1. Introduction
Increasing population growth, challenges relating to climate change, and improvements in energy policy demand a radical shift in how food is produced in order to sustainably feed a growing population on limited land [
1]. In order to accomplish this achievement, agroecological approaches to food production, which include circular economies based on natural and biological cycles of mineral nutrients, will be preferred over energy-intensive chemical crop fertilization methods.
Nitrogen fertilization is potentially a limiting factor in vegetable production. In organic management systems, the limited availability of approved and affordable fertilizer materials contributes to high production costs and may lead to reduced crop yields and farm profitability [
2]. In order to supply needed nitrogen, most organic farming systems include legume-based cover crops in crop rotations either during the growing season (intercropping or crop succession) or in non-producing seasons (fallow cover cropping) [
3]. Legume crops provide nitrogen to successive crops by hosting symbiotic bacteria which fix atmospheric nitrogen into plant-available forms. On average, legume-based cover crop systems may contribute 40–150 kg of nitrogen to succeeding crops when biomass is retained and incorporated into the soil [
4,
5]. While significant, vegetable crops often require substantially more nitrogen for optimum crop production [
5]. In addition, in order to attain maximum soil nitrogen fertility from planting cover crops, substantial land area is also required to be out of production for up to a full season, which reduces the land available for food production [
6]. Therefore, most vegetable producers in the northeastern USA rely on some form of fertilizer inputs to maintain farm profitability.
Another important mineral plant nutrient, phosphorus, is limiting in certain systems, such as field crop production in the midwestern USA, but is present in excess amounts in others. In the state of Vermont, located in the northeastern USA, phosphorus is present at such high levels that it is considered a potential pollutant on many farms [
7]. Dairy farming has been the primary agricultural activity in the state for over a century, and farmers imported substantial amounts of grain primarily from midwestern states to feed cows on those farms. From 1925 to 2012, Vermont farms accumulated an excess of over 1000 MT of excess phosphorous annually, with most of that coming from imported feed as opposed to fertilizer applications [
8]. Manure generated from dairy cows is typically applied to farmland as a fertilizer source, as it is high in nitrogen which supports regional production of corn and other field crops used on their farms. Manure, either fresh or composted, is a common fertility source used on vegetable farms [
9]. However, manure application historically has been based on crop nitrogen need but also provides substantial phosphorous to lands it is applied on, which accumulates over time [
8]. Vegetable growers must carefully consider fertilizer use on their crops, seeking to balance productivity, costs, regulatory limits of nitrogen and phosphorus, and the needs of their sites and soil type(s). Phosphorous tends to be relatively immobile in the environment but runs off of the land when soil particles it is adsorbed to are disturbed through tillage, erosion, or other disturbance [
10]. This excess phosphorous in waterways is a pollutant that is responsible for eutrophication in lakes and other water quality problems [
7].
Meanwhile, shepherds in New England contend with costs that currently do not contribute to their revenue. Shearing sheep is a necessary step in the husbandry of this small ruminant. Whether raised for meat or milk, annual shearing is required for their health. Since much of the clip produced in the northeast is coarse wool, not suitable for value-added products that require softness, such as yarn or clothing, it has little value. Since 1994, the market price for raw wool has been dropping, and it is now below the cost of shearing and transportation [
11]. A small percentage has the fine softness required for many small-batch cottage industries, but generally, if not sold to the local wool pool collection, many simply pile it in their barns or haul it out to the woods to be dumped [
12]. This represents a disconnect between crop production and broader land management functions that impedes the development of a closed-loop system that minimizes the use of off-farm products or inputs to sustain farm fertility.
