Vascular and Transpiration Flows Affecting Apricot (Prunus armeniaca L.) Fruit Growth
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Environment Data
2.2. Fruit and Shoot Seasonal Growth
2.3. Water Relations
2.4. Leaf Gas Exchanges
2.5. Xylem, Phloem and Transpiration Flows
2.6. Sap Flow Measurements
2.7. Fruit Surface Conductance and Dry Matter Content
2.8. Statistical Analysis
3. Results
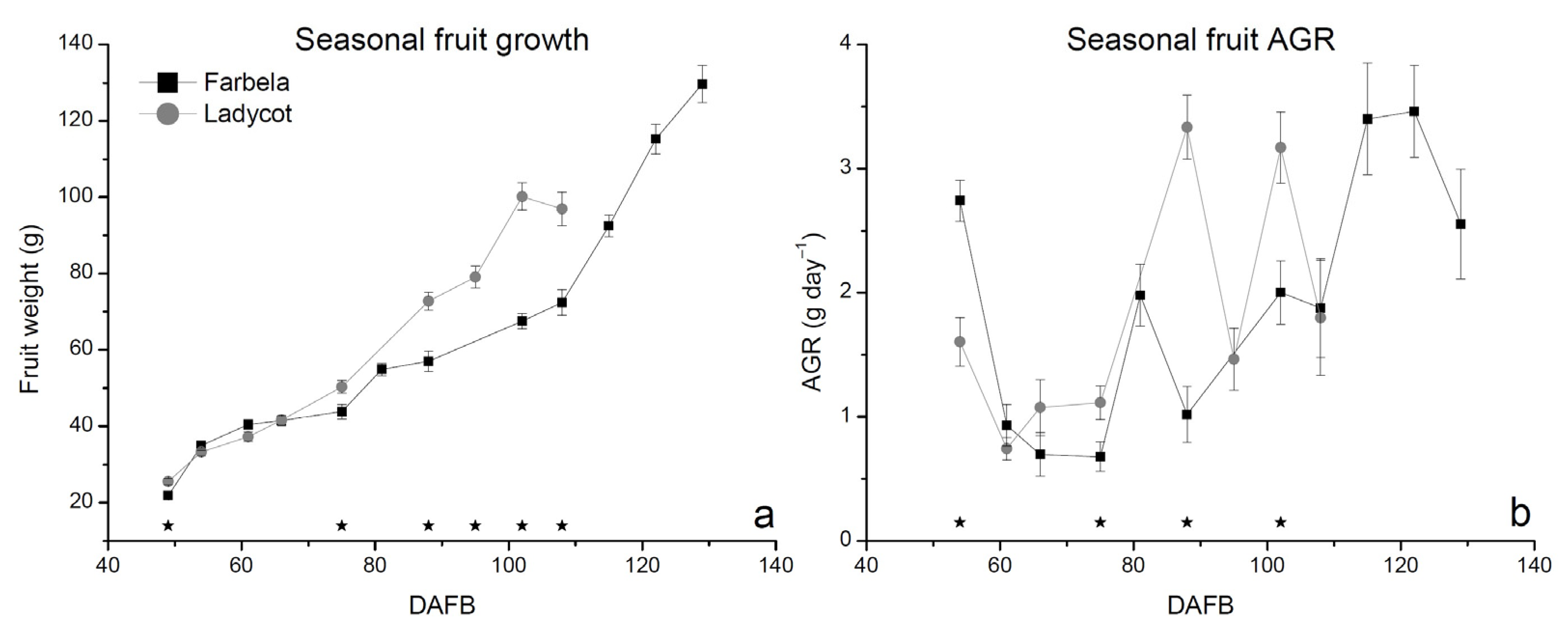
3.1. Fruit and Shoot Seasonal Growth
3.2. Water Relations
3.3. Leaf Gas Exchanges
3.4. Fruit Surface Conductance and Dry Matter
3.5. Sap Flow
3.6. Seasonal Vascular and Transpiration Flows to/from the Fruit
3.7. Daily Vascular and Transpiration Flows to/from the Fruit
4. Discussion
5. Conclusions
- (1)
- In Farbela, leaves and fruit tended to import water at different times during the day. Leaves were “stronger sinks” for water during the morning. However, the fruit became a “stronger sink” during the afternoon, thanks to their high transpiration, which likely reduced fruit turgor pressure and allowed higher xylem flow towards them. Furthermore, midday phloem inflow peaks to the fruit could have led to a further increase in xylem flow in the afternoon because of an accumulation of osmotically active solutes. As a result, fruit growth occurred mainly during the late afternoon and night;
- (2)
- In the early and mid-stages, the fruit growth strategy of Farbela was based on high water flows to and from the fruit. Transpiration and xylem flow decreased from the mid-stages but maintained relatively high values until harvest. In contrast, phloem flow became more important in the very last stages. Vascular flow showed the possibility of both symplasmic and apoplasmic phloem unloading; high water losses by transpiration could favour passive phloem unloading, while the recent report of several sugar transport genes in apricot suggests the simultaneous presence of an active phloem unloading as well.
