Arbuscular Mycorrhizal Fungi and Microbes Interaction in Rice Mycorrhizosphere
Abstract
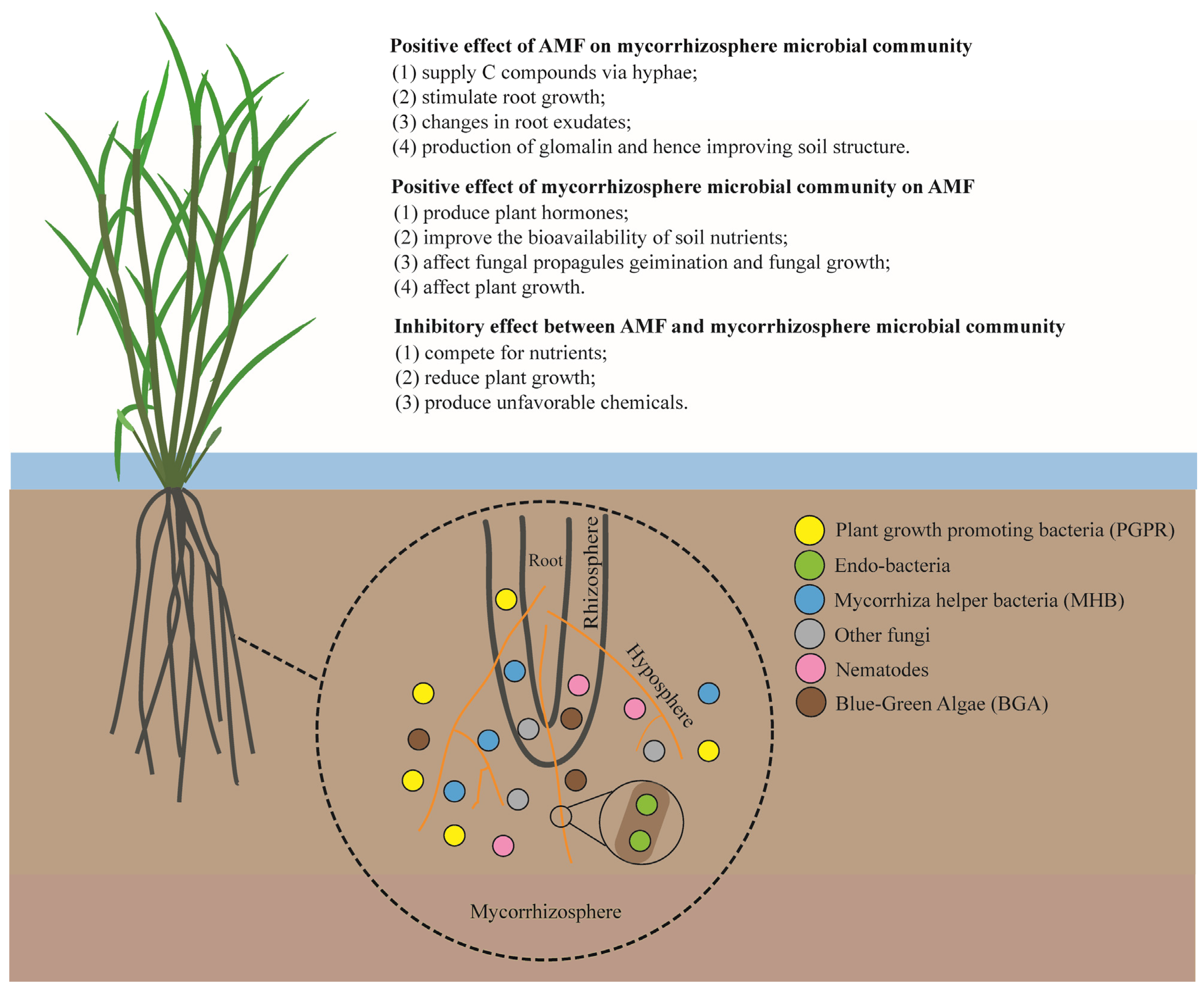
:1. Introduction
2. AMF in the Paddy Fields
3. Major Groups of Bacteria Interacting with AMF
3.1. PGPR
3.2. Endo-Bacteria
3.3. MHB
3.4. DB
4. Interactions between AMF and Other Fungi
5. Interactions between AMF and Other Microorganisms
6. AMF and Soil Microbial Community
7. Mechanisms Involved in Interactions between AMF and Other Soil Microbes
8. Potential Application of AMF Mutualism in Paddy Fields
9. Conclusions and Future Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAO. Rice Market Monitor. 2018. Available online: https://www.fao.org/3/I9243EN/i9243en.pdf (accessed on 25 April 2021).
