Soil Waterlogging Conditions Affect Growth, Water Status, and Chlorophyll “a” Fluorescence in Coffee Plants (Coffea arabica L.)
Abstract
:1. Introduction
2. Materials and Methods
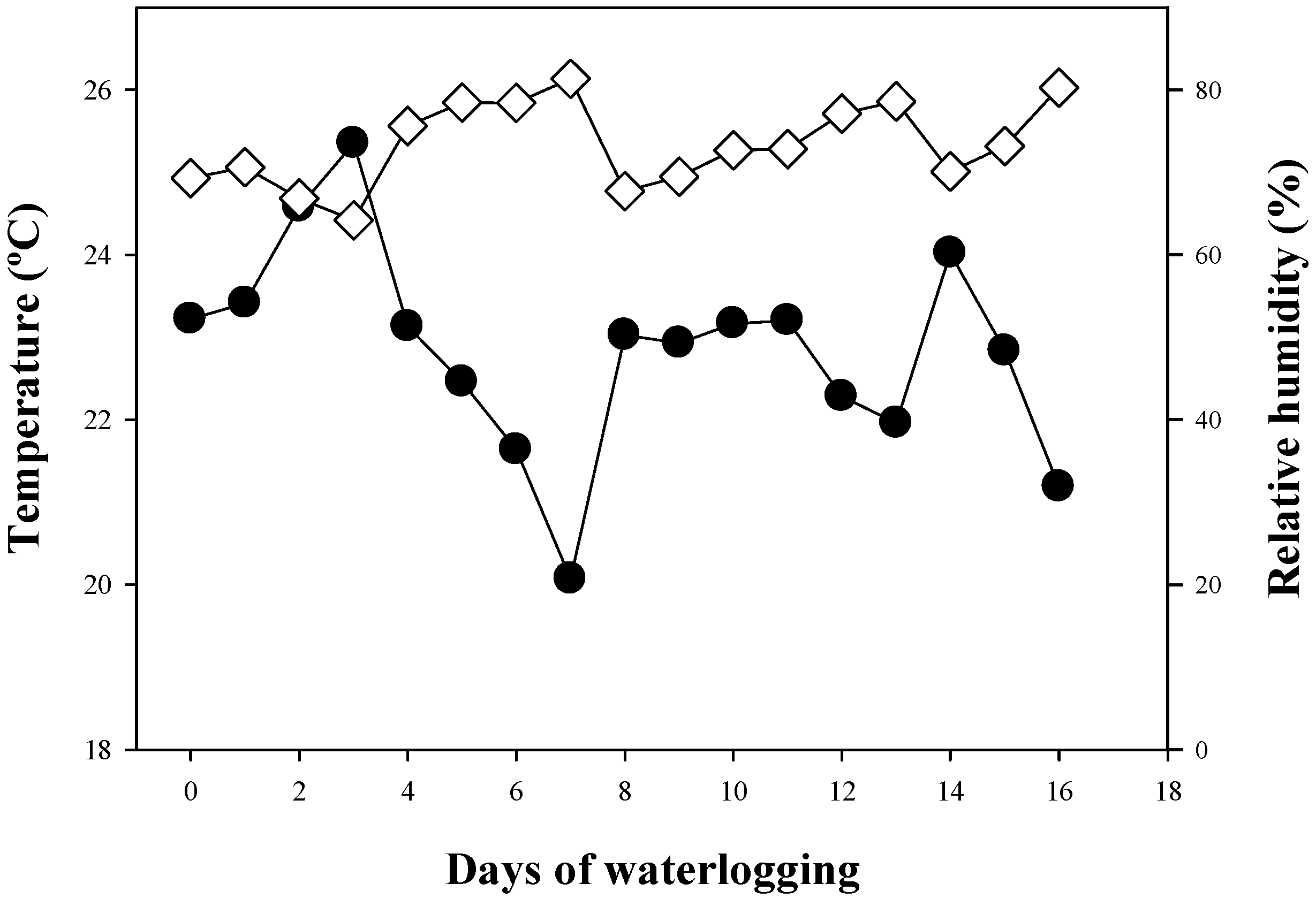
2.1. Plant Material, Growth Conditions, and Treatments
2.2. Growth Measurements
2.3. Physiological Measurements
2.3.1. Plants Water Relations
2.3.2. Chlorophyll “a” Fluorescence Parameters
2.4. Experiment Design and Statistical Analysis of Data
3. Results
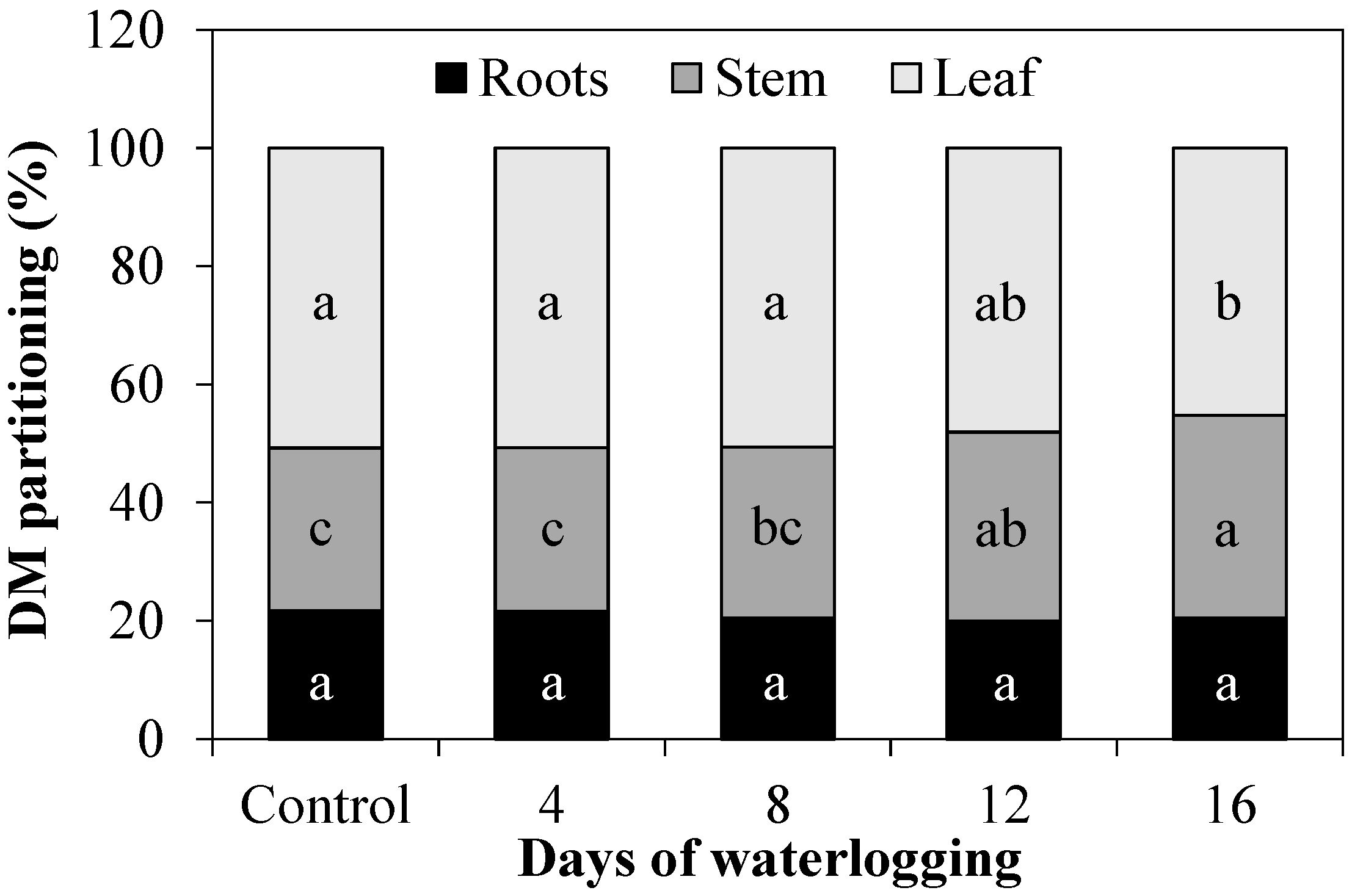
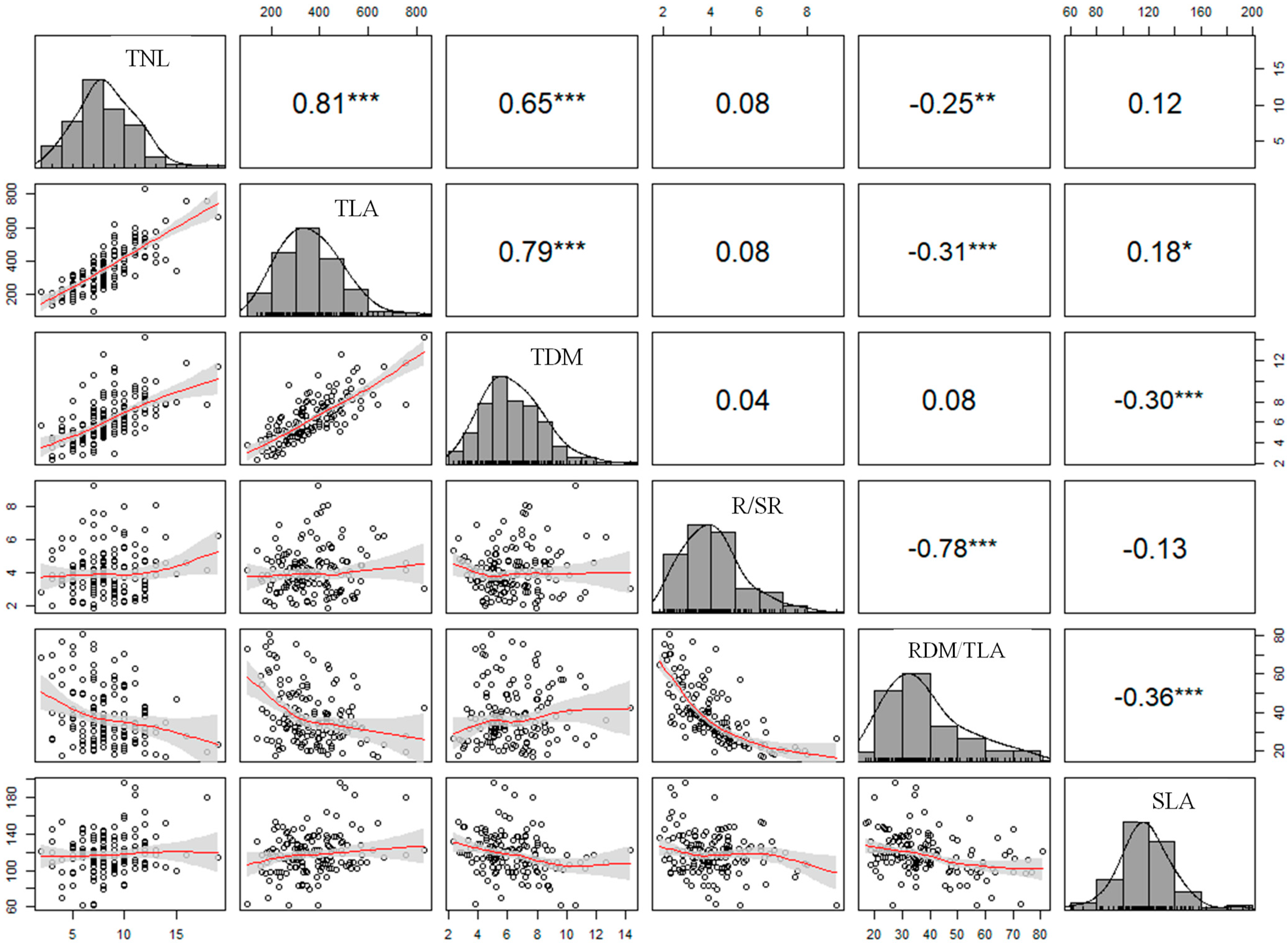
3.1. Growth Parameters and Symptoms
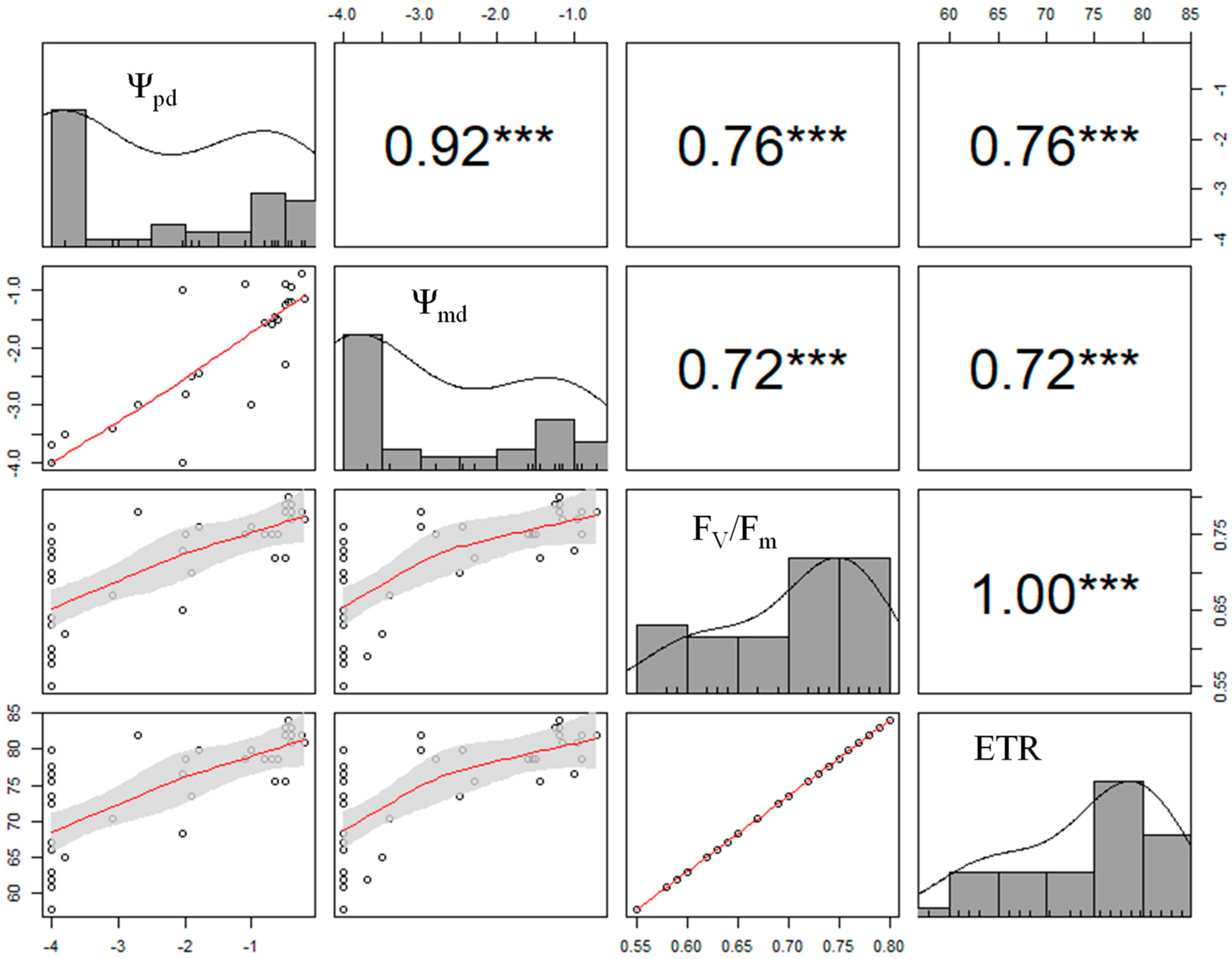
3.2. Plant Water Relations
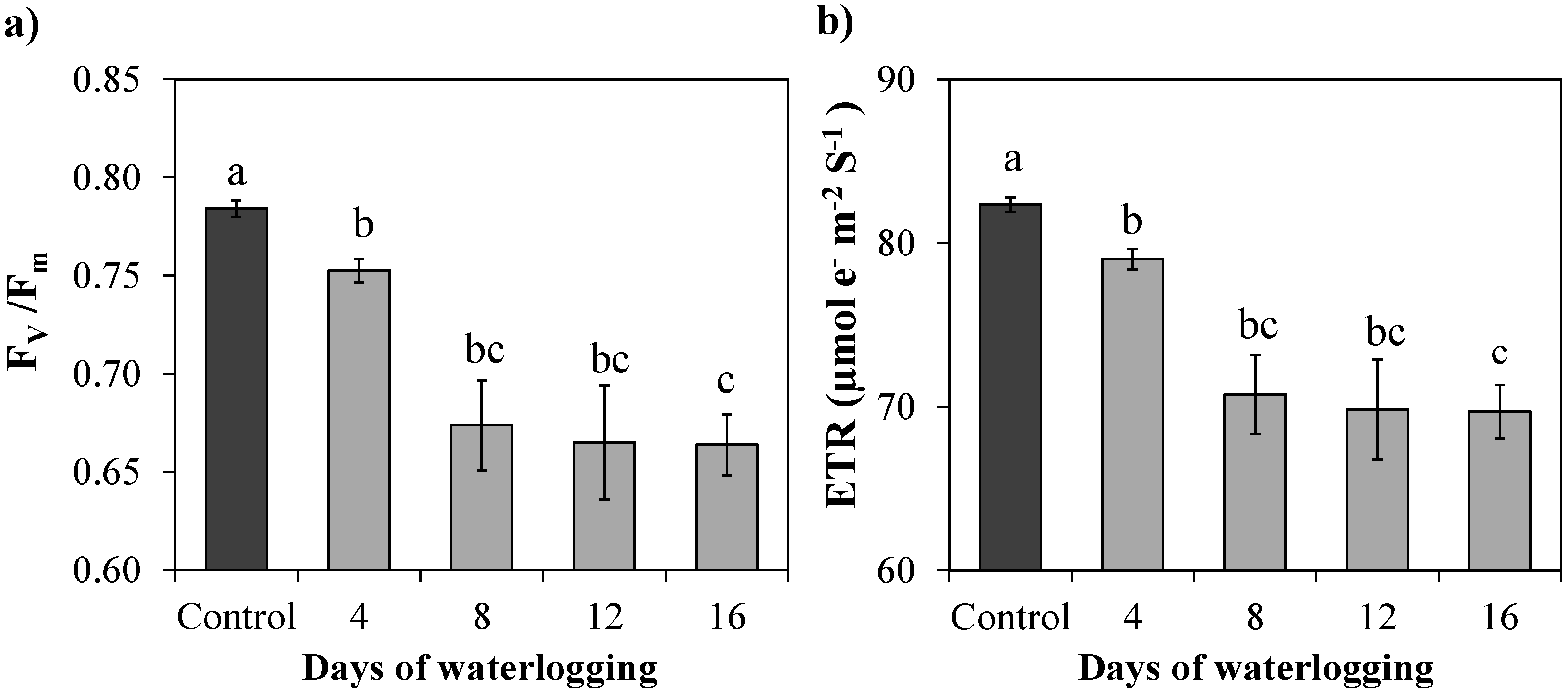
