 | - | 6.63 | - |
| qGY12 | 12 | 12_9178–12_9340 | 4.12 | - | −1.08 | - | 4.31 | - |
| qNP8.1 | 8 | 8_4554–8_4630 | 5.45 | - | 0.27 | - | 7.78 | - |
| qNP8.2 | 8 | 8_5461–RM547 | 5.24 | 5.44 | 0.26 | −0.26 | 7.47 | 7.24 |
| qNSP5.1 | 5 | 5_16443–5_16510 | 5.38 | - | −5.49 | - | 3.18 | - |
| qNSP5.2 | 5 | 5_18992–5_20037 | 5.01 | 7.98 | −5.28 | −5.46 | 2.94 | 3.14 |
| qNSP5.3 | 5 | 5_28029–5_28151 | 14.49 | - | −9.25 | - | 9.03 | - |
| qNSP6 | 6 | 6_3661–6_3912 | 4.59 | - | −5.04 | - | 2.67 | - |
| qNSP8.1 | 8 | 8_4554–8_4630 | 39.40 | 4.77 | −16.30 | 14.23 | 27.96 | 21.29 |
| qNSP8.2 | 8 | 8_24896–8_24911 | 14.23 | - | −9.33 | - | 8.81 | - |
| qNSP12 | 12 | 12_9178–12_9340 | 4.06 | - | −4.69 | - | 2.28 | - |
| qNGP5.1 | 5 | 5_848–5_893 | 5.76 | - | 5.43 | - | 3.74 | - |
| qNGP5.2 | 5 | 5_16443–5_16510 | 13.70 | - | −8.36 | - | 8.94 | - |
| qNGP5.3 | 5 | RM164–5_19469 | 4.72 | - | −4.78 | - | 2.92 | - |
| qNGP5.4 | 5 | 5_27266–5_27367 | 11.91 | - | −7.79 | - | 7.73 | - |
| qNGP6 | 6 | 6_4034–6_4235 | 4.46 | - | −4.68 | - | 2.79 | - |
| qNGP8.1 | 8 | 8_4554–8_4630 | 26.68 | 8.27 | −12.09 | 11.82 | 18.61 | 17.81 |
| qNGP8.2 | 8 | 8_24896–8_24911 | 10.54 | - | −7.43 | - | 6.77 | - |
| qNGP12 | 12 | 12_9178–12_9340 | 4.92 | - | −4.91 | - | 3.02 | - |
| qTGW2 | 2 | 2_26327–2_26472 | 26.48 | - | 0.49 | - | 6.91 | - |
| qTGW3.1 | 3 | 3_3746–3_3849 | 47.51 | - | −0.70 | - | 13.50 | - |
| qTGW3.2 | 3 | 3_16442–3_17040 | 106.14 | 5.23 | −1.26 | 0.03 | 43.64 | 0.03 |
| qTGW3.3 | 3 | 3_33197–3_34188 | 6.55 | - | 0.23 | - | 1.55 | - |
| qTGW4 | 4 | 4_29681–4_29848 | 4.65 | - | −0.19 | - | 1.07 | - |
| qTGW5 | 5 | 5_6827–5_7039 | 4.06 | - | 0.18 | - | 0.90 | - |
| qTGW6.1 | 6 | 6_12019–6_12037 | 7.15 | - | −0.25 | - | 1.71 | - |
| qTGW6.2 | 6 | 6_20638–6_21177 | 7.97 | 3.94 | −0.26 | 0.19 | 1.87 | 1.03 |
| qTGW6.3 | 6 | 6_26594–6_26697 | 4.43 | - | −0.19 | - | 1.02 | - |
| qTGW8.1 | 8 | 8_4142–8_4268 | 4.98 | 4.63 | −0.20 | 0.20 | 1.16 | 1.13 |
| qTGW8.2 | 8 | 8_26828–8_26848 | 11.57 | - | 0.32 | - | 2.78 | - |
| qTGW10 | 10 | 10_20601–10_20803 | 6.82 | 6.04 | −0.24 | 0.24 | 1.59 | 1.58 |
| qTGW11 | 11 | 11_24346–11_24472 | 7.49 | - | −0.25 | - | 1.76 | - |
| qGL2 | 2 | 2_28176–2_28509 | 8.92 | - | 0.058 | - | 1.10 | - |