- Ding, L.J.; Cui, H.L.; Nie, S.A.; Long, X.E.; Duan, G.L.; Zhu, Y.G. Microbiomes inhabiting rice roots and rhizosphere. FEMS Microbiol. Ecol. 2019, 95, fiz040. [Google Scholar] [CrossRef] [Green Version]
- Vallino, M.; Fiorilli, V.; Bonfante, P. Rice flooding negatively impacts root branching and arbuscular mycorrhizal colonization, but not fungal viability. Plant Cell Environ. 2014, 37, 557–572. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.Z.; Wang, Y.T.; Olsson, P.A. Arbuscular mycorrhiza under water-Carbon-phosphorus exchange between rice and arbuscular mycorrhizal fungi under different flooding regimes. Soil Boil. Biochem. 2019, 129, 169–177. [Google Scholar] [CrossRef]
- Campo, S.; Martín-Cardoso, H.; Olivé, M.; Pla, E.; Catala-Forner, M.; MartínezEixarch, M. Effect of root colonization by arbuscular mycorrhizal fungi on growth, productivity and blast resistance in rice. Rice 2020, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Tisarum, R.; Theerawitaya, C.; Samphumphuang, T.; Polispitak, K.; Thongpoem, P.; Singh, H.P.; Cha-Um, S. Alleviation of salt stress in upland rice (Oryza sativa L. ssp. indica cv. Leum Pua) using arbuscular mycorrhizal fungi inoculation. Front. Plant Sci. 2020, 11, 348. [Google Scholar] [CrossRef] [Green Version]
- Desiro, A.; Salvioli, A.; Ngonkeu, E.L.; Mondo, S.J.; Epis, S.; Faccio, A.; Kaech, A.; Pawlowska, T.E.; Bonfante, P. Detection of a novel intracellular microbiome hosted in arbuscular mycorrhizal fungi. ISME J. 2014, 8, 257–270. [Google Scholar] [CrossRef] [Green Version]
- Venice, F.; Ghignone, S.; Salvioli di Fossalunga, A.; Amselem, J.; Novero, M.; Xianan, X.; Toro, K.S.; Morin, E.; Lipzen, A.; Grigoriev, I.V.; et al. At the nexus of three kingdoms: The genome of the mycorrhizal fungus Gigaspora margarita provides insights into plant, endobacterial and fungal interactions. Environ. Microbiol. 2020, 22, 122–141. [Google Scholar] [CrossRef]
- Spatafora, J.W.; Chang, Y.; Benny, G.L.; Lazarus, K.; Smith, M.E.; Berbee, M.L.; Bonito, G.; Corradi, N.; Grigoriev, I.; Gryganskyi, A.; et al. A phylum-level phylogenetic classification of zygomycete fungi based on genome-scale data. Mycologia 2016, 108, 1028–1046. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: New York, NY, USA, 2010. [Google Scholar]
- Brundrett, M.C.; Tedersoo, L. Evolutionary history of mycorrhizal symbioses and global host plant diversity. New Phytol. 2018, 220, 1108–1115. [Google Scholar] [CrossRef]
- Ilag, L.L.; Rosales, A.M.; Elazegui, F.A.; Mew, T.W. Changes in the population of infective endomycorrhizal fungi in a rice-based cropping system. Plant Soil 1987, 103, 67–73. [Google Scholar] [CrossRef]
- Lumini, E.; Vallino, M.; Alguacil, M.M.; Romani, M.; Bianciotto, V. Different farming and water regimes in Italian rice fields affect arbuscular mycorrhizal fungal soil communities. J. Appl. Ecol. 2011, 21, 1696–1707. [Google Scholar] [CrossRef]
- Vallino, M.; Greppi, D.; Novero, M.; Bonfante, P.; Lupotto, E. Rice root colonisation by mycorrhizal and endophytic fungi in aerobic soil. Ann. Appl. Biol. 2009, 154, 195–204. [Google Scholar] [CrossRef]
- Wang, Y.T.; Li, T.; Li, Y.W.; Björn, L.O.; Rosendahl, S.; Olsson, P.A.; Li, S.S. Community dynamics of arbuscular mycorrhizal fungi in high-input and intensively irrigated rice cultivation systems. Appl. Environ. Microb. 2015, 81, 2958–2965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.W.; Wu, F.Y.; Li, H.; Chan, W.F.; Wu, S.C.; Wong, M.H. Mycorrhizal colonization status of lowland rice (Oryza sativa L.) in the southeastern region of China. Environ. Sci. Pollut. R. 2017, 24, 5268–5276. [Google Scholar] [CrossRef] [PubMed]
- Bernaola, L.; Cange, G.; Way, M.O.; Gore, J.; Hardke, J.; Stout, M. Natural colonization of rice by arbuscular mycorrhizal fungi in different production areas. Rice Sci. 2018, 25, 169–174. [Google Scholar] [CrossRef]
- Hajiboland, R.; Aliasgharzad, N.; Barzeghar, R. Influence of arbuscular mycorrhizal fungi on uptake of Zn and P by two contrasting rice genotypes. Plant Soil Environ. 2009, 55, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Ye, Z.H.; Chan, W.F.; Chen, X.W.; Wu, F.Y.; Wu, S.C.; Wong, M.H. Can arbuscular mycorrhizal fungi improve grain yield, As uptake and tolerance of rice grown under aerobic conditions? Environ. Pollut. 2011, 159, 2537–2545. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, A.; Cruz, C.; Pérez-Tienda, J.; Ferrol, N. Shedding light onto nutrient responses of arbuscular mycorrhizal plants: Nutrient interactions may lead to unpredicted outcomes of the symbiosis. Plant Sci. 2014, 221, 29–41. [Google Scholar] [CrossRef]