3.3. Chlorophyll “a” Fluorescence Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DaMatta, F.M.; Avila, R.T.; Cardoso, A.A.; Martins SC, V.; Ramalho, J.C. Physiological and Agronomic Performance of the Coffee Crop in the Context of Climate Change and Global Warming: A Review. J. Agric. Food Chem. 2018, 66, 5264–5274. [Google Scholar] [CrossRef] [PubMed]
- DaMatta, F.M.; Rahn, E.; Läderach, P.; Ghini, R.; Ramalho, J.C. Why could the coffee crop endure climate change and global warming to a greater extent than previously estimated? Clim. Chang. 2019, 152, 167–178. [Google Scholar] [CrossRef] [Green Version]
- Chemura, A.; Mudereri, B.T.; Yalew, A.W.; Gornott, C. Climate change and specialty coffee potential in Ethiopia. Sci. Rep. 2021, 11, 8097. [Google Scholar] [CrossRef] [PubMed]
- Toral-Juárez, M.A.; Avila, R.T.; Cardoso, A.A.; Brito, F.A.L.; Machado, K.L.G.; Almeida, W.L.; Souza, R.P.B.; Martins, S.C.V.; DaMatta, F.M. Drought-tolerant coffee plants display increased tolerance to waterlogging and post-waterlogging reoxygenation. Environ. Exp. Bot. 2021, 182, 104311. [Google Scholar] [CrossRef]
- Fischer, G.; Ramírez, F.; Casierra-Posada, F. Ecophysiological aspects of fruit crops in the era of climate change. A review. Agron. Colomb. 2016, 34, 190–199. [Google Scholar] [CrossRef]
- IPPC; Pörtner, H.-O.; Roberts, D.C.; Adams, H.; Adler, C.; Aldunce, P.; Ali, E.; Begum, R.A.; Betts, R.; Kerr, R.B.; et al. Climate Change 2022. Impacts, Adaptation and Vulnerability. Final Draft Version. 