As governments, their people, and farmers consider practices that reduce the impact of agriculture on climate change, integrated crop and livestock systems (ICLS) show promise toward reducing and even improving mitigating environmental damage. In a broad-ranging 2021 review of ICLS, Sekaraen et al. concluded that the integration of plant and animal agriculture systems could improve overall productivity while improving environmental outcomes [
13]. Traditionally, the fertility provided by animals to crops has been considered through nitrogen and other minerals in applied animal manures, either through grazing or the application of manure to fields [
14]. In most cases, the livestock utilized in cropping systems are a part of a profitable farming system, such as meat, dairy, or, where markets support it, fiber. In the case of sheep in the northeastern United States, the low value of wool and limited market for meat precludes sheep from being a profitable enterprise in the region [
15,
16]. However, a different paradigm may present a new consideration for using animals for land management and for using their fiber for an alternative: farm-grown fertility that could replace synthetic or imported organic fertilizers to meet nitrogen needs. Waste wool from textile factories or from on-farm sources is increasingly considered a potential fertilizer source. An article from the Utah Farm Bureau promotes the use of wool as a soil conditioner to improve water holding capacity, bulk density, and other soil quality characteristics [
17]. In a German study of wool slabs used as a substrate for hydroponic cucumber production, wool had a greater water holding capacity than other organic substrates [
18]. Another experiment in Poland studied waste wool as both a substrate and a fertility source and associated wool use with up to a 33% increase in crop yield [
19]. Scoured wool residues obtained from textile mills have been evaluated for their effects on soil properties and were found to improve bulk density, water holding capacity, and soil aggregation associated with wool products [
20]. Methods of processing waste wool into usable fertilizers through hydrolysis have been developed to improve the bioavailability of nutrients, product handling, and remove the pollutants contained in processed wool waste [
21,
22]. Numerous field trials in African and European sites have evaluated the effects of raw wool [
19,
23,
24,
25,
26], processed wool pellets [
27], and wool waste byproducts [
20,
22,
28,
29,
30] on the growth and productivity of field and vegetable crops. Overall, the application of wool at various rates has had beneficial effects on soil quality and plant performance with few, if any, negative side effects.
Findings from a USDA Rural Development-funded project conducted from 2016 to 2019 indicate an opportunity to address issues for both vegetable growers (access to low phosphorus fertilizers) and shepherds (potential markets for waste wool. The project authors sourced wool pellets from Wild Valley Farms (Croyden, UT, USA) with an NPK profile average of 9-0-4, supplying nitrogen, virtually no phosphorus, and contributing a small amount of potassium. The nitrogen is slowly released due to the physical properties of fibrous wool pellet’s slow breakdown. For many vegetable farmers in Vermont, this is an ideal combination. Many are over their regulatory limit for phosphorus and need a source of nitrogen that supplies the nutrient at times when it is needed by crops. A slower N-release rate helps to avoid the risk of nitrate leaching and runoff before plants can utilize the nutrient. Additionally, fifty percent of the weight of wool is carbon, and thus, minimally-processed pellets may also provide an opportunity for farmers to sequester carbon through their choice of fertilizer by incorporating the wool pellets into the soil [
31].
A key question of this is whether the use of minimally-processed wool pellets could improve vegetable plants’ productivity and utilize soil phosphorus, thus potentially preventing it from running off into nearby waterways. This preliminary research is intended to support the broader goal of developing a market or use for wool directly from local farms for use as a replacement fertilizer in organic vegetable systems in the northeastern USA.
2. Materials and Methods
2.1. Research Site, Plot Layout, and Crops Evaluated
The research was conducted in the 2021 growing season at two sites in Vermont, located in the northeastern USA. Site 1 (CCF) was located at a certified organic commercial vegetable farm in Orange County (lat 43.798275°, long −72.192763°). The soil was a well-drained riparian Hartland silt loam; cropping history consisted of mixed vegetables, including the annual winter rye cover crop. The field design includes permanent, minimum-tillage beds of ~1.2 m × 90 m. The tillage was performed with a BCS tractor and power harrow (BCS America, Oregon City, OR, USA). Site 2 was located at the University of Vermont Horticulture Research and Education Center (HREC) in Chittenden County (lat 44.430905°, long −73.206338°) and sited in the certified organic Catamount Educational Farm vegetable plots. The soil type at this site is an excessively well-drained lacustrine Windsor Adams loamy sand; cropping history is a two-year cycle of mixed vegetables alternated with oat/clover (summer) or rye/vetch (winter) cover crops, with the field in cover for all of the previous year. This field was prepared with a low tillage system including disc harrowing cover crop residue followed by pre-planting bed forming with a Perfecta field cultivator (Unverferth Mfg. Inc., Kalida, OH, USA). The standard bed size in the research field is 1.2 m 60 m.
Both sites were managed according to the growers’ standard management systems for plot preparation, irrigation, and weed management. Black plastic mulch was used for tomatoes at both sites, applied just prior to planting and after the bed preparation was completed. This obviated the need for hand weeding and reduced soil transpiration. The spinach was grown without mulch and hand-weeded twice at each site. At both sites, drip irrigation was applied to maintain at least 2.5 cm rainfall weekly throughout the growing season. Water was applied via drip tape calibrated at 8.32 l × 100 m−1 at four-hour intervals. Irrigation was typically run 2–3 days per week and up to five days per week when no rainfall occurred.