- Lin, A.; Zhang, X.; Yang, X. Glomus mosseae enhances root growth and Cu and Pb acquisition of upland rice (Oryza sativa L.) in contaminated soils. Ecotoxicology 2014, 23, 2053–2061. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Wang, L.; Ma, F.; Bloomfield, K.J.; Yang, J.; Atkin, O.K. Is resource allocation and grain yield of rice altered by inoculation with arbuscular mycorrhizal fungi? J. Plant Ecol. 2015, 8, 436–448. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Wang, L.; Ma, F.; Yang, J.; Atkin, O.K. Phenotypic plasticity in rice: Responses to fertilization and inoculation with arbuscular mycorrhizal fungi. J. Plant Ecol. 2015, 9, 107–116. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Bao, X.; Li, S. Effects of arbuscular mycorrhizal fungi on rice growth under different flooding and shading regimes. Front. Microbiol. 2021, 12, 3276. [Google Scholar] [CrossRef]
- Rambelli, A. The Rhizosphere of mycorrhizae. In Ectomycorrhizae; Marks, G.L., Koslowski, T.T., Eds.; Academic Press: New York, NY, USA, 1973; pp. 299–343. [Google Scholar]
- Priyadharsini, P.; Rojamala, K.; Ravi, R.K.; Muthuraja, R.; Nagaraj, K.; Muthukumar, T. Mycorrhizosphere: The extended rhizosphere and its significance. In Plant-Microbe Interaction: An Approach to Sustainable Agriculture; Choudhary, D., Varma, A., Tuteja, N., Eds.; Springer: Singapore, 2016. [Google Scholar]
- Linderman, R.G. Mycorrhizal interaction with the rhizosphere microflora: The Mycorrhizosphere Effect. Phytopathology 1988, 78, 366–371. [Google Scholar]
- Frey-Klett, P.; Chavatte, M.; Clausse, M.L.; Courrier, S.; Le Roux, C.; Raaijmakers, J.; Martinotti, M.G.; Pierrat, J.C.; Garbaye, J. Ectomycorrhizal symbiosis affects functional diversity of rhizosphere fluorescent pseudomonads. New Phytol. 2005, 165, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Hoeksema, J.D.; Chaudhary, V.B.; Gehring, C.A.; Johnson, N.C.; Karst, J.; Koide, R.T.; Pringle, A.; Zabinski, C.; Bever, J.D.; Moore, J.C.; et al. A meta-analysis of context-dependency in plant response to inoculation with mycorrhizal fungi. Ecol. Lett. 2010, 13, 394–407. [Google Scholar] [CrossRef]
- Ramírez-Viga, T.K.; Aguilar, R.; Castillo-Argüero, S.; Chiappa-Carrara, X.; Guadarrama, P.; Ramos-Zapata, J. Wetland plant species improve performance when inoculated with arbuscular mycorrhizal fungi: A meta-analysis of experimental pot studies. Mycorrhiza 2018, 28, 477–493. [Google Scholar] [CrossRef] [PubMed]
- Pivato, B.; Offre, P.; Marchelli, S.; Barbonaglia, B.; Mougel, C.; Lemanceau, P.; Berta, G. Bacterial effects on arbuscular mycorrhizal fungi and mycorrhiza development as influenced by the bacteria, fungi, and host plant. Mycorrhiza 2009, 19, 81–90. [Google Scholar] [CrossRef]
- Shrinkhala, M. Study on the Bioprotective Effect of Endomycorrhizae against M. graminicola in Rice. PhD Thesis, Catholic University, Leuven, Belgium, 2001. [Google Scholar]
- Ordoñez, Y.M.; Fernandez, B.R.; Lara, L.S.; Alia Rodriguez, A.; Uribe-Vélez, D.; Sanders, I.R. Bacteria with phosphate solubilizing capacity alter mycorrhizal fungal growth both inside and outside the root and in the presence of native microbial communities. PLoS ONE 2016, 11, e0154438. [Google Scholar] [CrossRef]
- Mangan, S.A.; Schnitzer, S.A.; Herre, E.A.; Mack, K.M.L.; Valencia, M.C.; Sanchez, E.I.; Bever, J.D. Negative plant-soil feedback predicts tree-species relative abundance in a tropical forest. Nature 2010, 466, 752–755. [Google Scholar] [CrossRef]
- van der Putten, W.H.; Bardgett, R.D.; Bever, J.D.; Bezemer, T.M.; Casper, B.B.; Fukami, T.; Kardol, P.; Klironomos, J.N.; Kulmatiski, A.; Schweitzer, J.A.; et al. Plant-soil feedbacks: The past, the present and future challenges. J. Ecol. 2013, 101, 265–276. [Google Scholar] [CrossRef]
- Breidenbach, B.; Pump, J.; Dumont, M.G. Microbial community structure in the rhizosphere of rice plants. Front. Microbiol. 2016, 6, 1537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheublin, T.R.; Sanders, I.R.; Keel, C.; van der Meer, J.R. Characterisation of microbial communities colonising the hyphal surfaces of arbuscular mycorrhizal fungi. ISME J. 2010, 4, 752–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miransari, M. Interactions between arbuscular mycorrhizal fungi and soil bacteria. Appl. Microbiol. Biot. 2011, 89, 917–930. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhou, J.; George, T.S.; Limpens, E.; Feng, G. Arbuscular mycorrhizal fungi conducting the hyphosphere bacterial orchestra. Trends Plant Sci. 2021, 27, 402–411. [Google Scholar] [CrossRef]
- Hussain, M.B.; Shah, S.H.; Matloob, A.; Mubaraka, R.; Ahmed, N.; Ahmad, I.; Haq, T.; Jamshaid, M.U. Rice Interactions with Plant Growth Promoting Rhizobacteria; Springer: Singapore, 2022; pp. 231–255. [Google Scholar]
- Artursson, V.; Finlay, R.D.; Jansson, J.K. Interactions between arbuscular mycorrhizal fungi and bacteria and their potential for stimulating plant growth. Environ. Microbiol. 2006, 8, 1–10. [Google Scholar] [CrossRef]
- Asiloglu, R.; Shiroishi, K.; Suzuki, K.; Oguz, C.T.; Murasee, T.; Harada, N. Protist-enhanced survival of a plant growth promoting rhizobacteria, Azospirillum sp. B510, and the growth of rice (Oryza sativa L.) plants. Appl. Soil Ecol. 2020, 154, 103599. [Google Scholar] [CrossRef]
- Ji, J.; Yuan, D.; Jin, C.; Wang, G.; Li, X.Z.; Guan, C.F. Enhancement of growth and salt tolerance of rice seedlings (Oryza sativa L.) by regulating ethylene production with a novel halotolerant PGPR strain Glutamicibacter sp. YD01 containing ACC deaminase activity. Acta. Physiol. Plant 2020, 42, 1–17. [Google Scholar] [CrossRef]
