Agronomic Performance of Grain Sorghum (Sorghum bicolor (L.) Moench) Cultivars under Intensive Fish Farm Effluent Irrigation
Abstract
1. Introduction
2. Materials and Methods
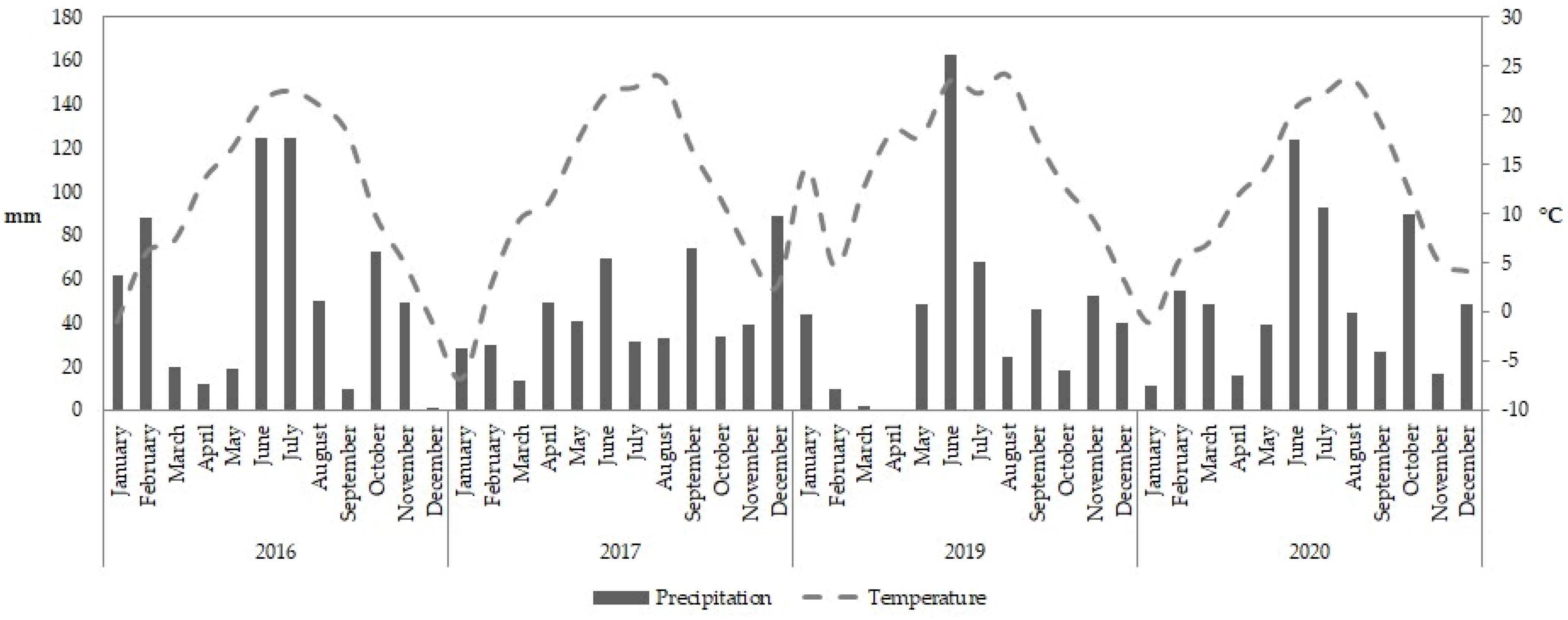
2.1. Site Description and Climatic Conditions
2.2. The Plant Material and Experimental Design
2.3. Assay of Phenologycal Parameters and Mineral Content
2.4. Statictical Analyses
3. Results
3.1. Changes in Soil Parameters during the Experiment
3.2. Results of the Main Macroelement Content of Sorghum Plant Parts
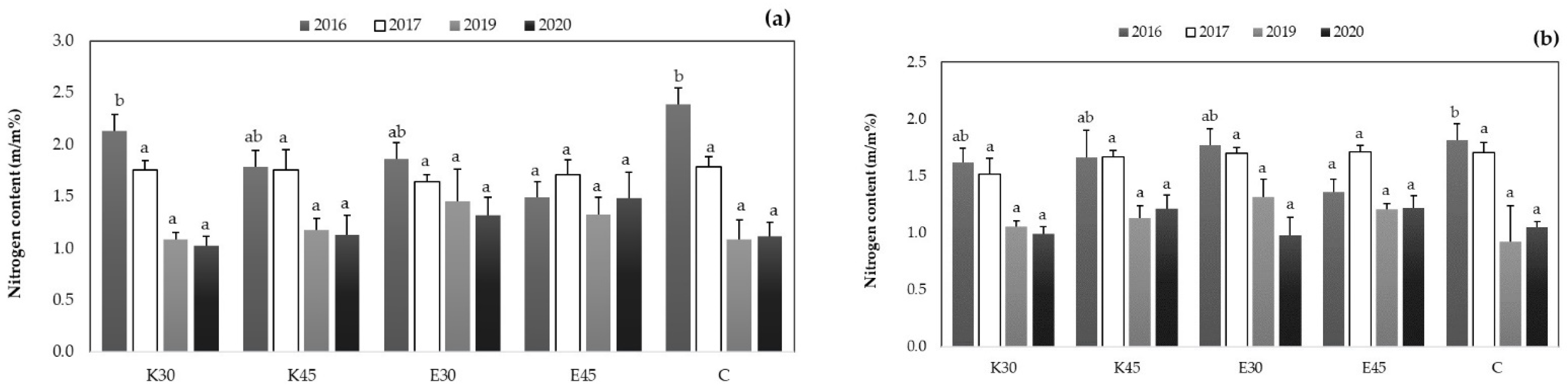
3.2.1. Changing of Nitrogen Content in Different Plant Parts
3.2.2. Changing of Phosphorus Content in Sorghum Plant Part
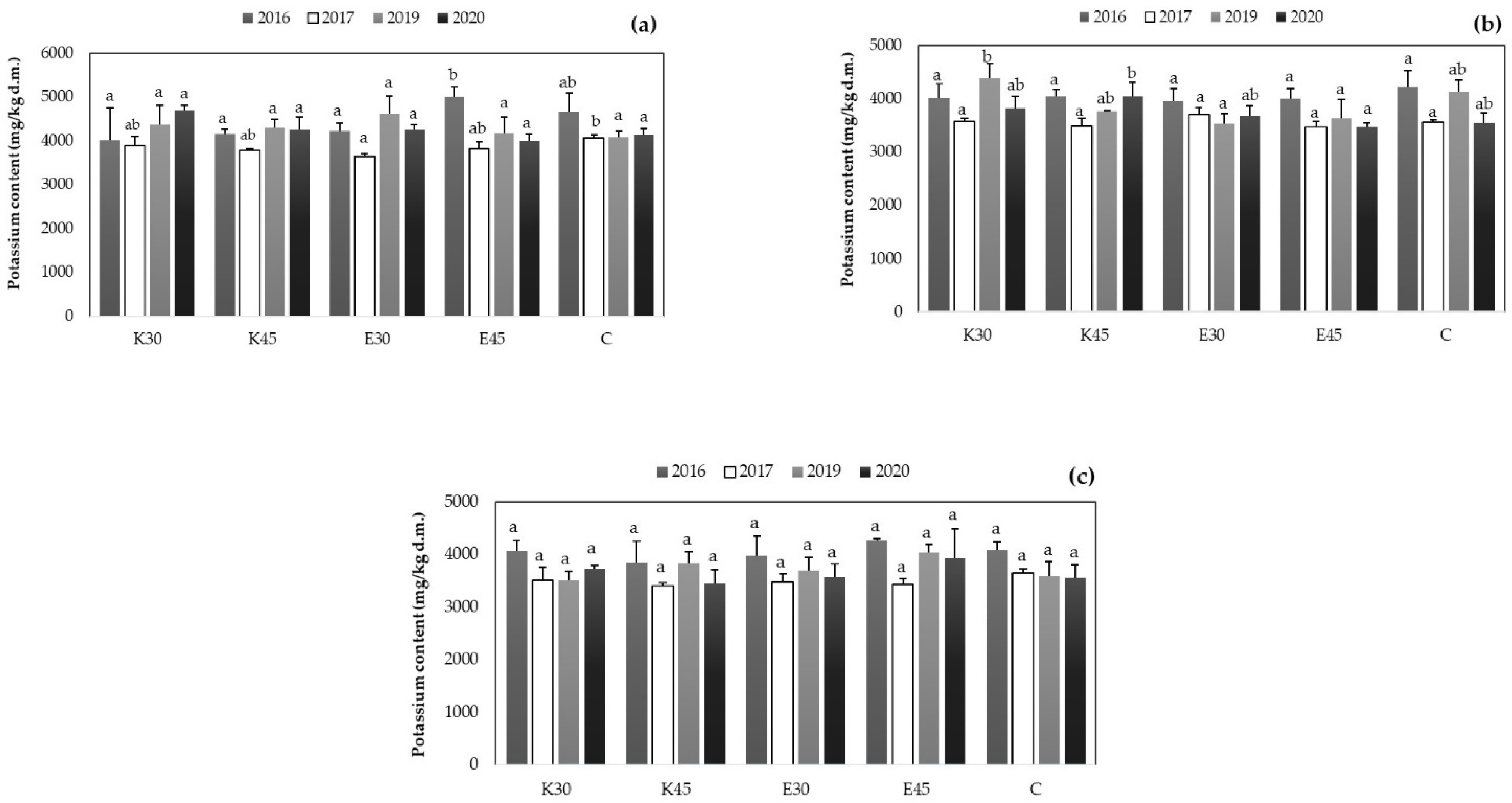
3.2.3. Changing of Potassium Content in Sorghum Plant Part
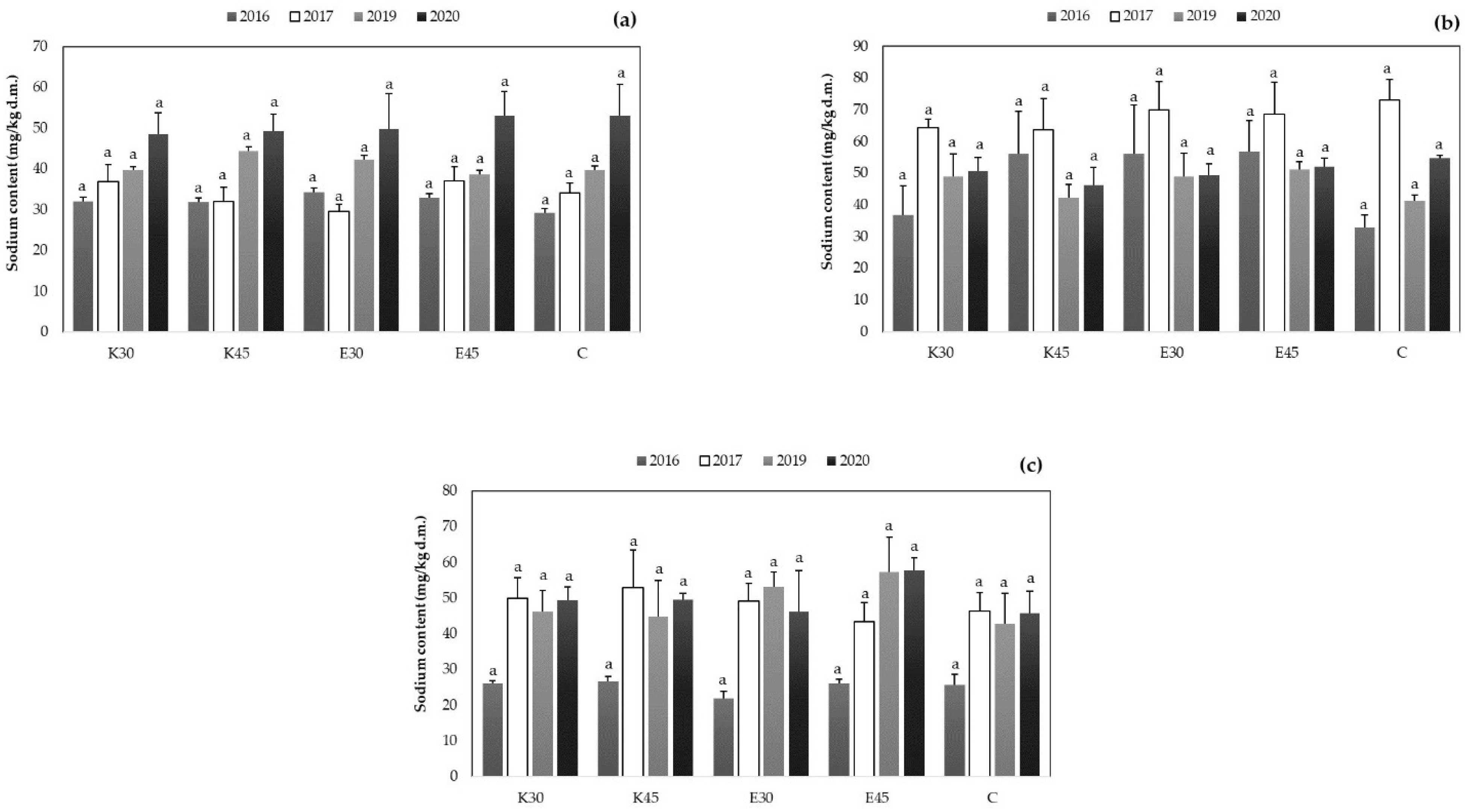
3.2.4. Changing of Sodium Content in Sorghum Plant Part
3.3. Phenological Results
3.3.1. Development of Relative Chlorophyll Content
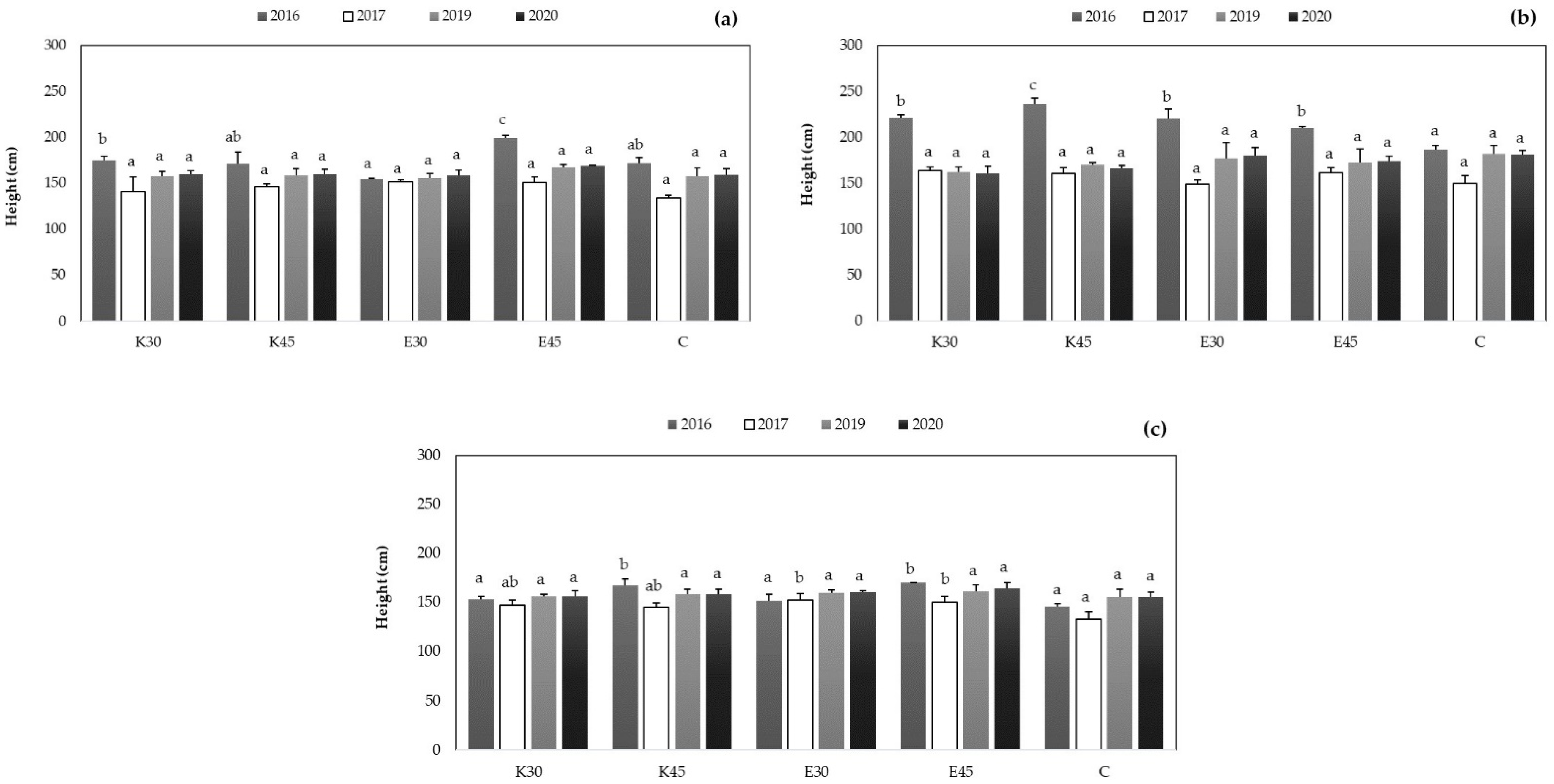
3.3.2. Determination of Growth Parameter during the Seasons
3.4. Development of Biomass Product over the Four Experimental Years
3.4.1. Development of the Green Mass of the Three Sorghum Cultivars during the Experimental Years
3.4.2. Improvement of the Grain Yield of the Three Sorghum Cultivars during the Experimental Years
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Heaton, E.A.; Clifton-Brown, J.; Voigt, T.B.; Jones, M.B.; Long, S.P. Miscanthus for Renewable Energy Generation: European Union Experience and Projections for Illinois. Mitig. Adapt. Strateg. Glob. Chang. 2004, 9, 433–451. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Dolat, A.; Steinberger, Y.; Wang, X.; Osman, A.; Xie, G.H. Biomass Yield and Changes in Chemical Composition of Sweet Sorghum Cultivars Grown for Biofuel. Field Crops Res. 2009, 111, 55–64. [Google Scholar] [CrossRef]
- Ghatak, H.R. Biorefineries from the Perspective of Sustainability: Feedstocks, Products, and Processes. Renew. Sustain. Energy Rev. 2011, 15, 4042–4052. [Google Scholar] [CrossRef]
- Owusu-Sekyere, E.; Scheepers, M.E.; Jordaan, H. Economic Water Productivities Along the Dairy Value Chain in South Africa: Implications for Sustainable and Economically Efficient Water-Use Policies in the Dairy Industry. Ecol. Econ. 2017, 134, 22–28. [Google Scholar] [CrossRef]
- Simon, S.; Wiegmann, K. Modelling Sustainable Bioenergy Potentials from Agriculture for Germany and Eastern European Countries. Biomass Bioenergy 2009, 33, 603–609. [Google Scholar] [CrossRef]