2022, p. 3676. Available online: https://www.ipcc.ch/report/ar6/wg2/ (accessed on 7 March 2022).
- Bastianin, A.; Lanza, A.; Manera, M. Economic impacts of El Niño southern oscillation: Evidence from the Colombian coffee market. Agric. Econ. 2018, 49, 623–633. [Google Scholar] [CrossRef] [Green Version]
- Ceballos-Sierra, F.; Dall’Erba, S. The effect of climate variability on Colombian coffee productivity: A dynamic panel model approach. Agric. Syst. 2021, 190, 103126. [Google Scholar] [CrossRef]
- Poveda, G.; Jaramillo, A.; Gil, M.M.; Quiceno, N.; Mantilla, R.I. Seasonally in ENSO-related precipitation, river discharges, soil moisture, and vegetation index in Colombia. Water Resour. Res. 2001, 37, 2169–2178. [Google Scholar] [CrossRef]
- Hoyos, N.; Escobar, J.; Restrepo, J.C.; Arango, A.M.; Ortiz, J.C. Impact of the 2010–2011 La Niña phenomenon in Colombia, South America: The human toll of an extreme weather event. Appl. Geogr. 2013, 39, 16–25. [Google Scholar] [CrossRef]
- Peña-Quiñones, A.J.; Valencia-Arbeláez, J.A.; Ramírez-Carabalí, C. Caracterización del efecto de «El Niño» y «La Niña» sobre la lluvia de la zona cafetera colombiana. Luna Azul 2014, 39, 89–104. [Google Scholar] [CrossRef]
- Federación Nacional de Cafeteros de Colombia. FNC en Estadística Cafetera. 2021. Available online: https://federaciondecafeteros.org/wp/estadisticas-cafeteras (accessed on 11 March 2022).