- Xiao, A.W.; Li, Z.; Li, W.C.; Ye, Z.H. The effect of plant growth-promoting rhizobacteria (PGPR) on arsenic accumulation and the growth of rice plants (Oryza sativa L.). Chemosphere 2020, 242, 125136. [Google Scholar]
- Okonji, C.J.; Sakariyawo, O.S.; Okeleye, K.A.; Osunbiyi, A.G.; Ajayi, E.O. Effects of arbuscular mycorrhizal fungal inoculation on soil properties and yield of selected rice varieties. J. Agri. Sci. 2018, 63, 153–170. [Google Scholar] [CrossRef]
- Yu, H.; Liu, X.; Yang, C.; Peng, Y.S.; Yu, X.L.; Gu, H.; Zheng, X.F.; Wang, C.; Xiao, F.S.; Shu, L.F.; et al. Co-symbiosis of arbuscular mycorrhizal fungi (AMF) and diazotrophs promote biological nitrogen fixation in mangrove ecosystems. Soil Biol. Biochem. 2021, 161, 108382. [Google Scholar] [CrossRef]
- Primieri, S.; Magnoli, S.M.; Koffel, T.; Stürmer, S.L.; Bever, J.D. Perennial, but not annual legumes synergistically benefit from infection with arbuscular mycorrhizal fungi and rhizobia: A meta-analysis. New Phytol. 2022, 233, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Mäder, P.; Kaiser, F.; Adholeya, A.; Singh, R.; Uppal, H.S.; Sharma, A.K.; Srivastava, R.; Sahaid, V.; Aragnoe, M.; Wiemkenf, A.; et al. Inoculation of root microorganisms for sustainable wheat–rice and wheat–black gram rotations in India. Soil Biology Biochem. 2011, 43, 609–619. [Google Scholar] [CrossRef]
- Ruíz-Sánchez, M.; Armada, E.; Muñoz, Y.; de Salamone, I.E.G.; Aroca, R.; Ruíz-Lozano, J.M.; Azcón, R. Azospirillum and arbuscular mycorrhizal colonization enhance rice growth and physiological traits under well-watered and drought conditions. J. Plant Physiol. 2011, 168, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- PremKumari, S.M.; Prabina, B.J. Impact of the mixed consortium of indigenous arbuscular mycorrhizal fungi (AMF) on the growth and yield of rice (ORYZA SATIVA L.) under the system of rice intensification (SRI). Inter. J. Environ. Agri. Biotechnol. 2017, 2, 238743. [Google Scholar] [CrossRef]
- Norouzinia, F.; Ansari, M.H.; Aminpanah, H.; Firozi, S. Alleviation of soil salinity on physiological and agronomic traits of rice cultivars using Arbuscular mycorrhizal fungi and Pseudomonas strains under field conditions. J. Neotrop. Agri. 2020, 7, 25–42. [Google Scholar] [CrossRef] [Green Version]
- Hoseinzade, H.; Ardakani, M.R.; Shahdi, A.; Rahmani, H.A.; Noormohammadi, G.; Miransari, M. Rice (Oryza sativa L.) nutrient management using mycorrhizal fungi and endophytic Herbaspirillum seropedicae. J. Integr. Agr. 2016, 15, 1385–1394. [Google Scholar] [CrossRef] [Green Version]
- Beura, K.; Pradhan, A.K.; Ghosh, G.K.; Kohli, A.; Singh, M. Root architecture, yield and phosphorus uptake by rice: Response to rock phosphate enriched compost and microbial inoculants. Int. Res. J. Pure Appl. Chem. 2020, 21, 33–39. [Google Scholar] [CrossRef]
- Lasudee, K.; Tokuyama, S.; Lumyong, S.; Pathom-Aree, W. Actinobacteria associated with arbuscular mycorrhizal Funneliformis mosseae spores, taxonomic characterization and their beneficial traits to plants: Evidence obtained from mung bean (Vigna radiata) and thai jasmine rice (Oryza sativa). Front. Microbiol. 2018, 9, 1247. [Google Scholar] [CrossRef]
- Baby, U.I.; Manibhushanrao, K. Influence of organic amendments on arbuscular mycorrhizal fungi in relation to rice sheath blight disease. Mycorrhiza 1996, 6, 201–206. [Google Scholar] [CrossRef]
- Bernaola, L.; Cosme, M.; Schneider, R.W.; Stout, M. Belowground inoculation with arbuscular mycorrhizal fungi increases local and systemic susceptibility of rice plants to different pest organisms. Front. Plant Sci. 2018, 9, 747. [Google Scholar] [CrossRef]
- Radwan, F.I.; El-Seoud, A.I.; El-Ham, A.B. Response of two rice cultivars to Blue Green Algae, a-mycorrhizae inoculation and mineral nitrogen fertilizer. Middle Eastern Russian J. Plant Sci. Biotechnol. 2008, 2, 29–34. [Google Scholar]
- Bonfante, P.; Desirò, A. Who lives in a fungus? The diversity, origins and functions of fungal endobacteria living in Mucoromycota. ISME J. 2017, 11, 1727–1735. [Google Scholar] [CrossRef] [PubMed]
- Mondo, S.J.; Toomer, K.H.; Morton, J.B.; Lekberg, Y.; Pawlowska, T.E. Evolutionary stability in a 400-million-year-old heritable facultative mutualism. Evolution 2012, 66, 2564–2574. [Google Scholar] [CrossRef]
- Naumann, M.; Schüler, A.; Bonfante, P. The obligate endobacteria of arbuscular mycorrhizal fungi are ancient heritable components related to the Mollicutes. ISME J. 2010, 4, 862–871. [Google Scholar] [CrossRef] [Green Version]
- Hamedi, J.; Mohammadipanah, F. Biotechnological application and taxonomical distribution of plant growth promoting actinobacteria. J. Ind. Microbiol. Biotechnol. 2015, 42, 157–171. [Google Scholar] [CrossRef]
- Qin, S.; Feng, W.W.; Wang, T.T.; Ding, P.; Xing, K.; Jiang, J.H. Plant growth-promoting effect and genomic analysis of the beneficial endophyte Streptomyces sp. KLBMP 5084 isolated from halophyte Limonium sinense. Plant Soil 2017, 416, 117–132. [Google Scholar] [CrossRef]