- Sadia, B.; Saeed Awan, F.; Saleem, F.; Razzaq, A.; Irshad, B. Sorghum an Important Annual Feedstock for Bioenergy. In Biomass Bioenergy-Recent Trends Future Chall; El-Fatah Abomohra, A., Ed.; IntechOpen: London, UK, 2019; ISBN 978-1-78923-987-4. Available online: https://www.researchgate.net/publication/337694786_Sorghum_an_Important_Annual_Feedstock_for_Bioenergy (accessed on 13 May 2022).
- Staggenborg, S.A.; Dhuyvetter, K.C.; Gordon, W.B. Grain Sorghum and Corn Comparisons: Yield, Economic, and Environmental Responses. Agron. J. 2008, 100, 1600–1604. [Google Scholar] [CrossRef]
- Assefa, Y.; Staggenborg, S.A.; Prasad, V.P.V. Grain Sorghum Water Requirement and Responses to Drought Stress: A Review. Crop Manag. 2010, 9, 1–11. [Google Scholar] [CrossRef]
- Plénet, D.; Cruz, P. Maize and Sorghum. In Diagnosis of the Nitrogen Status in Crops; Lemaire, G., Ed.; Springer: Berlin/Heidelberg, Germany, 1997; pp. 93–106. ISBN 978-3-642-64506-8. [Google Scholar]
- Gosling, S.N.; Arnell, N.W. A Global Assessment of the Impact of Climate Change on Water Scarcity. Clim. Chang. 2016, 134, 371–385. [Google Scholar] [CrossRef]
- Mekonnen, M.M.; Hoekstra, A.Y. Four Billion People Facing Severe Water Scarcity. Sci. Adv. 2016, 2, e1500323. [Google Scholar] [CrossRef]
- O’Connor, G.A.; Elliott, H.A.; Bastian, R.K. Degraded Water Reuse: An Overview. J. Environ. Qual. 2008, 37, S-157–S-168. [Google Scholar] [CrossRef]
- Oster, J.D. Irrigation with Poor Quality Water. Agric. Water Manag. 1994, 25, 271–297. [Google Scholar] [CrossRef]
- Kestemont, P. Different Systems of Carp Production and Their Impacts on the Environment. Aquaculture 1995, 129, 347–372. [Google Scholar] [CrossRef]
- WHO. A Regional Overview of Wastewater Management and Reuse in the Eastern Mediterranean Region; CEHA, 2005. Available online: https://apps.who.int/iris/bitstream/handle/10665/116463/dsa759.pdf (accessed on 13 May 2022).
- Kolozsvári, I.; Kun, Á.; Jancsó, M.; Bakti, B.; Bozán, C.; Gyuricza, C. Utilization of Fish Farm Effluent for Irrigation Short Rotation Willow (Salix alba L.) under Lysimeter Conditions. Forests 2021, 12, 457. [Google Scholar] [CrossRef]
- Lin, Y.-F.; Jing, S.-R.; Lee, D.-Y.; Wang, T.-W. Nutrient Removal from Aquaculture Wastewater Using a Constructed Wetlands System. Aquaculture 2002, 209, 169–184. [Google Scholar] [CrossRef]
- Tsuchihashi, N.; Goto, Y. Cultivation of Sweet Sorghum (Sorghum bicolor (L.) Moench) and Determination of Its Harvest Time to Make Use as the Raw Material for Fermentation, Practiced during Rainy Season in Dry Land of Indonesia. Plant Prod. Sci. 2004, 7, 442–448. [Google Scholar] [CrossRef]
- Paterson, A.H. Genomics of Sorghum. Int. J. Plant Genom. 2008, 2008, 1362451. [Google Scholar] [CrossRef]