- (3)
- Despite the sap flow and fruit growth dynamics being monitored only in Farbela, Ladycot showed similar water relations and leaf gas exchanges throughout the season, despite differences in the shoot length. Moreover, the relatively high values of Ladycot fruit surface conductance led to the hypothesise that vascular flow dynamics in this cultivar might not be too different from those of Farbela.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Badenes, M.L.; Byrne, D.H. Fruit Breeding; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; ISBN 9781441907639. [Google Scholar]
- Breen, K.C.; Tustin, D.S.; van Hooijdonk, B.M.; Stanley, C.J.; Scofield, C.; Wilson, J.M.; Oliver, M.J.; Dayatilake, G.A. Use of physiological principles to guide precision orchard management and facilitate increased yields of premium quality fruit. Acta Hortic. 2021, 1314, 241–252. [Google Scholar] [CrossRef]
- Perez-Sarmiento, F.; Alcobendas, R.; Mounzer, O.; Alarcon, J.; Nicolas, E. Effects of regulated deficit irrigation on physiology and fruit quality in apricot trees. Span. J. Agric. Res. 2010, 8, 86–94. [Google Scholar] [CrossRef]
- Pérez-Sarmiento, F.; Mirás-Avalos, J.M.; Alcobendas, R.; Alarcón, J.J.; Mounzer, O.; Nicolás, E. Effects of regulated deficit irrigation on physiology, yield and fruit quality in apricot trees under mediterranean conditions. Span. J. Agric. Res. 2016, 14, 28. [Google Scholar] [CrossRef]
- Morandi, B.; Rieger, M.; Grappadelli, L.C. Vascular flows and transpiration affect peach (Prunus persica Batsch.) fruit daily growth. J. Exp. Bot. 2007, 58, 3941–3947. [Google Scholar] [CrossRef] [PubMed]
- Blanke, M.M.; Lenz, F. Fruit photosynthesis. Plant. Cell Environ. 1989, 12, 31–46. [Google Scholar] [CrossRef]
- Münch, E. Die Stoffbewegungen in der Pflanze (Gustav Fischer, Jena). Curr. Opin. Plant Biol. 1930, 43, 36–42. [Google Scholar]
- Minchin, P.E.H.; Thorpe, M.R. Is Phloem Transport Due to a Hydrostatic Pressure Gradient? Supporting Evidence from Pressure Chamber Experiments. Funct. Plant Biol. 1987, 14, 397–402. [Google Scholar] [CrossRef]
- Minchin, P.E.H.; Thorpe, M.R. What determines carbon partitioning between competing sinks? J. Exp. Bot. 1996, 47, 1293–1296. [Google Scholar] [CrossRef]
- Patrick, J.W. Sieve element unloading: Cellular pathway, mechanism and control. Physiol. Plant. 1990, 78, 298–308. [Google Scholar] [CrossRef]
- Patrick, J.W. Phloem Unloading: Sieve Element Unloading and Post-Sieve Element Transport. Annu. Rev. Plant Biol. 1997, 48, 191–222. [Google Scholar] [CrossRef]
- Lalonde, S.; Tegeder, M.; Throne-Holst, M.; Frommer, W.B.; Patrick, J.W. Phloem loading and unloading of sugars and amino acids. Plant Cell Environ. 2003, 26, 37–56. [Google Scholar] [CrossRef]
- Jones, H.G.; Higgs, K.H. Surface conductance and water balance of developing apple (Malus pumila mill.) fruits. J. Exp. Bot. 1982, 33, 67–77. [Google Scholar] [CrossRef]
- Lescourret, F.; Génard, M.; Habib, R.; Fishman, S. Variation in surface conductance to water vapor diffusion in peach fruit and its effects on fruit growth assessed by a simulation model. Tree Physiol. 2001, 21, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Gibert, C.; Lescourret, F.; Génard, M.; Vercambre, G.; Pérez Pastor, A. Modelling the effect of fruit growth on surface conductance to water vapour diffusion. Ann. Bot. 2005, 95, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Fishman, S.; Génard, M. A biophysical model of fruit growth: Simulation of seasonal and diurnal dynamics of mass. Plant Cell Environ. 1998, 21, 739–752. [Google Scholar] [CrossRef]
- Morandi, B.; Losciale, P.; Manfrini, L.; Zibordi, M.; Anconelli, S.; Pierpaoli, E.; Corelli Grappadelli, L. Leaf gas exchanges and water relations affect the daily patterns of fruit growth and vascular flows in Abbé Fétel pear (Pyrus communis L.) trees. Sci. Hortic. 2014, 178, 106–113. [Google Scholar] [CrossRef]