- Bonfante, P. Plants, mycorrhizal fungi and endobacteria: A dialog among cells and genomes. Biol. Bull. 2003, 204, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Salvioli, A.; Ghignone, S.; Novero, M.; Navazio, L.; Venice, F.; Bagnaresi, P.; Bonfante, P. Symbiosis with an endobacterium increases the fitness of a mycorrhizal fungus, raising its bioenergetic potential. ISME J. 2016, 10, 130–144. [Google Scholar] [CrossRef]
- Garbaye, J. Mycorrhization helper bacteria: A new dimension to the mycorrhizal symbiosis. Acta Bot. Gallica. 1994, 141, 517–521. [Google Scholar] [CrossRef] [Green Version]
- Turrini, A.; Avio, L.; Giovannetti, M.; Agnolucci, M. Functional complementarity of arbuscular mycorrhizal fungi and associated microbiota: The challenge of translational research. Front. Plant Sci. 2018, 9, 1407. [Google Scholar] [CrossRef]
- Gupta, S.K.; Chakraborty, A.P. Mycorrhiza helper bacteria: Future prospects. Int. J. Res. Rev. 2020, 7, 387–391. [Google Scholar]
- Sangwan, S.; Prasanna, R. Mycorrhizae helper bacteria: Unlocking their potential as bioenhancers of plant-arbuscular mycorrhizal fungal associations. Microb. Ecol. 2021, 1–10. Available online: https://linkspringer.53yu.com/article/10.1007/s00248-021-01831-7 (accessed on 25 April 2022). [CrossRef] [PubMed]
- Rillig, M.C.; Lutgen, E.R.; Ramsey, P.W.; Klironomos, J.N.; Gannon, J.E. Microbiota accompanying different arbuscular mycorrhizal fungal isolates influence soil aggregation. Pedobiologia 2005, 49, 251–259. [Google Scholar] [CrossRef]
- Frey-Klett, P.; Garbaye, J.; Tarkka, M. The mycorrhiza helper bacteria revisited. New Phytol. 2007, 176, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Berg, G. Plant–microbe interactions promoting plant growth and health: Perspectives for controlled use of microorganisms in agriculture. Appl. Microbiol. Biotechnol. 2009, 84, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Panneerselvam, P.; Kumar, U.; Sugitha, T.C.K.; Parameswaran, C.; Sahoo, S.; Binodh, A.K.; Jahan, A.; Anandan, A. Arbuscular mycorrhizal fungi (AMF) for sustainable rice production. In Advances in Soil Microbiology: Recent Trends and Future Prospects; Springer: Singapore, 2017; pp. 99–126. [Google Scholar]
- Bharadwaj, D.P.; Alström, S.; Lundquist, P.O. Interactions among Glomus irregulare, arbuscular mycorrhizal spore-associated bacteria, and plant pathogens under in vitro conditions. Mycorrhiza 2012, 22, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.; Abd_Allah, E.F.; Alqarawi, A.A.; Al-Huqail, A.A.; Wirth, S.; Egamberdieva, D. The interaction between arbuscular mycorrhizal fungi and endophytic bacteria enhances plant growth of Acacia gerrardii under salt stress. Front. Microbiol. 2016, 7, 1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larimer, A.L.; Clay, K.; Bever, J.D. Synergism and context dependency of interactions between arbuscular mycorrhizal fungi and rhizobia with a prairie legume. Ecology 2014, 95, 1045–1054. [Google Scholar] [CrossRef] [Green Version]
- Nehl, D.B.; Allen, S.J.; Brown, J.F. Deleterious rhizosphere bacteria: An integrating perspective. Appl. Soil Ecol. 1997, 5, 1–20. [Google Scholar] [CrossRef]
- Aguilar-Paredes, A.; Valdés, G.; Nuti, M. Ecosystem functions of microbial consortia in sustainable agriculture. Agronomy 2020, 10, 1902. [Google Scholar] [CrossRef]
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The rhizosphere microbiome: Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, S.; Li, H.; Wang, R.; Zhang, K.; Xu, J. Fungi-nematode interactions: Diversity, ecology, and biocontrol prospects in agriculture. J. Fungi 2020, 6, 206. [Google Scholar] [CrossRef]
- Geisen, S.; Quist, C.W. Microbial-Faunal interactions in the rhizosphere. In Rhizosphere Biology: Interactions between Microbes and Plants; Springer: Singapore, 2021; pp. 237–253. [Google Scholar]
- Schonbeck, M. Soil health and organic farming; Organic Farming Research Foundation: Santa Cruz, CA, USA, 2017. [Google Scholar]
- Brahmaprakash, G.P.; Sahu, P.K.; Lavanya, G.; Nair, S.S.; Gangaraddi, V.K.; Gupta, A. Microbial functions of the rhizosphere. In Plant-Microbe Interactions in Agro-Ecological Perspectives; Springer: Singapore, 2017; pp. 177–210. [Google Scholar]
- Innocenti, G.; Sabatini, M.A. Collembola and plant pathogenic, antagonistic and arbuscular mycorrhizal fungi: A review. B. Insectol. 2018, 71, 71–76. [Google Scholar]
- Weil, R.; Brady, N. The Nature and Properties of Soils, 15th ed.; Prentice Hall International: Hoboken, NJ, USA, 2017. [Google Scholar]
- Ashmrita, M.; Radha, S. Blue-green algal biofertilizer and growth response of rice plants. Int. J. Plant Sci. 2017, 12, 68–71. [Google Scholar]
- Ojha, S.K.; Benjamin, J.C.; Singh, A.K. Role on biofertilizer (Blue green algae) in paddy crop. J. Pharmacogn. Phytochem. 2018, 7, 830–832. [Google Scholar]
- Jacoby, R.; Peukert, M.; Succurro, A.; Koprivova, A.; Kopriva, S. The role of soil microorganisms in plant mineral nutrition-current knowledge and future directions. Front. Plant Sci. 2017, 8, 1617. [Google Scholar] [CrossRef] [Green Version]