| qGL3.1 | 3 | 3_3746–3_3849 | 23.87 | - | −0.100 | - | 3.16 | - |
| qGL3.2 | 3 | 3_8094–3_8436 | 4.44 | - | −0.040 | - | 0.53 | - |
| qGL3.3 | 3 | 3_16442–3_17040 | 162.25 | 36.58 | −0.435 | 0.0004 | 60.43 | 0.0001 |
| qGL3.4 | 3 | 3_31352–3_31647 | 3.93 | - | 0.038 | - | 0.47 | - |
| qGL4 | 4 | 4_19863–4_19890 | 4.09 | - | −0.039 | - | 0.50 | - |
| qGL5.1 | 5 | 5_7928–5_8536 | 4.82 | - | 0.042 | - | 0.58 | - |
| qGL5.2 | 5 | 5_19815–5_20037 | 23.40 | 10.73 | −0.099 | −0.103 | 3.17 | 3.46 |
| qGL5.3 | 5 | 5_20171–5_20351 | 44.60 | 7.15 | 0.139 | 0.122 | 6.28 | 4.86 |
| qGL6 | 6 | 6_458–6_572 | 8.04 | - | 0.055 | - | 0.99 | - |
| qGL7 | 7 | 7_27901–7_27914 | 6.29 | - | −0.048 | - | 0.76 | - |
| qGL8 | 8 | 8_3569.1–8_3569.2 | 4.67 | - | −0.041 | - | 0.55 | - |
| qGL10 | 10 | 10_20601–10_20803 | 27.76 | - | −0.107 | - | 3.73 | - |
| qGL11.1 | 11 | 11_5422–11_5467 | 10.07 | - | 0.062 | - | 1.25 | - |
| qGL11.2 | 11 | 11_10868–11_16240 | 4.46 | - | 0.041 | - | 0.53 | - |
| qGL12 | 12 | 12_25885–12_26263 | 13.78 | - | −0.073 | - | 1.73 | - |
| qGW2.1 | 2 | 2_25614–2_25767 | 52.84 | - | 0.033 | - | 21.36 | - |
| qGW2.2 | 2 | 2_35156–2_35425 | 3.92 | - | 0.008 | - | 1.32 | - |
| qGW3.1 | 3 | 3_4336–3_4381 | 6.92 | 5.45 | −0.011 | 0.009 | 2.21 | 1.61 |
| qGW3.2 | 3 | 3_22495–3_22634 | 18.64 | 12.97 | 0.018 | −0.017 | 6.29 | 5.33 |
| qGW3.3 | 3 | 3_34881–3_35292 | 9.85 | 7.24 | 0.013 | −0.011 | 3.19 | 2.27 |
| qGW4.1 | 4 | 4_20301–4_20353 | 6.20 | - | 0.010 | - | 1.95 | - |
| qGW4.2 | 4 | 4_29681–4_30701 | 16.69 | 10.50 | −0.017 | 0.014 | 5.59 | 3.97 |
| qGW6.1 | 6 | PB9–6_10875 | 7.05 | 4.53 | −0.011 | 0.008 | 2.27 | 1.26 |
| qGW6.2 | 6 | 6_13038–Si13070 | 6.77 | - | −0.011 | - | 2.16 | - |
| qGW7 | 7 | 7_4209–7_4482 | 9.77 | - | 0.013 | - | 3.15 | - |
| qGW9 | 9 | 9_19391–9_20428 | 4.50 | - | −0.009 | - | 1.44 | - |
| qGW11 | 11 | 11_22486–11_23129 | 11.65 | 5.09 | −0.014 | −0.012 | 3.78 | 2.87 |
| qGW12 | 12 | 12_25491–12_25509 | 17.92 | - | 0.018 | - | 6.07 | - |
| qRLW2 | 2 | 2_24568–2_24751 | 6.50 | - | −0.029 | - | 1.38 | - |
| qRLW3.1 | 3 | 3_16442–3_17040 | 122.43 | 23.20 | −0.194 | −0.006 | 61.83 | 0.06 |
| qRLW3.2 | 3 | 3_22495–3_22634 | 5.39 | 4.89 | −0.027 | 0.021 | 1.16 | 0.70 |
| qRLW4.1 | 4 | 4_15980–4_17226 | 6.09 | - | −0.028 | - | 1.32 | - |