At each site, one bed each of spinach (Spinacia oleracea, cvr. ‘Kolibri’) and tomato (Solanum lycopersicum, cvr. ‘Skyway’) was grown for use in the trial. Both crops were seeded in the greenhouse for later transplanting into the research plot. The spinach was grown with three rows per bed with 0.3 m between rows and 0.3 m between plants within the row, for a plant density of 11.1 plants·m−2. Tomatoes were planted in one single row per bed with 0.6 m between plants, for a total plant density of 1.4 plants·m−2. Spinach was transplanted on 4 May and 26 April for CCF and HREC sites, respectively. Tomatoes were transplanted on 24 May at CCF and on 23 June at HREC, with the later date selected in order to better align with the farm’s fall markets.
The primary variable in this study was the type and/or amount of fertilizer used to provide supplemental nutrition to each crop beyond that supplied by the soil (
Table 1). Wool pellets were sourced from Wild Valley Farms (Croyden, UT, USA). Both fertilizers were analyzed by the University of Maine Analytical Laboratory (Orono, ME) prior to developing treatments. The commercial fertilizer used in the grower standard treatments was Pro-Booster 10-0-0 (North Country Organics, Bradford, VT, USA). Pro-Booster is a mixed, pelleted fertilizer blend certified for use in organic production systems. It is derived from vegetable protein meals (such as alfalfa meal, cocoa meal, cottonseed meal, kelp meal, peanut meal, and soybean meal), animal protein meals (such as blood meal, crab meal, dried whey, feather meal, and fish meal), pasteurized poultry litter, and nitrate of soda [
32]. These sources, particularly nitrate of soda, which is a mined source of nitrate from Chile, as well as blood and poultry litter, are relatively quick-release forms of nitrogen, which contrasts this product from the slower-release nitrogen found in wool products [
5,
9,
30].
In addition to soil building through cover cropping and prior-season compost application, farm managers at both research sites use this product as their standard fertilizer to meet crop nitrogen needs beyond that provided through decomposition of soil organic matter (SOM).
For each crop, fertility treatments were applied based on either the growers’ standard nitrogen rate using both wool (WL-GS) and commercial fertilizer (GS), the wool pellet manufacturer’s recommended rate (WL-25), or no fertility was applied to the non-treated control (NTC) treatment (
Table 2). The wool pellet manufacturer’s recommended rate of 25 lbs of material per 100 feet of crop bed (11.3 kg × 30 m
−1) provides an equivalent of ~302 kg actual N·ha
−1 based on a bed width of 1 m, which is greater than typically recommended for either tomatoes or spinach [
5]. The site managers agreed upon a standard target rate of 130 kg N·ha
−1 for both crops and both sites, which is within standard regional recommendations [
5]. Because of variations in the analyzed nitrogen content compared to the advertised levels, the GS treatment received 129 kg·ha
−1, WL-GS received 138 kg, and 25,320 kg·ha
−1 of total N was applied to WL-25. These treatments and rates were selected to test (a) the performance of wool pellets vs. standard commercial fertilizers at typically-used field rates and (b) the performance of wool pellets and standard commercial fertilizers against a non-treated control that relied solely on soil nutrients to meet crop needs; and (c) performance of wool pellets at the manufacturer’s recommended rates vs. standard nitrogen rates supplied by either wool pellets or commercial fertilizer.
Immediately prior to applying plastic mulch, the fertilizer treatments were applied to the plots. The fertilizer was incorporated mechanically after application with a BCS power harrow attachment. Each crop was grown in a single bed at each site, and experimental treatments were assigned in a randomized complete block design with blocking sequential down the length of the row. The treatments were applied to a 3.3 m length of the bed, which represented the experimental plot and broadcast by hand across the width of the bed. The total area for each plot was 4 m2. Each plot was separated by 1 m from the next adjacent plot to avoid the contamination of fertilizer treatments between plots. Each treatment was replicated four times at each site, for a total of 16 plots (four treatments × four replications each).