- Fitter, A.H.; Garbaye, J. Interactions between mycorrhizal fungi and other soil organisms. Plant Soil 1994, 159, 123–132. [Google Scholar] [CrossRef]
- Ye, S.; Yang, Y.; Xin, G.; Wang, Y.; Ruan, L.; Ye, G. Studies of the Italian ryegrass–rice rotation system in southern China: Arbuscular mycorrhizal symbiosis affects soil microorganisms and enzyme activities in the Lolium mutiflorum L. rhizosphere. Appl. Soil Ecol. 2015, 90, 26–34. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, N.; Fan, J.; Wang, F.; George, T.S.; Feng, G. Arbuscular mycorrhizal fungi stimulate organic phosphate mobilization associated with changing bacterial community structure under field conditions. Environ. Microbiol. 2018, 20, 2639–2651. [Google Scholar] [CrossRef]
- Al-Maliki, S.; Ebreesum, H. Changes in soil carbon mineralization, soil microbes, roots density and soil structure following the application of the arbuscular mycorrhizal fungi and green algae in the arid saline soil. Rhizosphere 2020, 14, 100203. [Google Scholar] [CrossRef]
- Park, Y.S.; Ryu, C.M. Understanding plant social networking system: Avoiding deleterious microbiota but calling beneficials. Int. J. Mol. Sci. 2021, 22, 3319. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.W.; Wu, L.; Luo, N.; Mo, C.H.; Wong, M.H.; Li, H. Arbuscular mycorrhizal fungi and the associated bacterial community influence the uptake of cadmium in rice. Geoderma 2019, 337, 749–757. [Google Scholar] [CrossRef]
- Svenningsen, N.B.; Watts-Williams, S.J.; Joner, E.J.; Battini, F.; Efthymiou, A.; Cruz-Paredes, C.; Nybroe, O.; Jakobsen, I. Suppression of the activity of arbuscular mycorrhizal fungi by the soil microbiota. ISME J. 2018, 12, 1296–1307. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, D.A.; da Silva, T.F.; Pylro, V.S.; Salles, J.F.; Andreote, F.D.; Dini-Andreote, F. Soil microbial diversity affects the plant-root colonization by arbuscular mycorrhizal fungi. Microb. Ecol. 2021, 82, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Makaure, B.T.; Aremu, A.O.; Magadlela, A. Soil nutritional status drives the co-occurrence of nodular bacterial species and arbuscular mycorrhizal fungi modulating plant nutrition and growth of Vigna unguiculata L.(Walp) in grassland and savanna ecosystems in KwaZulu-Natal, South Africa. J. Soil Sci. Plant Nut. 2022, 1–16. Available online: https://linkspringer.53yu.com/article/10.1007/s42729-022-00763-6 (accessed on 25 April 2022). [CrossRef]
- Gomes, M.P.; Marques, R.Z.; Nascentes, C.C.; Scotti, M.R. Synergistic effects between arbuscular mycorrhizal fungi and rhizobium isolated from As-contaminated soils on the As-phytoremediation capacity of the tropical woody legume Anadenanthera peregrina. Int. J. Phytoremediat 2020, 22, 1362–1371. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, K.; Sulewska, H.; Błaszczyk, L.; Basińska-Barczak, A.; Mikołajczak, K.; Salamon, S.; Szymańska, G.; Dryjański, L. Growth and photosynthetic activity of selected spelt varieties (Triticum aestivum ssp. spelta L.) cultivated under drought conditions with different endophytic core microbiomes. Int. J. Mol. Sci. 2020, 21, 7987. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.F.; Upadhyaya, A. A survey of soils for aggregate stability and glomalin, a glycoprotein produced by hyphae of arbuscular mycorrhizal fungi. Plant Soil 1998, 198, 97–107. [Google Scholar] [CrossRef]
- Hussain, Q.; Pan, G.X.; Liu, Y.Z.; Zhang, A.; Li, L.Q.; Zhang, X.H.; Jin, Z.J. Microbial community dynamics and function associated with rhizosphere over periods of rice growth. Plant Soil Environ. 2021, 58, 55–61. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Ge, T.; Hu, Y.; Zhou, P.; Wang, T.; Shibistova, O.; Guggenberger, G.; Su, Y.; Wu, J. Fate of rice shoot and root residues, rhizodeposits, and microbial assimilated carbon in paddy soil-part 2: Turnover and microbial utilization. Plant Soil 2017, 416, 243–257. [Google Scholar] [CrossRef]
- Zhu, Z.; Ge, T.; Liu, S.; Hu, Y.; Ye, R.; Xiao, M.; Tong, C.; Kuzyakov, Y.; Wu, J. Rice rhizodeposits affect organic matter priming in paddy soil: The role of N fertilization and plant growth for enzyme activities, CO2 and CH4 emissions. Soil Biol. Biochem. 2018, 116, 369–377. [Google Scholar] [CrossRef]
- Jamiołkowska, A.; Księżniak, A.; Hetman, B.; Kopacki, M.; Skwaryło-Bednarz, B.; Gałązka, A.; Thanoon, A.H. Interactions of arbuscular mycorrhizal fungi with plants and soil microflora. Acta Sci. Pol. Hortorum Cultus 2017, 16, 89–95. [Google Scholar] [CrossRef]
- Xu, J.; Liu, S.; Song, S.; Guo, H.; Tang, J.; Yong, J.W.H.; Ma, Y.; Chen, X. Arbuscular mycorrhizal fungi influence decomposition and the associated soil microbial community under different soil phosphorus availability. Soil Biol. Biochem. 2018, 120, 181–190. [Google Scholar] [CrossRef]
- Lareen, A.; Burton, F.; Schäfer, P. Plant root-microbe communication in shaping root microbiomes. Plant Mol. Biol. 2016, 90, 575–587. [Google Scholar] [CrossRef] [Green Version]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.S. Plant growth-promoting rhizobacteria: Context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef] [Green Version]