- Ovalle-Rivera, O.; Läderach, P.; Bunn, C.; Obersteiner, M.; Schroth, G. Projected Shifts in Coffea arabica Suitability among Major Global Producing Regions Due to Climate Change. PLoS ONE 2015, 10, e0124155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham, Y.; Reardon-Smith, K.; Mushtaq, S.; Cockfield, G. The impact of climate change and variability on coffee production: A systematic review. Clim. Chang. 2019, 156, 609–630. [Google Scholar] [CrossRef]
- Tian, L.; Bi, W.; Ren, X.; Li, W.; Sun, L.; Li, J. looding has more adverse effects on the stem structure and yield of spring maize (Zea mays L.) than waterlogging in Northeast China. Eur. J. Agron. 2020, 117, 126054. [Google Scholar] [CrossRef]
- Silveira, H.R.D.O.; de Souza, K.R.D.; Alves, J.D.; de Santos, M.O.; Andrade, C.A.; Bomfim, S.C. Gas exchange and carbohydrate partitioning in coffee seedlings under waterlogging. Cienc. Agrotecnol. 2015, 39, 138–146. [Google Scholar] [CrossRef]
- Silveira, H.R.; de Santos, M.O.; Alves, J.D.; de Souza, K.R.D.; Andrade, C.A.; Alves, R.G.M. Growth effects of water excess on coffee seedlings (Coffea arabica L.). Acta Sci. Agron. 2014, 36, 211–218. [Google Scholar] [CrossRef]
- Loreti, E.; van Veen, H.; Perata, P. Plant responses to flooding stress. Curr. Opin. Plant Biol. 2016, 33, 64–71. [Google Scholar] [CrossRef] [Green Version]
- Yamauchi, T.; Colmer, T.D.; Pedersen, O.; Nakazono, M. Regulation of Root Traits for Internal Aeration and Tolerance to Soil Waterlogging-Flooding Stress. Plant Physiol. 2018, 176, 1118–1130. [Google Scholar] [CrossRef] [Green Version]
- Maldonado, C.E.M.; Giraldo, L.Á. Resistencia genética a la enfermedad de la cereza del café en variedades cultivadas en Colombia. Rev. Cenicafé 2020, 71, 69–90. [Google Scholar] [CrossRef]
- Federación Nacional de Cafeteros de Colombia. Publicaciones en Informe de Gestión 2020. 2021. Available online: https://federaciondecafeteros.org/wp/tipos/informes/ (accessed on 11 March 2022).
- Arcila-Pulgarín, J.; Buhr, L.; Bleiholder, H.; Hack, H.; Meier, U.; Wicke, H. Application of the extended BBCH scale for the description of the growth stages of coffee (Coffea spp.). Ann. Appl. Biol. 2002, 141, 19–27. [Google Scholar] [CrossRef]
- Sadeghian, K.S.S. Manejo integrado de nutrientes para una caficultura sostenible. Suelos Ecuat. 2014, 44, 74–89. [Google Scholar]
- Gaitán, B.A.L.; Villegas, G.C.; Rivillas, O.C.A.; Hincapié, G.E. Almácigos de café: Calidad fitosanitaria, manejo y siembra en el campo. Av. Téc. 2011, 404, 8. Available online: http://hdl.handle.net/10778/350 (accessed on 9 February 2022).
- Unigarro-Muñoz, C.A.; Hernández-Arredondo, J.D.; Montoya-Restrepo, E.C.; Medina-Rivera, R.D.; Ibarra-Ruales, L.N.; Carmona-González, C.Y.; Flórez-Ramos, C.P. Estimation of leaf area in coffee leaves (Coffea arabica L.) of the Castillo® variety. Bragantia 2015, 74, 412–416. [Google Scholar] [CrossRef] [Green Version]
- Avila, R.T.; de Almeida, W.L.; Costa, L.C.; Machado, K.L.G.; Barbosa, M.L.; de Souza, R.P.B.; Martino, P.B.; Juárez, M.A.T.; Marçal, D.M.S.; Martins, S.C.V.; et al. Elevated air [CO2] improves photosynthetic performance and alters biomass accumulation and partitioning in drought-stressed coffee plants. Environ. Exp. Bot. 2020, 177, 104137. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.-M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Revelle, W. Psych: Procedures for Personality and Psychological Research; R Package Version 2.0.8; Northwestern University: Evanston, IL, USA, 2020; Available online: https://CRAN.r-project.org/package=psych (accessed on 14 March 2022).