2.2. Soil Testing
Within each experimental spinach plot, five 2.5 cm × 15 cm soil cores were collected on the day of harvest, combined, and mixed into a single composite sample. The soil was collected by sweeping away the organic material on the immediate surface, then inserting the probe 15 cm into the soil surface. That core was collected and added to the composite sample for the plot. The soil samples were weighed on the day of collection, then held in mesh bags in a drying oven at 60 °C for one week until dry. The samples were then submitted to the University of Maine Analytical Lab (Orono, ME, USA) for chemical analysis. The soil organic matter was measured through the loss of weight on ignition and the mineral nutrients were measured using a modified Morgan’s extraction method [
33].
2.3. Crop Yield and Quality
2.3.1. Spinach Yield
Upon maturity, all of the spinach was harvested by cutting all leaves at the soil line. Two harvest measurements were collected. First, ten plants were harvested from the center of the plot, and the total number of leaves per plant was counted and weighed on a field scale to the closest 0.1 g and placed in a labeled paper bag for subsequent analysis. The second harvest collection comprised the remaining plants in the central 1 m section of the bed. All of the plants were harvested and weighed on a field scale to the nearest 0.1 g, and the two harvest weights were added together to calculate the total harvest yield (g·m−2).
2.3.2. Spinach Tissue Analysis
Spinach leaves collected from the five-plant samples were dried in their paper bags in a drying oven at 60 °C for one week. After drying, the sample was re-weighed to determine the dry matter weight. The dried plant tissues were ground and submitted to the University of Vermont Agriculture and Environmental Testing Laboratory (Burlington, VT, USA) for an analysis of nitrogen, phosphorus, potassium, calcium, magnesium, boron, zinc, manganese, copper, aluminum, and iron content. The total N was determined using the Dumas standard combustion [
34]. All of the other nutrients were measured by dry ashing [
35]. Solution analysis was by plasma emission.
2.3.3. Tomato Yield
Tomato yield data were only collected at the HREC site. Each week, beginning 1 September and continuing for six weeks, all ripe fruit, determined by visual assessment for red color, were harvested from the three plants in the middle of each experimental plot. The fruit were individually weighed to the nearest 0.1 g. On the final harvest date of 6 October, all of the fruit were stripped from the plant, and the assessment also included ripe and unripe values based on fruit color. All of the observations were completed by the same research assistant in order to minimize the variation among subjective parameters. Immediately after the final harvest, the three plants from the center of each experimental field were cut at soil level and placed in labeled paper bags. Where necessary, the plants were cut into pieces to fit into one bag. Aboveground plant material was held in a drying oven at 60 °C for one week until fully dried, then weighed to the nearest 0.1 g.
2.3.4. Tomato Quality and Juice Analysis
Following weighing, the fruit were visually evaluated for splitting, the incidence of rot, and grade. The grades assigned included 1st grade (no blemishes), 2nd grade (minor blemishes but fruit skin not compromised nor rot present), and cull (rotten, cracked, or severely disfigured fruit). Five ripe fruit for each experimental plot from the final harvest were selected randomly for juice analysis. The fruit were processed into one bulk sample per experimental plot with a Breville Juice Fountain Elite juicer (Breville USA, Torrance, CA, USA). The juice samples were frozen until evaluation, then thawed and centrifuged prior to testing. The samples were analyzed for their sugar content (soluble solids (SS), ° brix) using a PEN refractometer (Atago, Bellevue, WA, USA), pH with a digital pH meter (Accumet AB15, Fisher Scientific, Waltham, MA, USA), and titratable acidity (TA, citric acid equivalent) by titrating with 0.1 M of sodium hydroxide to pH 8.2. The total polyphenols (tannin) were assessed by enzymatic assay (Total Phenols UniFLEX kit, Unitech Scientific, Hawaiian Gardens, CA, USA) and analysis by spectrophotometer (Hach DR 6000, Loveland, CO, USA). All of the methods used standard procedures for juice evaluation [
36].
2.3.5. Tomato Plant Dry Matter Weight
Immediately after the final fruit harvest, tomato plants were cut at ground level and removed. Plants were placed in paper bags and held in a walk-in drying oven at 60 °C for one week until fully dried, then weighed to the nearest 0.1 g.