- Mace, E.S.; Tai, S.; Gilding, E.K.; Li, Y.; Prentis, P.J.; Bian, L.; Campbell, B.C.; Hu, W.; Innes, D.J.; Han, X.; et al. Whole-Genome Sequencing Reveals Untapped Genetic Potential in Africa’s Indigenous Cereal Crop Sorghum. Nat. Commun. 2013, 4, 2320. [Google Scholar] [CrossRef]
- Teetor, V.H.; Duclos, D.V.; Wittenberg, E.T.; Young, K.M.; Chawhuaymak, J.; Riley, M.R.; Ray, D.T. Effects of Planting Date on Sugar and Ethanol Yield of Sweet Sorghum Grown in Arizona. Ind. Crops Prod. 2011, 34, 1293–1300. [Google Scholar] [CrossRef]
- Murray, S.C.; Rooney, W.L.; Hamblin, M.T.; Mitchell, S.E.; Kresovich, S. Sweet Sorghum Genetic Diversity and Association Mapping for Brix and Height. Plant Genome 2009, 2, 48–62. [Google Scholar] [CrossRef]
- Vasilakoglou, I.; Dhima, K.; Karagiannidis, N.; Gatsis, T. Sweet Sorghum Productivity for Biofuels under Increased Soil Salinity and Reduced Irrigation. Field Crops Res. 2011, 120, 38–46. [Google Scholar] [CrossRef]
- Al-Jaloud, A.A.; Hussain, G.; Al-Saati, A.J.; Karimulla, S. Effect of Wastewater Irrigation on Mineral Composition of Corn and Sorghum Plants in a Pot Experiment. J. Plant Nutr. 1995, 18, 1677–1692. [Google Scholar] [CrossRef]
- Flörke, M.; Schneider, C.; McDonald, R.I. Water Competition between Cities and Agriculture Driven by Climate Change and Urban Growth. Nat. Sustain. 2018, 1, 51–58. [Google Scholar] [CrossRef]
- Qi, D.; Yan, J.; Zhu, J. Effect of a Reduced Fertilizer Rate on the Water Quality of Paddy Fields and Rice Yields under Fishpond Effluent Irrigation. Agric. Water Manag. 2020, 231, 105999. [Google Scholar] [CrossRef]
- Abdelraouf, R.E.; Abou-Hussein, S.D.; Badr, M.A.; El-Tohamy, N.M. Safe and Sustainable Fertilization Technology with Using Fish Water Effluent as a New Bio-Source for Fertilizing. Acta Hortic. 2016, 1142, 41–48. [Google Scholar] [CrossRef]
- Guimarães, M.J.M.; Simões, W.L.; Tabosa, J.N.; dos Santos, J.E.; Willadino, L. Cultivation of Forage Sorghum Varieties Irrigated with Saline Effluent from Fish-Farming under Semiarid Conditions. Rev. Bras. Eng. Agrícola Ambient. 2016, 20, 461–465. [Google Scholar] [CrossRef][Green Version]
- Sou/Dakouré, M.Y.; Mermoud, A.; Yacouba, H.; Boivin, P. Impacts of Irrigation with Industrial Treated Wastewater on Soil Properties. Geoderma 2013, 200–201, 31–39. [Google Scholar] [CrossRef]
- Wang, M.; Chen, S.; Chen, L.; Wang, D.; Zhao, C. The Responses of a Soil Bacterial Community under Saline Stress Are Associated with Cd Availability in Long-Term Wastewater-Irrigated Field Soil. Chemosphere 2019, 236, 124372. [Google Scholar] [CrossRef]