- Morandi, B.; Manfrini, L.; Losciale, P.; Zibordi, M.; Corelli Grappadelli, L. Changes in vascular and transpiration flows affect the seasonal and daily growth of kiwifruit (Actinidia deliciosa) berry. Ann. Bot. 2010, 105, 913–923. [Google Scholar] [CrossRef]
- Mantell, A.; Goldshmidt, E.E.; Monselise, S.P. Turnover of tritiated water in calamondin plants [Citrus madurensis]. J. Am. Soc. Hortic. Sci. 1980, 105, 741–744. [Google Scholar]
- Huang, X.-M.; Huang, H.-B.; Gao, F.-F. The growth potential generated in citrus fruit under water stress and its relevant mechanisms. Sci. Hortic. 2000, 83, 227–240. [Google Scholar] [CrossRef]
- Lang, A. Xylem, phloem and transpiration flows in developing apple fruit. J. Exp. Bot. 1990, 41, 645–651. [Google Scholar] [CrossRef]
- Bondada, B.R.; Matthews, M.A.; Shackel, K.A. Functional xylem in the post-veraison grape berry. J. Exp. Bot. 2005, 56, 2949–2957. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.; Smith, J.P.; Bondada, B.R. Ripening grape berries remain hydraulically connected to the shoot. J. Exp. Bot. 2006, 57, 2577–2587. [Google Scholar] [CrossRef] [PubMed]
- Brüggenwirth, M.; Winkler, A.; Knoche, M. Xylem, phloem, and transpiration flows in developing sweet cherry fruit. Trees Struct. Funct. 2016, 30, 1821–1830. [Google Scholar] [CrossRef]
- Zhang, X.; Schmidt, R.E. The impact of growth regulators on the a-tocopherol status in water-stressed Poapratensis L. Int. Turfgrass Soc. Res. J. 1997, 8, 1364–1373. [Google Scholar]
- Hallett, I.C.; Sutherland, P.W. Structure and Development of Kiwifruit Skins; The University of Chicago Press: Chicago, IL, USA, 2014; Volume 166, pp. 693–704. [Google Scholar]
- Montanaro, G.; Dichio, B.; Xiloyannis, C.; Celano, G. Light influences transpiration and calcium accumulation in fruit of kiwifruit plants (Actinidia deliciosa var. deliciosa). Plant Sci. 2006, 170, 520–527. [Google Scholar] [CrossRef]
- Li, S.H.; Génard, M.; Bussi, C.; Lescourret, F.; Laurent, R.; Besset, J.; Habib, R. Preliminary study on transpiration of peaches and nectarines. Gartenbauwissenschaft 2002, 67, 39–43. [Google Scholar]
- Wu, B.H.; Génard, M.; Kervella, J.; Li, S.H.; Laurent, R. Relationship between skin speckle, soluble solids content and transpiration rate in nectarines. Eur. J. Hortic. Sci. 2003, 68, 83–85. [Google Scholar]
- Montanaro, G.; Dichio, B.; Xiloyannis, C. Significance of fruit transpiration on calcium nutrition in developing apricot fruit. J. Plant Nutr. Soil Sci. 2010, 173, 618–622. [Google Scholar] [CrossRef]
- Iqbal, S.; Ni, X.; Bilal, M.S.; Shi, T.; Khalil-ur-Rehman, M.; Zhenpeng, P.; Jie, G.; Usman, M.; Gao, Z. Identification and expression profiling of sugar transporter genes during sugar accumulation at different stages of fruit development in apricot. Gene 2020, 742, 144584. [Google Scholar] [CrossRef]
- Turner, N.; Long, M. Errors Arising From Rapid Water Loss in the Measurement of Leaf Water Potential by the Pressure Chamber Technique. Funct. Plant Biol. 1980, 7, 527. [Google Scholar] [CrossRef]
- McCutchan, H.; Shackel, K.A. Stem-water Potential as a Sensitive Indicator of Water Stress in Prune Trees (Prunus domestica L. cv. French). J. Am. Soc. Hortic. Sci. 1992, 117, 607–611. [Google Scholar] [CrossRef]
- Naor, A.; Klein, I.; Doron, I. Stem water potential and apple size. J. Am. Soc. Hortic. Sci. 1995, 120, 577–582. [Google Scholar] [CrossRef]
- Morandi, B.; Manfrini, L.; Zibordi, M.; Noferini, M.; Fiori, G.; Grappadelli, L.C. A low-cost device for accurate and continuous measurements of fruit diameter. HortScience 2007, 42, 1380–1382. [Google Scholar] [CrossRef]