2.4. Statistical Analysis
The data were statistically analyzed using JMP 15.0 (SAS Institute, Cary, NC, USA). Where multiple observations were made for each treatment replicate, data were tabulated to generate a mean of means or summed when appropriate (e.g., to determine tomato yield) for each replicate, and statistics were completed on the summarized dataset. All of the datasets analyzed for the analysis of variance contained sixteen observations or calculated values per parameter, corresponding to four replicates of each of the four fertilizer treatments applied. For all analyses, a one-way ANOVA was completed, with the fertilizer treatment as a fixed effect and the parameter of interest as a dependent variable. If the overall F-test in the ANOVA was below an α error of 0.05, post-hoc means comparisons for each treatment were conducted using Tukey’s adjustment to maintain an overall α = 0.05.
4. Discussion
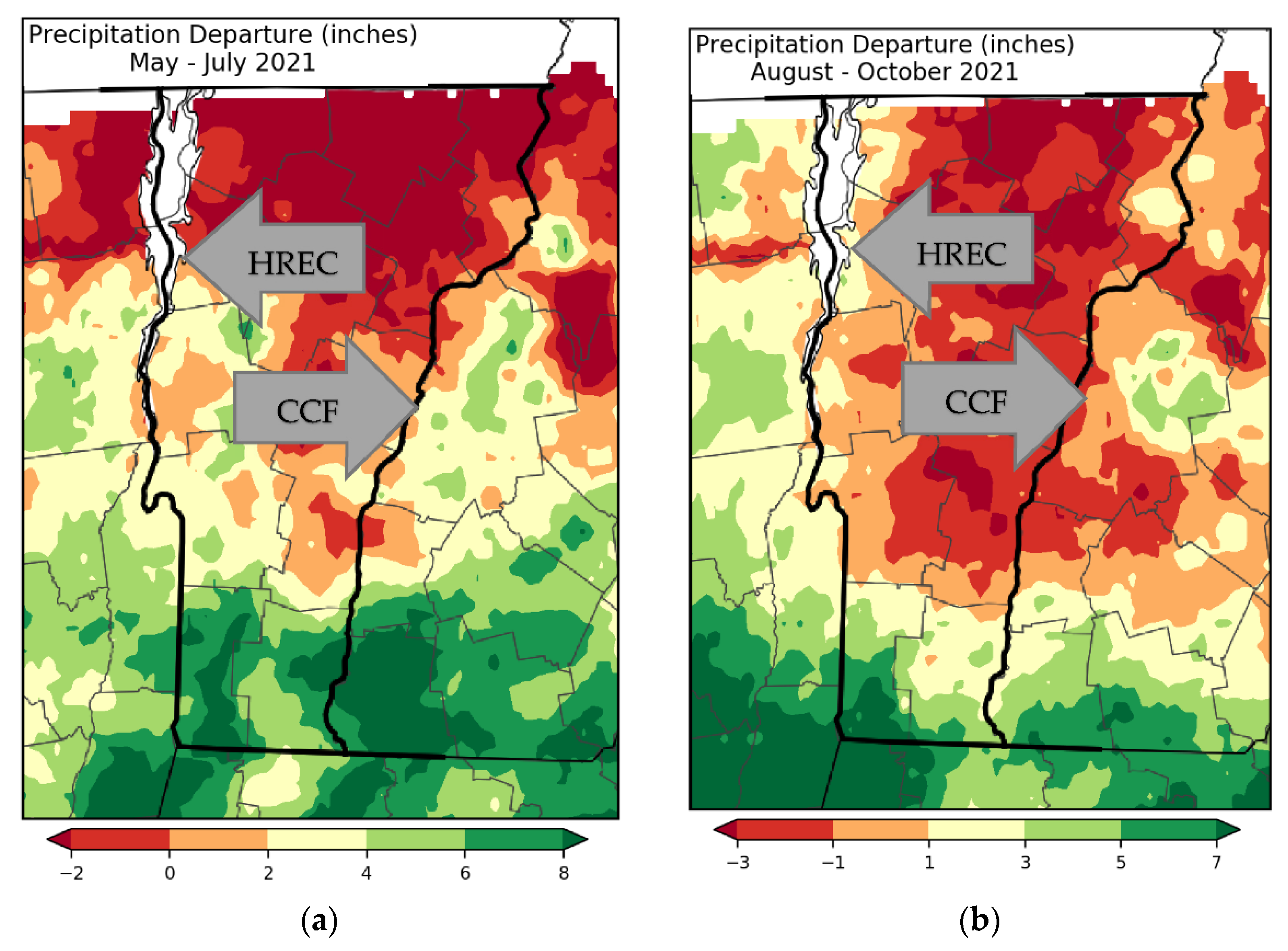
General growing conditions between the two sites, including weather and soil factors, were not particularly different, save for two important considerations. First, SOM at CC was approximately twice that observed at HREC. This is an important consideration in that plant-available nitrogen is released during the decomposition of organic matter, and thus, native fertility prior to fertilizer treatment was greater overall for crops grown at CC compared to HREC. While the experimental design of this trial does not lend itself to direct comparisons between the two study sites, this factor likely explains the differences in magnitude for yield at each site. Another primary difference among the sites is the level of phosphorus in the soil. HREC has high phosphorus (35–43 ppm) owing to legacy applications of dairy manure that were performed for decades. This level is above the 20 ppm that triggers regulation under Vermont Required Agriculture Practices regulations [
38]. Soil phosphorus at CC (4.7–8.1 ppm) was below regulatory thresholds and is still at an optimum level for vegetable production in the region [
5]. Within each site, there were no differences in soil chemistry due to the application of any fertilizer treatment, save for aluminum at CC, which is not an essential plant nutrient, and which was below toxicity levels at both sites [
5].