- Yang, C.; Wang, X.; Miao, F.; Li, Z.; Tang, W.; Sun, J. Assessing the Effect of Soil Salinization on Soil Microbial Respiration and Diversities under Incubation Conditions. Appl. Soil Ecol. 2020, 155, 103671. [Google Scholar] [CrossRef]
- Arienzo, M.; Christen, E.W.; Quayle, W.; Kumar, A. A Review of the Fate of Potassium in the Soil–Plant System after Land Application of Wastewaters. J. Hazard. Mater. 2009, 164, 415–422. [Google Scholar] [CrossRef]
- Egner, H.; Riem, H.; Domingo, W. Untersuchungen Über Die Chemische Bodenanalyse Als Grundlage Für Die Beurteilung Des Nährstoffzustandes Der Böden. II. Chemische Extraktionsmethoden Zur Phosphor Und Kaliumbestimmung. K. Lantbr. Ann. 1960, 26, 199–215. [Google Scholar]
- Campos, F.S.; Araújo, G.G.L.; Simões, W.L.; Gois, G.C.; Machado Guimarães, M.J.; da Silva, T.G.F.; Rodrigues Magãlhaes, A.L.; Oliveira, G.F.; de Almeida Araujo, C.; Silva, T.S.; et al. Mineral and Fermentative Profile of Forage Sorghum Irrigated with Brackish Water. Commun. Soil Sci. Plant Anal. 2021, 52, 1353–1362. [Google Scholar] [CrossRef]
- Malhotra, H.; Vandana; Sharma, S.; Pandey, R. Phosphorus Nutrition: Plant Growth in Response to Deficiency and Excess. In Plant Nutrients and Abiotic Stress Tolerance; Hasanuzzaman, M., Fujita, M., Oku, H., Nahar, K., Hawrylak-Nowak, B., Eds.; Springer: Singapore, 2018; pp. 171–190. ISBN 978-981-10-9043-1. [Google Scholar]
- Gordon, W.B.; Whitney, D.A. Effects of Phosphorus Application Method and Rate on Furrow-irrigated Ridge-tilled Grain Sorghum. J. Plant Nutr. 2000, 23, 23–34. [Google Scholar] [CrossRef]
- Marschner, H.; Marschner, P. Mineral Nutrition of Higher Plants, 3rd ed.; Elsevier: London, UK; Academic Press: Waltham, MA, USA, 2012; ISBN 978-0-12-384905-2. [Google Scholar]
- Chhipa, B.; Lal, P. Na/K Ratios as the Basis of Salt Tolerance in Wheat. Aust. J. Agric. Resour. Econ. 1995, 46, 533. [Google Scholar] [CrossRef]
- Ahman, S.; Islam Khan, N.; Iqbal, M.Z.; Hussain, A.; Hassan, M. Salt Tolerance of Cotton (Gossypium hirsutum L.). Asian J. Plant Sci. 2002, 1, 715–719. [Google Scholar] [CrossRef]
- Iqbal, N.; Ashraf, M.Y.; Javed, F.; Martinez, V.; Ahmad, K. Nitrate Reduction and Nutrient Accumulation in Wheat Grown in Soil Salinized with Four Different Salts. J. Plant Nutr. 2006, 29, 409–421. [Google Scholar] [CrossRef]
- Rao, D.L.N. The Effects of Salinity and Sodicity upon Nodulation and Nitrogen Fixation in Chickpea (Cicer arietinum). Ann. Bot. 2002, 89, 563–570. [Google Scholar] [CrossRef]
- Rout, N.P.; Shaw, B.P. Salt Tolerance in Aquatic Macrophytes: Possible Involvement of the Antioxidative Enzymes. Plant Sci. 2001, 160, 415–423. [Google Scholar] [CrossRef]