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Singh, R.; Shelke, G.; Kumar, A.; Jha, P. Biochemistry and genetics of ACC deaminase: A weapon to “stress ethylene” produced in plants. Front. Microbiol. 2015, 6, 937. [Google Scholar] [CrossRef]
- Ali, S.; Kim, W.-C. Plant growth promotion under water: Decrease of Waterlogging-Induced ACC and Ethylene Levels by ACC Deaminase-Producing Bacteria. Front. Microbiol. 2018, 9, 1096. [Google Scholar] [CrossRef]
- Pandey, A.K.; Singh, A.G.; Gadhiya, A.R.; Kumar, S.; Singh, D.; Mehta, R. Chapter 17—Current approaches in horticultural crops to mitigate waterlogging stress. In Stress Tolerance in Horticultural Crops; Rai, E.A.C., Rai, A., Rai, K.K., Rai, V.P., Kumar, A., Eds.; Woodhead Publishing: Sawston, UK, 2021; pp. 289–299. [Google Scholar] [CrossRef]
- Jackson, M.B. Ethylene and Responses of Plants to Soil Waterlogging and Submergence. Annu. Rev. Plant Physiol. 1985, 36, 145–174. [Google Scholar] [CrossRef]
- Irfan, M.; Hayat, S.; Hayat, Q.; Afroz, S.; Ahmad, A. Physiological and biochemical changes in plants under waterlogging. Protoplasma 2010, 241, 3–17. [Google Scholar] [CrossRef]
- Ashraf, M.A. Waterlogging stress in plants: A review. Afr. J. Agric. Res. 2012, 7, 1976–1981. [Google Scholar] [CrossRef]
- Pan, J.; Sharif, R.; Xu, X.; Chen, X. Mechanisms of Waterlogging Tolerance in Plants: Research Progress and Prospects. Front. Plant Sci. 2021, 11, 627331. [Google Scholar] [CrossRef]
- Ferner, E.; Rennenberg, H.; Kreuzwieser, J. Effect of flooding on C metabolism of flood-tolerant (Quercus robur) and non-tolerant (Fagus sylvatica) tree species. Tree Physiol. 2012, 32, 135–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shukor, N.N.; Abdul-Hamid, H.; Abdu, A.; Ismail, M.K. Waterlogging Effects on Growth and Physiological Characteristics of Azadirachta excelsa Seedlings. Am. J. Plant Physiol. 2014, 9, 78–94. [Google Scholar] [CrossRef] [Green Version]
- El-Yazel, M.A.S.; EI-Shewy, A.A.; Matter, F.M.A. Impact of water logging condition on seedling of five timber tree species. Hortic. Int. J. 2022, 6, 37–45. [Google Scholar] [CrossRef]
- Sairam, R.K.; Kumutha, D.; Ezhilmathi, K.; Deshmukh, P.S.; Srivastava, G.C. Physiology and biochemistry of waterlogging tolerance in plants. Biol. Plant. 2008, 52, 401. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E.; Maller, I.A.; Murphy, A. Plant Physiology and Development, 6th ed.; Sinauer Associates Inc.: Sunderland, MA, USA, 2015; p. 692. [Google Scholar]
- Schildwacht, P.M. Is a decreased water potential after withholding oxygen to roots the cause of the decline of leaf-elongation rates in Zea mays L. and Phaseolus vulgaris L.? Planta 1989, 177, 178–184. [Google Scholar] [CrossRef]
- Pierce, M.; Raschke, K. Correlation between loss of turgor and accumulation of abscisic acid in detached leaves. Planta 1980, 148, 174–182. [Google Scholar] [CrossRef]
- Tounekti, T.; Mahdhi, M.; Al-Turki, T.A.; Khemira, H. Water relations and photo-protection mechanisms during drought stress in four coffee (Coffea arabica) cultivars from southwestern Saudi Arabia. S. Afr. J. Bot. 2018, 117, 17–25. [Google Scholar] [CrossRef]
- Ratzmann, G.; Zakharova, L.; Tietjen, B. Optimal leaf water status regulation of plants in drylands. Sci. Rep. 2019, 9, 3768. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, A.A.; Brodribb, T.J.; Kane, C.N.; DaMatta, F.M.; McAdam, S.A.M. Osmotic adjustment and hormonal regulation of stomatal responses to vapour pressure deficit in sunflower. AoB Plants 2020, 12, plaa025. [Google Scholar] [CrossRef]
- Yao, G.-Q.; Nie, Z.-F.; Turner, N.C.; Li, F.-M.; Gao, T.-P.; Fang, X.-W.; Scoffoni, C. Combined high leaf hydraulic safety and efficiency provides drought tolerance in Caragana species adapted to low mean annual precipitation. New Phytol. 2021, 229, 230–244. [Google Scholar] [CrossRef]
- Almeida, W.L.; Ávila, R.T.; Pérez-Molina, J.P.; Barbosa, M.L.; Marçal, D.M.S.; de Souza, R.P.B.; Martino, P.B.; Cardoso, A.A.; Martins, S.C.V.; DaMatta, F.M. The interplay between irrigation and fruiting on branch growth and mortality, gas exchange and water relations of coffee trees. Tree Physiol. 2021, 41, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Melke, A.; Fetene, M. Eco-physiological basis of drought stress in coffee (Coffea arabica, L.) in Ethiopia. Theor. Exp. Plant Physiol. 2014, 26, 225–239. [Google Scholar] [CrossRef]