Statistically significant differences in spinach yield among the treatments were few, but the design of the experiment and general soil conditions may not have been conducive to detecting differences. At HREC, the mean spinach yields from GS and WL-25 were 61% and 47% greater than NTC, despite no differences detected. The magnitude of these differences would be economically important, and an experiment with a greater number of replications may detect differences among these treatments’ populations. All of the fertility treatments ranked higher than NTC, with an overall mean increase of 47–149% in yield over the non-fertilized treatment.
At CC, where SOM was roughly twice that for HREC across all treatments, no statistically significant differences in spinach yield were observed, and the range of means was smaller than at HREC. The NTC treatment at CC ranked only second-lowest after WL-GS. However, the GS treatment had twice the observed yield of the WL-GS, despite both of those treatments being adjusted for equal nitrogen application. This is likely due to the short season for spinach production at both sites, with 41 and 43 days of field growth from transplanting to harvest for HREC and CC, respectively. Raw wool is a relatively slow-release nitrogen material that requires microbial degradation in order to convert complex proteins and other nitrogen-containing compounds into plant-available forms [
39]. At CC, the base soil fertility may have been sufficient to provide adequate nitrogen for plant growth even without fertilization, but the lower SOM at HREC likely provided limited nitrogen that prevented adequate crop growth without supplemental fertilization.
Increased soil temperature would likely result in a greater nitrogen release from wool pellets. Soil temperature readings were collected at each site at different times in the season and are not comparable to one another; however, the plots at HREC were protected with row cover to prevent insect damage, and those at CC were not. This would suggest that temperatures may have been warmer at HREC, which could have contributed to increased nitrogen mineralization from the WL treatments. Despite a lack of statistical difference between NTC and WL-GS, there was a dose-response observed in the rankings at HREC, where the amount of nitrogen applied correlated with the crop yield. At CC, cooler temperatures owing to the lack of row cover compared to HREC may have allowed for less nitrogen mineralization from the wool pellets, which would explain the lower relative spinach yield for the lower-nitrogen WL-GS treatment, despite the lack of statistically significant results.
Laboratory analysis of fertilizer samples showed similar levels of many nutrients. Nitrogen, calcium, magnesium, boron, manganese, and zinc were within 40% of one another among the two products; wool pellets had higher levels of carbon, potassium, and sodium but less phosphorus than Pro-Booster. The effects of the fertility treatments on the mineral nutrient uptake into plant tissues in spinach were inconsistent. At CC, only potassium was affected by fertility treatment, with WL-25 having the greatest foliar potassium compared to GS. There was no dose-response evident, although the fertility treatments were only calculated and ranked by the amount of applied nitrogen. NTC plants were intermediate in potassium, above WL-GS and GS treatments, and below WL-25 but not statistically significant from any other. More differences were observed at HREC, which lends support to the hypothesis that the sandier, lower SOM soil there would respond more easily to fertility treatments. However, as in the past example, there is no clear indication that increasing levels of fertilizer treatment increased most mineral nutrients in plant tissues. The primary nutrient that was affected in a clear dose-response manner is nitrogen, which was present at HREC at greater levels in leaves that received greater amounts of wool fertilizer. This suggests greater mineralization and/or uptake of nitrogen from wool sources compared to standard commercial organic fertilizer. Unfortunately, soil mineral nitrogen was not measured in this experiment, which substantially limits the interpretation of these results.