- Sakuratani, T. A Heat Balance Method for Measuring Water Flux in the Stem of Intact Plants. J. Agric. Meteorol. 1981, 37, 9–17. [Google Scholar] [CrossRef]
- Peressotti, A.; Ham, J.M. A dual-heater gauge for measuring sap flow with an improved heat-balance method. Agron. J. 1996, 88, 149–155. [Google Scholar] [CrossRef]
- Morandi, B.; Manfrini, L.; Lugli, S.; Tugnoli, A.; Boini, A.; Perulli, G.D.; Bresilla, K.; Venturi, M.; Corelli Grappadelli, L. Sweet cherry water relations and fruit production efficiency are affected by rootstock vigor. J. Plant Physiol. 2019, 237, 43–50. [Google Scholar] [CrossRef]
- Greenspan, M.D.; Shackel, K.A.; Matthews, M.A. Developmental changes in the diurnal water budget of the grape berry exposed to water deficits. Plant. Cell Environ. 1994, 17, 811–820. [Google Scholar] [CrossRef]
- Montanaro, G.; Dichio, B.; Xiloyannis, C.; Lang, A. Fruit transpiration in kiwifruit: Environmental drivers and predictive model. AoB Plants 2012, 2012, pls036. [Google Scholar] [CrossRef]
- Corelli Grappadelli, L.; Morandi, B.; Manfrini, L.; O’Connell, M. Apoplasmic and simplasmic phloem unloading mechanisms: Do they co-exist in Angeleno plums under demanding environmental conditions? J. Plant Physiol. 2019, 237, 104–110. [Google Scholar] [CrossRef]
- Knoche, M.; Peschel, S.; Hinz, M.; Bukovac, M.J. Studies on water transport through the sweet cherry fruit surface: Characterizing conductance of the cuticular membrane using pericarp segments. Planta 2000, 212, 127–135. [Google Scholar] [CrossRef]
- Dražeta, L.; Lang, A.; Hall, A.J.; Volz, R.K.; Jameson, P.E. Causes and effects of changes in xylem functionality in apple fruit. Ann. Bot. 2004, 93, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Mazzeo, M.; Dichio, B.; Clearwater, M.J.; Montanaro, G.; Xiloyannis, C. Hydraulic resistance of developing Actinidia fruit. Ann. Bot. 2013, 112, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Carbone, K.; Ciccoritti, R.; Paliotta, M.; Rosato, T.; Terlizzi, M.; Cipriani, G. Chemometric classification of early-ripening apricot (Prunus armeniaca, L.) germplasm based on quality traits, biochemical profiling and in vitro biological activity. Sci. Hortic. 2018, 227, 187–195. [Google Scholar] [CrossRef]
- Alarcón, J.J.; Domingo, R.; Green, S.R.; Sánchez-Blanco, M.J.; Rodríguez, P.; Torrecillas, A. Sap flow as an indicator of transpiration and the water status of young apricot trees. Plant Soil 2000, 227, 77–85. [Google Scholar] [CrossRef]
- Morandi, B.; Manfrini, L.; Losciale, P.; Zibordi, M.; Corelli-Grappadelli, L. The positive effect of skin transpiration in peach fruit growth. J. Plant Physiol. 2010, 167, 1033–1037. [Google Scholar] [CrossRef] [PubMed]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giovannini, A.; Venturi, M.; Gutiérrez-Gordillo, S.; Manfrini, L.; Corelli-Grappadelli, L.; Morandi, B. Vascular and Transpiration Flows Affecting Apricot (Prunus armeniaca L.) Fruit Growth. Agronomy 2022, 12, 989. https://doi.org/10.3390/agronomy12050989
Giovannini A, Venturi M, Gutiérrez-Gordillo S, Manfrini L, Corelli-Grappadelli L, Morandi B. Vascular and Transpiration Flows Affecting Apricot (Prunus armeniaca L.) Fruit Growth. Agronomy. 2022; 12(5):989. https://doi.org/10.3390/agronomy12050989
Chicago/Turabian StyleGiovannini, Andrea, Melissa Venturi, Saray Gutiérrez-Gordillo, Luigi Manfrini, Luca Corelli-Grappadelli, and Brunella Morandi. 2022. "Vascular and Transpiration Flows Affecting Apricot (Prunus armeniaca L.) Fruit Growth" Agronomy 12, no. 5: 989. https://doi.org/10.3390/agronomy12050989
APA StyleGiovannini, A., Venturi, M., Gutiérrez-Gordillo, S., Manfrini, L., Corelli-Grappadelli, L., & Morandi, B. (2022). Vascular and Transpiration Flows Affecting Apricot (Prunus armeniaca L.) Fruit Growth. Agronomy, 12(5), 989. https://doi.org/10.3390/agronomy12050989