- Barickman, T.C.; Simpson, C.R.; Sams, C.E. Waterlogging Causes Early Modification in the Physiological Performance, Carotenoids, Chlorophylls, Proline, and Soluble Sugars of Cucumber Plants. Plants 2019, 8, 160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, L.; Bittencourt, P.R.L.; Oliveira, R.S.; Junior, M.B.M.; Barros, F.V.; Ribeiro, R.V.; Mazzafera, P. Plant pneumatics: Stem air flow is related to embolism—New perspectives on methods in plant hydraulics. New Phytol. 2016, 211, 357–370. [Google Scholar] [CrossRef] [Green Version]
- Chávez-Arias, C.C.; Gómez-Caro, S.; Restrepo-Díaz, H. Physiological, Biochemical and Chlorophyll Fluorescence Parameters of Physalis Peruviana L. Seedlings Exposed to Different Short-Term Waterlogging Periods and Fusarium Wilt Infection. Agronomy 2019, 9, 213. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-C.; Li, M.-S.; Chen, K.-T.; Lin, Y.-H.; Ko, S.-S. Photosynthetic and Morphological Responses of Sacha Inchi (Plukenetia volubilis L.) to Waterlogging Stress. Plants 2022, 11, 249. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef] [Green Version]
- Björkman, O.; Demmig, B. Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origins. Planta 1987, 170, 489–504. [Google Scholar] [CrossRef]
- Zhang, R.D.; Zhou, Y.F.; Yue, Z.X.; Chen, X.F.; Cao, X.; Xu, X.X.; Xing, Y.F.; Jiang, B.; Ai, X.Y.; Huang, R.D. Changes in photosynthesis, chloroplast ultrastructure, and antioxidant metabolism in leaves of sorghum under waterlogging stress. Photosynthetica 2019, 57, 1076–1083. [Google Scholar] [CrossRef] [Green Version]
- Demmig-Adams, B.; Adams, W.W.; Winter, K.; Meyer, A.; Schreiber, U.; Pereira, J.S.; Krüger, A.; Czygan, F.-C.; Lange, O.L. Photochemical efficiency of photosystem II, photon yield of O2 evolution, photosynthetic capacity, and carotenoid composition during the midday depression of net CO2 uptake in Arbutus unedo growing in Portugal. Planta 1989, 177, 377–387. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Santos, M.; Coelho, L.S.; Carvalho, G.R.; Botelho, C.E.; Torres, L.F.; Vilela, D.J.M.; Andrade, A.C.; Silva, V.A. Photochemical efficiency correlated with candidate gene expression promote coffee drought tolerance. Sci. Rep. 2021, 11, 7436. [Google Scholar] [CrossRef] [PubMed]




| pH | Texture | Organic Matter | Clay | Sands | Silt | N | Al | K | Ca | Mg | P | S | B |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | cmol (+) kg−1 | ppm | |||||||||||
| 5.0 | Sandy loam | 9.5 | 15.7 | 58.1 | 26.1 | 0.4 | 0.4 | 0.1 | 0.7 | 0.3 | 2.2 | 16 | 0.38 |
| Days of Waterlogging | Parameters | |||
|---|---|---|---|---|
| LAR | SLA | R/SR | RDM/TLA | |
| Control | 0.50 ± 0.10 a | 109 ± 3.50 b | 3.89 ± 0.19 a | 41.10 ± 2.40 a |
| 4 | 0.50 ± 0.01 a | 122 ± 3.76 ab | 3.82 ± 0.23 a | 37.30 ± 2.32 a |
| 8 | 0.50 ± 0.01 a | 126 ± 5.63 a | 4.26 ± 0.31 a | 34.80 ± 2.40 a |
| 12 | 0.48 ± 0.01 ab | 117 ± 3.39 ab | 4.40 ± 0.27 a | 37.20 ± 2.81 a |
| 16 | 0.45 ± 0.01 b | 118 ± 3.26 ab | 4.17 ± 0.21 a | 36.86 ± 3.19 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
León-Burgos, A.F.; Unigarro, C.A.; Balaguera-López, H.E. Soil Waterlogging Conditions Affect Growth, Water Status, and Chlorophyll “a” Fluorescence in Coffee Plants (Coffea arabica L.). Agronomy 2022, 12, 1270. https://doi.org/10.3390/agronomy12061270
León-Burgos AF, Unigarro CA, Balaguera-López HE. Soil Waterlogging Conditions Affect Growth, Water Status, and Chlorophyll “a” Fluorescence in Coffee Plants (Coffea arabica L.). Agronomy. 2022; 12(6):1270. https://doi.org/10.3390/agronomy12061270
Chicago/Turabian StyleLeón-Burgos, Andrés Felipe, Carlos Andrés Unigarro, and Helber Enrique Balaguera-López. 2022. "Soil Waterlogging Conditions Affect Growth, Water Status, and Chlorophyll “a” Fluorescence in Coffee Plants (Coffea arabica L.)" Agronomy 12, no. 6: 1270. https://doi.org/10.3390/agronomy12061270
APA StyleLeón-Burgos, A. F., Unigarro, C. A., & Balaguera-López, H. E. (2022). Soil Waterlogging Conditions Affect Growth, Water Status, and Chlorophyll “a” Fluorescence in Coffee Plants (Coffea arabica L.). Agronomy, 12(6), 1270. https://doi.org/10.3390/agronomy12061270