- Zhalnina, K.; Louie, K.B.; Hao, Z.; Mansoori, N.; da Rocha, U.N.; Shi, S.; Cho, H.; Karaoz, U.; Loqué, D.; Bowen, B.P.; et al. Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat. Microbiol. 2018, 3, 470–480. [Google Scholar] [CrossRef] [Green Version]
- Matsushima, C.; Shenton, M.; Kitahara, A.; Wasaki, J.; Oikawa, A.; Cheng, W.; Ikeo, K.; Tawaraya, K. Multiple analysis of root exudates and microbiome in rice (Oryza sativa) under low P conditions. Arch. Microbiol. 2021, 203, 5599–5611. [Google Scholar] [CrossRef]
- Mitra, D.; Guerra Sierra, B.E.; Khoshru, B.; Santos Villalobos, S.D.L.; Belz, C.; Chaudhary, P.; Shahri, F.N.; Djebaili, R.; Adeyemi, N.O.; El-Ballat, E.M.; et al. Impacts of arbuscular mycorrhizal fungi on rice growth, development, and stress management with a particular emphasis on strigolactone effects on root development. Commun. Soil Sci. Plan. 2021, 52, 1591–1621. [Google Scholar] [CrossRef]
- Nasir, F.; Tian, L.; Shi, S.; Chang, C.L.; Ma, L.; Gao, Y.Z.; Tian, C.J. Strigolactones positively regulate defense against Magnaporthe oryzae in rice (Oryza sativa). Plant Physiol. Bioch. 2019, 142, 106–116. [Google Scholar] [CrossRef]
- Mandal, S.M.; Chakraborty, D.; Dey, S. Phenolic acids act as signaling molecules in plant-microbe symbioses. Plant Signal. Behav. 2010, 5, 359–368. [Google Scholar] [CrossRef] [Green Version]
- Frankenberger, W.T.; Arshad, M. Phytohormones in Soils: Microbial Production and Function; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Khan, N.; Bano, A.; Ali, S.; Babar, M.A. Crosstalk amongst phytohormones from planta and PGPR under biotic and abiotic stresses. Plant Growth Regul. 2020, 90, 189–203. [Google Scholar] [CrossRef]
- Lugtenberg, B.; Rozen, D.E.; Kamilova, F. Wars between microbes on roots and fruits. F1000Research 2017, 6, 343. [Google Scholar] [CrossRef] [Green Version]
- Doni, F.; Mispan, M.S.; Suhaimi, N.S.M.; Ishak, N.; Uphoff, N. Roles of microbes in supporting sustainable rice production using the system of rice intensification. Appl. Microbiol. Biot. 2019, 103, 5131–5142. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Kai, M.; Effmert, U.; Piechulla, B. Bacterial-plant-interactions: Approaches to unravel the biological function of bacterial volatiles in the rhizosphere. Front. Microbiol. 2016, 7, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naseem, H.; Bano, A. Role of plant growth-promoting rhizobacteria and their exopolysaccharide in drought tolerance of maize. J. Plant Interact. 2014, 9, 689–701. [Google Scholar] [CrossRef] [Green Version]
- Helman, Y.; Chernin, L. Silencing the mob: Disrupting quorum sensing as a means to fight plant disease. Mol. Plant Pathol. 2015, 16, 316–329. [Google Scholar] [CrossRef]
- Ma, Y.; Oliveira, R.S.; Freitas, H.; Zhang, C. Biochemical and molecular mechanisms of plant-microbe-metal interactions: Relevance for phytoremediation. Front. Plant Sci. 2016, 7, 918. [Google Scholar] [CrossRef]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant-microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef]
- Pandit, A.; Adholeya, A.; Cahill, D.; Brau, L.; Kochar, M. Microbial biofilms in nature: Unlocking their potential for agricultural applications. J. Appl. Microbiol. 2020, 129, 199–211. [Google Scholar] [CrossRef] [Green Version]
- Tisserant, E.; Malbreil, M.; Kuo, A.; Kohler, A.; Symeonidi, A.; Balestrini, R.; Charron, P.; Duensing, N.; dit Frey, N.F.; Gianinazzi-Pearson, V. Genome of an arbuscular mycorrhizal fungus provides insight into the oldest plant symbiosis. Proc. Natl. Acad. Sci. 2013, 110, 20117–20122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berendsen, R.L.; Pieterse, C.M.; Bakker, P.A. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Fagundes, C.T.; Amaral, F.A.; Teixeira, A.L.; Souza, D.G.; Teixeira, M.M. Adapting to environmental stresses: The role of the microbiota in controlling innate immunity and behavioral responses. Immunol. Rev. 2012, 245, 250–264. [Google Scholar] [CrossRef] [PubMed]
- Cameron, D.D.; Neal, A.L.; van Wees, S.C.; Ton, J. Mycorrhiza-induced resistance: More than the sum of its parts? Trends Plant Sci. 2013, 18, 539–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuti, M.; Giovannetti, G. Borderline products between bio-fertilizers/bio-effectors and plant protectants: The role of microbial consortia. J. Agric. Sci. Technol. A 2015, 5, 305–315. [Google Scholar]
- Pangesti, N.; Reichelt, M.; van de Mortel, J.E.; Kapsomenou, E.; Gershenzon, J.; van Loon, J.J.; Dicke, M.; Pineda, A. Jasmonic acid and ethylene signaling pathways regulate glucosinolate levels in plants during rhizobacteria-induced systemic resistance against a leaf-chewing herbivore. J. Chem. Ecol. 2016, 42, 1212–1225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baez-Rogelio, A.; Morales-García, Y.E.; Quintero-Hernández, V.; Muñoz-Rojas, J. Next generation of microbial inoculants for agriculture and bioremediation. Microb. Biotechnol. 2017, 10, 19–21. [Google Scholar]