- Calone, R.; Sanoubar, R.; Lambertini, C.; Speranza, M.; Vittori Antisari, L.; Vianello, G.; Barbanti, L. Salt Tolerance and Na Allocation in Sorghum bicolor under Variable Soil and Water Salinity. Plants 2020, 9, 561. [Google Scholar] [CrossRef]
- Sixto, H.; Grau, J.M.; Alba, N.; Alía, R. Response to Sodium Chloride in Different Species and Clones of Genus Populus L. Forestry 2005, 74, 93–104. [Google Scholar] [CrossRef]
- Shannon, M.C.; Grieve, C.M. Tolerance of Vegetable Crops to Salinity. Sci. Hortic. 1998, 78, 5–38. [Google Scholar] [CrossRef]
- Chookhampaeng, S. The Effect of Salt Stress on Growth, Chlorophyll Content Proline Content and Antioxidative Enzymes of Pepper (Capsicum annuum L.) Seedling. Eur. J. Sci. Res. 2011, 49, 103–109. [Google Scholar]
- Sevengor, S.; Yasar, F.; Kusvuran, S.; Ellialtioglu, S. The Effect of Salt Stress on Growth, Chlorophyll Content, Lipid Peroxidation and Antioxidative Enzymes of Pumpkin Seedling. Afr. J. Agric. Res. 2011, 6, 4920–4924. [Google Scholar] [CrossRef]
- Padilla, F.M.; de Souza, R.; Peña-Fleitas, M.T.; Gallardo, M.; Giménez, C.; Thompson, R.B. Different Responses of Various Chlorophyll Meters to Increasing Nitrogen Supply in Sweet Pepper. Front. Plant Sci. 2018, 9, 1752. [Google Scholar] [CrossRef] [PubMed]
- Almodares, A.; Sharif, M.E. Effects of Irrigation Water Qualities on Biomass and Sugar Contents of Sugar Beet and Sweet Sorghum Cultivars. J. Environ. Biol. 2007, 28, 213–218. [Google Scholar] [PubMed]
- Katerji, N.; Van Hoorn, J.W.; Hamdy, A.; Mastrorilli, M.M.; Mou, E. Osmotic Adjustment of Sugar Beets in Response to Soil Salinity and Its Influence on Stomatal Conductance, Growth and Yield. Agric. Water Manag. 1997, 34, 57–69. [Google Scholar] [CrossRef]
- Hussein, M.M.; Abdel-Kader, A.A.; Kady, K.A.; Youssef, R.A.; Alva, A.K. Sorghum Response to Foliar Application of Phosphorus and Potassium with Saline Water Irrigation. J. Crop Improv. 2010, 24, 324–336. [Google Scholar] [CrossRef]
- Ayers, R.S.; Westcot, D.W. Water Quality for Agriculture; FAO Irrigation and Drainage Paper; Food and Agriculture Organization of the United Nations: Rome, Italy, 1985; ISBN 978-92-5-102263-4. [Google Scholar]


| Depth of the Soil Layer | ||
|---|---|---|
| Soil Parameter | 0–30 cm | 30–60 cm |
| pH (KCL) | 7.23 ± 0.06 | 7.15 ± 0.05 |
| Texture | clay loam | clay loam |
| EC (µS/cm) | 410 ± 28 | 458 ± 30 |
| Total carbonate content (m/m%) | 1.96 ± 0.97 | 1.41 ± 0.51 |
| Total organic carbon content (m/m%) | 1.21 ± 0.08 | 1.33 ± 0.09 |
| KCL-NO2− + NO3−-N (mg/kg) | 3.47 ± 0.56 | 4.42 ± 0.63 |
| AL-P2O5 (mg/kg) | 2350 ± 607 | 3013 ± 395 |