For many other nutrients, the levels in spinach leaf tissue were lower for the wool treatments than for either the NTC or GS treatments. Though not consistently statistically lower, observed differences in magnesium, phosphorus, boron, and calcium compared to NTC may indicate tie-up in the soil due to microbes actively digesting and degrading the wool pellets. An evaluation of changes in the soil chemistry, and subsequent differences in plant-available nutrients, would likely require a longer-term experiment than conducted here. For example, Suruchi et al. (2014) found increased nutrient levels in soil and plant tissues with the application of raw wool when measured over multiple growing seasons [
24], and Voncina et al. (2014) detected nitrogen release for two years following the application of raw wool to asparagus [
25]. In a study of flower crops grown in Nova Scotia, Canada, the application of wool increased the crop yield over the next two seasons by 1.7 to 3.5 times, and soil and plant-tissue nitrogen increases were recorded for that time as well [
40].
Tomatoes served an important but limited purpose in this trial. Because one site was lost to pest pressure, this crop was not replicated beyond one limited trial. This limits these data to only providing supporting evidence of the better-replicated spinach trial, but any conclusions drawn about the effect of wool fertilizers on tomatoes should be preliminary. In this experiment, tomatoes served as a longer-season crop than spinach, and a positive effect of fertilization was observed on their crop yield and plant growth. Similar to spinach at HREC, there was a dose-response observed with the increasing nitrogen application, although only the NTC and WL-25 treatments were statistically different from one another. It is important to note that when the target nitrogen application of 130 kg × ha
−1 was applied, the wool pellets were comparable to standard commercial fertilizer. This is similar to the results seen with spinach, at least at HREC, where there was no difference between the nitrogen-dose equivalent GS and the WL-GS treatments for crop yield. In tomato, the crop yield did not appear to be a function primarily of fruit number, as there were no statistical differences among treatments for that parameter, but all fertilized treatments did have 25–35% more fruit than the NTC. Fruit size was likely a significant determinant in total crop yield, and WL-GS had a larger fruit weight than both non-wool treated treatments, and WL-25 ranked second-largest but was not different from any other treatment. An effect on fruit size attributable to the application of wool pellets was not observed by Ordiales, et al. [
27], but many prior studies have shown improvement in tomato fruit size with increased quantity and consistency of soil moisture [
41,
42,
43,
44]. Past studies of wool as both a growing substrate and as a soil additive have shown improved soil moisture retention [
18,
20]. Soil moisture data were collected in this experiment, but the equipment used was unreliable, so the results are not included in this paper. Further study should include the meticulous collection of soil moisture data, either continuously via electroconductivity-based soil moisture meters or irrometers, to better elucidate the effects of wool pellets on the soil moisture conditions.
In contrast to crop yield and fruit size, tomato plant growth, as measured by dry matter weight of aboveground plants post-harvest, was increased by commercial fertilizer in the GS treatment compared to the NTC and the wool treatments were intermediate. As was discussed above, many of the nitrogen courses included in Pro-Booster are rapidly-available forms, including natural soda nitrate, which is chemically similar to many fast-acting synthetic nitrogen sources. Future research trials evaluating wool pellet fertilizers must include mineralizable soil nitrogen and nitrogen uptake in order to best test the availability of nitrogen from wool pellet fertilizers to plants. A common consideration in plant production is that nitrogen fertilization favors vegetative growth over fruit production, and that mantra appears to be supported by the data in this trial. Increased or excess nitrogen is also associated with decreased fruit quality [
45,
46,
47,
48], but there were no differences observed in this study for important fruit quality parameters on tomato, including fruit splitting, rotting, grade distribution, or juice chemistry (SS, pH, TA), and continued research is warranted to verify the observations made in this study. The total polyphenols in juice samples were affected by the fertilizer treatments, with the highest levels in NTC and the lowest in WL-25, and the other treatments were intermediate. Polyphenols are a large category of complex phytochemicals, including nutraceutical [
49] and plant protective compounds [
50,
51]. Increased polyphenol content is generally associated with mild plant stress [
52], and in tomato specifically, increased soil nitrogen is associated with decreased polyphenol production [
53,
54]. This is congruent with the results observed in this study and is worth consideration for continued research on crops where polyphenols are an important component of fruit quality, e.g., tomato, apple, grape, etc., for nutraceutical, flavor, or wine quality considerations.