| qRLW4.2 | 4 | 4_30701–4_32392 | 7.33 | - | 0.032 | - | 1.67 | - |
| qRLW6 | 6 | RM276–6_6539 | 9.18 | - | 0.035 | - | 2.01 | - |
| qRLW8 | 8 | 8_5753–8_5949 | 4.98 | - | 0.025 | - | 1.06 | - |
| qRLW10 | 10 | 10_20225–10_20341 | 13.72 | - | −0.043 | - | 3.08 | - |
| qRLW11 | 11 | 11_7662–11_7931.1 | 13.36 | - | 0.042 | - | 2.99 | - |
| qRLW12 | 12 | 12_25640–12_25804 | 18.48 | - | −0.050 | - | 4.23 | - |
| Group | Pi26–Pi25 a | Pib | Pita | n | LBR | GY | |
|---|---|---|---|---|---|---|---|
| 2019 | 2020 | ||||||
| 1 | F | N | N | 27 | 0.1 ± 0.3 a | 32.38 ± 6.42 ab | 33.85 ± 5.99 ab |
| 2 | F | F | N | 23 | 0.1 ± 0.3 a | 31.03 ± 5.49 ab | 34.62 ± 6.63 ab |
| 3 | F | N | F | 34 | 0.1 ± 0.3 a | 34.10 ± 6.63 a | 39.05 ± 9.74 a |
| 4 | F | F | F | 28 | 0.0 ± 0.1 a | 33.90 ± 5.30 ab | 36.65 ± 8.72 ab |
| 5 | N | N | N | 17 | 5.9 ± 0.6 b | 30.23 ± 4.80 b | 32.79 ± 5.92 b |
| 6 | N | F | N | 19 | 6.4 ± 1.3 b | 31.63 ± 4.78 ab | 35.52 ± 7.06 ab |
| 7 | N | N | F | 39 | 6.3 ± 1.2 b | 32.08 ± 5.84 ab | 35.33 ± 6.73 ab |
| 8 | N | F | F | 18 | 6.4 ± 1.4 b | 30.84 ± 3.07 ab | 35.33 ± 5.64 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanyange, L.; Fan, Y.-Y.; Zhang, Z.-H.; Huang, D.-R.; Huang, T.-X.; Zhuang, J.-Y.; Zhu, Y.-J. Genetic Association between Blast Resistance and Yield Traits in Rice Detected Using a High-Density Bin Map. Agronomy 2022, 12, 1173. https://doi.org/10.3390/agronomy12051173
Kanyange L, Fan Y-Y, Zhang Z-H, Huang D-R, Huang T-X, Zhuang J-Y, Zhu Y-J. Genetic Association between Blast Resistance and Yield Traits in Rice Detected Using a High-Density Bin Map. Agronomy. 2022; 12(5):1173. https://doi.org/10.3390/agronomy12051173
Chicago/Turabian StyleKanyange, Lydia, Ye-Yang Fan, Zhen-Hua Zhang, De-Run Huang, Ting-Xu Huang, Jie-Yun Zhuang, and Yu-Jun Zhu. 2022. "Genetic Association between Blast Resistance and Yield Traits in Rice Detected Using a High-Density Bin Map" Agronomy 12, no. 5: 1173. https://doi.org/10.3390/agronomy12051173
APA StyleKanyange, L., Fan, Y.-Y., Zhang, Z.-H., Huang, D.-R., Huang, T.-X., Zhuang, J.-Y., & Zhu, Y.-J. (2022). Genetic Association between Blast Resistance and Yield Traits in Rice Detected Using a High-Density Bin Map. Agronomy, 12(5), 1173. https://doi.org/10.3390/agronomy12051173