| AL-K2O (mg/kg) | 627 ± 137 | 957 ± 195 |
| AL-Na (mg/kg) | 45.0 ± 11.0 | 56.2 ± 13.6 |
| EC | NH4-N | N | P | K | Na | SAR | |
|---|---|---|---|---|---|---|---|
| (µS/cm) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | ||
| Effluent water | 1307.0 | 22.5 | 29.2 | 3.9 | 6.4 | 275.5 | 12.1 |
| Körös River oxbow lake water | 371.0 | 0.6 | 2.1 | 0.2 | 3.7 | 31.2 | 1.2 |
| Date of Sowing | Irrigation Water Doses (mm) | Number of Irrigation | Amount of Water Applied by Irrigation (mm) | Precipitation during the Growing Season (mm) | Amount of Additional Irrigation (Körös River) during Germination (mm) | Total Amount of Available Water (mm) | |
|---|---|---|---|---|---|---|---|
| 2016 | 4 May | 30 | 5 | 150 | 296 | 120 | 566 |
| 45 | 225 | 641 | |||||
| 2017 | 2 May | 30 | 6 | 180 | 144 | 80 | 404 |
| 45 | 270 | 494 | |||||
| 2019 | 7 May | 30 | 8 | 240 | 208 | 40 | 488 |
| 45 | 360 | 608 | |||||
| 2020 | 27 April | 30 | 4 | 120 | 288 | 90 | 498 |
| 45 | 180 | 558 |
| 2020 | E30 | E45 | K30 | K45 | C | |
|---|---|---|---|---|---|---|
| 0–30 cm | p1 | |||||
| pH (KCl) | 7.28 ± 0.04 b | 7.30 ± 0.06 b | 7.22 ± 0.02 ab | 7.25 ± 0.04 ab | 7.18 ± 0.03 a | * |
| EC (µS/cm) | 355 ± 38 a | 352 ± 38 a | 411 ± 38 ab | 402 ± 38 ab | 464 ± 38 b | ** |
| Total carbonate content (m/m%) | 2.31 ± 0.67 | 2.02 ± 0.93 | 1.90 ± 0.23 | 2.35 ± 0.23 | 1.43 ± 0.24 | n.s. |
| Total organic carbon (m/m%) | 1.09 ± 0.08 | 1.08 ± 0.05 | 1.18 ± 0.05 | 1.16 ± 0.04 | 1.23 ± 0.07 | n.s. |
| KCL-NO2− + NO3−-N (mg/kg) | 3.01 ± 0.45 | 3.45 ± 0.81 | 3.44 ± 0.95 | 3.85 ± 1.04 | 3.42 ± 0.64 | n.s. |
| AL-P2O5 (mg/kg) | 1243 ± 262 a | 1247 ± 159 a | 1880 ± 285 ab | 1543 ± 179 bc | 2300 ± 225 c | ** |
| AL-K2O (mg/kg) | 350 ± 75 a | 333 ± 45 a | 475 ± 67 ab | 460 ± 31 ab | 509 ± 41 b | ** |
| AL-Na (mg/kg) | 113.7 ± 18.9 ab | 122.9 ± 21.3 b | 89.2 ± 21.0 ab | 82.1 ± 17.9 a | 86.8 ± 23.3 a | ** |
| 30–60 cm | p1 | |||||
| pH (KCl) | 7.13 ± 0.10 | 7.08 ± 0.03 | 7.16 ± 0.06 | 7.17 ± 0.07 | 7.12 ± 0.05 | n.s. |
| EC (µS/cm) | 458 ± 38 | 476 ± 38 | 464 ± 38 | 452 ± 38 | 451 ± 38 | n.s. |
| Total carbonate content (m/m%) | 1.92 ± 1.16 | 1.41 ± 0.74 | 1.74 ± 0.52 | 1.91 ± 1.10 | 1.40 ± 0.47 | n.s. |
| Total organic carbon (m/m%) | 1.30 ± 0.18 | 1.42 ± 0.06 | 1.29 ± 0.07 | 1.30 ± 0.14 | 1.40 ± 0.06 | n.s. |
| KCL-NO2− + NO3−-N (mg/kg) | 3.55 ± 0.32 | 3.37 ± 0.31 | 3.22 ± 0.92 | 3.26 ± 0.66 | 3.53 ± 0.36 | n.s. |
| AL-P2O5 (mg/kg) | 2100 ± 447 | 2447 ± 716 | 2373 ± 234 | 2127 ± 318 | 3010 ± 828 | n.s. |
| AL-K2O (mg/kg) | 612 ± 114 | 607 ± 94 | 635 ± 93 | 727 ± 66 | 640 ± 68 | n.s. |