- Masoero, G.; Giovannetti, G. Biotechnologies based on microbial consortia. Atti Dell’Accademia Di Agricoltura Di Torino 2011, 153, 111–127. [Google Scholar]
- Masoero, G.; Giovannetti, G. In vivo Stem pH can testify the acidification of the maize treated by mycorrhizal and microbial consortium. J. Enviro. Agri. Sci. 2015, 3, 23–30. [Google Scholar]
- Chinnusamy, M.; Kaushik, B.D.; Prasanna, R. Growth, nutritional, and yield parameters of wetland rice as influenced by microbial consortia under controlled conditions. J. Plant Nutr. 2006, 29, 857–871. [Google Scholar]
- Earanna, N.; Muruli, K. Field evaluation of nursery bed inoculated arbuscular mycorrhiza and rootdip inoculated Azotobacter chroococcum and Aspergillus awamori on aerobic rice. J. Appl. Nat. Sci. 2011, 3, 58–61. [Google Scholar] [CrossRef]
- Santhosh, G.P.; Siddaram, M.D.; Shubha, S. Interaction effect of consortium of Azospirillum, PSB and AM fungus with reduced levels of N & P fertilizers on growth of direct seeded rice. J. Eco-friendly Agri. 2018, 13, 22–26. [Google Scholar]
- Upadhyay, M.K.; Yadav, P.; Shukla, A.; Srivastava, S. Utilizing the potential of microorganisms for managing arsenic contamination: A feasible and sustainable approach. Front. Environ. Sci. 2018, 6, 24. [Google Scholar] [CrossRef] [Green Version]
- Ashnaei, S.P. Plant growth promoting rhizobacteria and Rhizophagus irregularis: Biocontrol of rice blast in wild type and mycorrhiza-defective mutant. J. Plant Protect. Res. 2019, 3, 362–375. [Google Scholar]
- Sawers, R.J.; Gutjahr, C.; Paszkowski, U. Cereal mycorrhiza: An ancient symbiosis in modern agriculture. Trends Plant Sci. 2008, 13, 93–97. [Google Scholar] [CrossRef] [Green Version]
| Type of Microorganisms | Microbe Species | AMF Species | Effects | Reference |
|---|---|---|---|---|
| Bacteria | A. Lipoferum, B. megaterium | Glomus sp., Gigasporasp sp. and Acaulospora sp. | Rice growth↑, grain yield↑ | [50] |
| A. brasilense, B. cepacia | F. mosseae | Rice growth↑, grain yield↑, P uptake↑ | [53] | |
| H. seropedicae | F. mosseae | Rice growth↑ | [52] | |
| P. fluorescens, P. putida | R. irregularis | Grain yield↑, salinity↓ | [51] | |
| P. jessenii, P. synxantha | A natural AMF consortium (Mnat) and a single-spore AMF strain (Mss2) | No change | [48] | |
| S. thermocarboxydus | F. mosseae | Rice growth↑ | [54] | |
| Fungi | R. solani | R. fasciculatum, F. mosseae, G. aggregatum, G. fulvum, G. candida, and G. gigantea | AMF spore density and infection↑, sheath blight↓ | [55] |
| R. irregularis, F. mosseae, G. deserticola, R. fasciculatum, S. dussii, and G. microaggregatum | Sheath blight↑ | [56] | ||
| Soil faunas and other microbes | M. graminicola | R. irregularis, F. mossae | F. mossae decreased M. graminicola multiplication, while R. irregularis did not | [32] |
| Blue-Green Algae | G. microcarpium | Rice growth↑, grain yield↑ | [57] |
| Type of Microorganisms | Full Name | Abbreviations |
|---|---|---|
| AMF | Rhizophagus irregularis | R. irregularis |
| Funneliformis mosseae | F. mosseae | |
| Gigaspora candida | G. candida | |
| Glomus aggregatum | G. aggregatum | |
| Glomus caledonium | G. caledonium | |
| Glomus coronatum | G. coronatum | |
| Glomus deserticola | G. deserticola | |
| Glomus fulvum | G. fulvum | |
| Gigaspora gigantea | G. gigantea | |
| Glomus microaggregatum | G. microaggregatum | |
| Glomus viscosum | G. viscosum | |
| Rhizophagus fasciculatum | R. fasciculatum | |
| Sclerocystis dussii | S.dussii | |
| Bacteria | Azospirillum brasilense | Brasilense |
| Azospirillum lipoferum | A. lipoferum | |
| Bacillus megaterium | B. megaterium | |
| Bacillus subtilis | B. subtilis | |
| Burkholderia cepacia | B. cepacia | |
| Herbaspirillum seropedicae | H. seropedicae | |
| Pseudomonas fluorescens | P. fluorescens | |
| Pseudomonas jessenii | P. jessenii | |
| Pseudomonas putida | P. putida | |
| Pseudomonas synxantha | P. synxantha | |
| Streptomyces thermocarboxydus | S. thermocarboxydus | |
| Fungi | Rhizoctonia solani | R. solani |
| Magnaporthe oryzae | M. oryzae | |
| Soil fauna | Meloidogyne graminicola | M. graminicola |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, X.; Zou, J.; Zhang, B.; Wu, L.; Yang, T.; Huang, Q. Arbuscular Mycorrhizal Fungi and Microbes Interaction in Rice Mycorrhizosphere. Agronomy 2022, 12, 1277. https://doi.org/10.3390/agronomy12061277
Bao X, Zou J, Zhang B, Wu L, Yang T, Huang Q. Arbuscular Mycorrhizal Fungi and Microbes Interaction in Rice Mycorrhizosphere. Agronomy. 2022; 12(6):1277. https://doi.org/10.3390/agronomy12061277
Chicago/Turabian StyleBao, Xiaozhe, Jixiang Zou, Bin Zhang, Longmei Wu, Taotao Yang, and Qing Huang. 2022. "Arbuscular Mycorrhizal Fungi and Microbes Interaction in Rice Mycorrhizosphere" Agronomy 12, no. 6: 1277. https://doi.org/10.3390/agronomy12061277
APA StyleBao, X., Zou, J., Zhang, B., Wu, L., Yang, T., & Huang, Q. (2022). Arbuscular Mycorrhizal Fungi and Microbes Interaction in Rice Mycorrhizosphere. Agronomy, 12(6), 1277. https://doi.org/10.3390/agronomy12061277