| AL-Na (mg/kg) | 122.2 ± 14.7 ab | 127.0 ± 18.2 b | 88.9 ± 20.0 a | 95.6 ± 24.2 ab | 89.9 ± 20.0 a | ** |
| Variety of Grain Sorghum | Treatments | Average SPAD Values | |||
|---|---|---|---|---|---|
| 2016 | 2017 | 2019 | 2020 | ||
| ‘Alföldi 1’ | K30 | 50.7 ± 5.8 a | 51.7 ± 6.2 a | 42.8 ± 6.7 a | 42.5 ± 6.1 a |
| K45 | 52.6 ± 7.5 a | 52.1 ± 11.6 a | 48.5 ± 9.0 bc | 49.9 ± 8.8 bc | |
| E30 | 52.6 ± 8.0 a | 49.0 ± 6.7 a | 44.0 ± 7.2 ab | 44.5 ± 7.7 ab | |
| E45 | 50.0 ± 6.1 a | 49.1 ± 6.7 a | 52.4 ± 4.5 c | 53.2 ± 4.7 c | |
| C | 52.8 ± 9.1 a | 49.8 ± 7.8 a | 44.3 ± 9.2 ab | 45.3 ± 9.4 ab | |
| ‘Farmsugro 180’ | K30 | 46.3 ± 7.3 a | 49.8 ± 5.7 a | 44.8 ± 5.1 b | 45.6 ± 5.5 b |
| K45 | 50.0 ± 9.0 a | 51.0 ± 5.8 a | 39.1 ± 5.4 a | 41.0 ± 5.6 a | |
| E30 | 49.5 ± 10.1 a | 50.7 ± 6.2 a | 38.9 ± 5.0 a | 41.1 ± 4.6 a | |
| E45 | 47.7 ± 8.8 a | 52.5 ± 6.9 a | 47.1 ± 5.1 b | 48.0 ± 4.9 b | |
| C | 47.6 ± 10.2 a | 49.8 ± 7.4 a | 46.8 ± 5.0 b | 47.7 ± 4.6 b | |
| ‘GK Emese’ | K30 | 51.9 ± 7.0 a | 49.2 ± 5.7 a | 45.7 ± 6.3 b | 45.5 ± 6.2 b |
| K45 | 56.5 ± 7.3 a | 50.1 ± 7.3 a | 40.0 ± 7.3 a | 37.8 ± 8.0 a | |
| E30 | 52.4 ± 7.2 a | 47.3 ± 7.6 a | 41.0 ± 6.9 ab | 38.0 ± 10.2 a | |
| E45 | 53.1 ± 7.1 a | 48.6 ± 6.8 a | 44.8 ± 6.8 b | 45.0 ± 7.5 b | |
| C | 55.8 ± 7.3 a | 48.4 ± 7.5 a | 42.8 ± 6.7 ab | 43.0 ± 6.6 ab | |
| Variety of Grain Sorghum | Applied Treatments | 2016 | 2017 | 2019 | 2020 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Green Mass Weight (g/Plant) Mean ± SD | p-Value | Green Mass Weight (g/Plant) Mean ± SD | p-Value | Green Mass Weight (g/Plant) Mean ± SD | p-Value | Green Mass Weight (g/Plant) Mean ± SD | p-Value | ||
| ‘Alföldi 1’ | K30 | 301 ± 9 a | n.s | 422 ± 79 a | n.s. | 315 ± 58 a | n.s. | 379 ± 104 a | n.s. |
| K45 | 302 ± 49 a | 451 ± 92 a | 357 ± 54 a | 420 ± 123 a | |||||
| E30 | 296 ± 62 a | 436 ± 100 a | 386 ± 81 a | 402 ± 31 a | |||||
| E45 | 365 ± 108 a | 476 ± 117 a | 329 ± 93 a | 376 ± 173 a | |||||
| C | 320 ± 80 a | 349 ± 89 a | 397 ± 70 a | 269 ± 55 a | |||||
| ‘Farmsugro 180’ | K30 | 583 ± 81 b | * | 427 ± 48 a | n.s. | 331 ± 36 a | n.s. | 397 ± 80 a | n.s. |
| K45 | 528 ± 96 ab | 489 ± 105 a | 308 ± 52 a | 383 ± 62 a | |||||
| E30 | 539 ± 91 ab | 460 ± 65 a | 346 ± 37 a | 422 ± 107 a | |||||
| E45 | 433 ± 84 a | 458 ± 71 a | 330 ± 33 a | 461 ± 60 a | |||||
| C | 540 ± 58 ab | 411 ± 74 a | 321 ± 60 a | 448 ± 53 a | |||||
| ‘GK Emese’ | K30 | 244 ± 70 a | n.s | 427 ± 98 a | n.s. | 435 ± 74 ab | ** | 347 ± 86 a | n.s. |
| K45 | 229 ± 47 a | 418 ± 74 a | 531 ± 83 abc | 303 ± 65 a | |||||
| E30 | 256 ± 20 a | 401 ± 75 a | 573 ± 96 c | 335 ± 70 a | |||||
| E45 | 209 ± 25 a | 421 ± 61 a | 555 ± 59 bc | 401 ± 140 a | |||||
| C | 227 ± 27 a | 339 ± 67 a | 421 ± 63 a | 330 ± 56 a | |||||
| Variety of Grain Sorghum | Applied Treatments | 2016 | 2017 | 2019 | 2020 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Grain Yield (g/Plant) Mean ± SD | p-Value | Grain Yield (g/Plant) Mean ± SD | p-Value | Grain Yield (g/Plant) Mean ± Std. Deviation | p-Value | Grain Yield (g/Plant) Mean ± Std. Deviation | p-Value | ||
| ‘Alföldi 1’ | K30 | 125 ± 16 bc | *** | 123 ± 18 a | n.s. | 68 ± 17 a | n.s. | 102 ± 38 a | n.s. |
| K45 | 128 ± 21 c | 115 ± 9 a | 83 ± 21 a | 109 ± 53 a | |||||
| E30 | 138 ± 17 c | 116 ± 17 a | 91 ± 16 a | 105 ± 7 a | |||||
| E45 | 100 ± 14 ab | 120 ± 24 a | 67 ± 11 a | 106 ± 42 a | |||||
| C | 82 ± 10 a | 104 ± 22 a | 83 ± 11 a | 89 ± 46 a | |||||
| ‘Farmsugro 180’ | K30 | 75 ± 6 a | *** | 94 ± 11 a | n.s. | 88 ± 16 a | n.s. | 67± 13 ab | * |
| K45 | 86 ± 2 b | 105 ± 25 a | 88 ± 13 a | 57 ± 12 a | |||||
| E30 | 88 ± 3 b | 90 ± 19 a | 96 ± 17 a | 64 ± 23 ab | |||||
| E45 | 87 ± 3 b | 81 ± 8 a | 93 ± 16 a | 81 ± 21 ab | |||||
| C | 70 ± 6 a | 86 ± 13 a | 94 ± 12 a | 91 ± 21 b | |||||
| ‘GK Emese’ | K30 | 140 ± 13 a | n.s. | 109 ± 27 a | n.s. | 80 ± 18 a | n.s. | 94 ± 22 a | n.s. |
| K45 | 129 ± 26 a | 106 ± 17 a | 75 ± 18 a | 77 ± 13 a | |||||
| E30 | 110 ± 52 a | 112 ± 15 a | 77 ± 15 a | 86 ± 19 a | |||||
| E45 | 127 ± 15 a | 107 ± 17 a | 87 ± 9 a | 110 + 46 a | |||||
| C | 100 ± 19 a | 98 ± 13 a | 73 ± 19 a | 94 ± 15 a | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolozsvári, I.; Kun, Á.; Jancsó, M.; Palágyi, A.; Bozán, C.; Gyuricza, C. Agronomic Performance of Grain Sorghum (Sorghum bicolor (L.) Moench) Cultivars under Intensive Fish Farm Effluent Irrigation. Agronomy 2022, 12, 1185. https://doi.org/10.3390/agronomy12051185
Kolozsvári I, Kun Á, Jancsó M, Palágyi A, Bozán C, Gyuricza C. Agronomic Performance of Grain Sorghum (Sorghum bicolor (L.) Moench) Cultivars under Intensive Fish Farm Effluent Irrigation. Agronomy. 2022; 12(5):1185. https://doi.org/10.3390/agronomy12051185
Chicago/Turabian StyleKolozsvári, Ildikó, Ágnes Kun, Mihály Jancsó, Andrea Palágyi, Csaba Bozán, and Csaba Gyuricza. 2022. "Agronomic Performance of Grain Sorghum (Sorghum bicolor (L.) Moench) Cultivars under Intensive Fish Farm Effluent Irrigation" Agronomy 12, no. 5: 1185. https://doi.org/10.3390/agronomy12051185
APA StyleKolozsvári, I., Kun, Á., Jancsó, M., Palágyi, A., Bozán, C., & Gyuricza, C. (2022). Agronomic Performance of Grain Sorghum (Sorghum bicolor (L.) Moench) Cultivars under Intensive Fish Farm Effluent Irrigation. Agronomy, 12(5), 1185. https://doi.org/10.3390/agronomy12051185






