Effect of Selenium Nanocomposites Based on Natural Polymer Matrices on the Biomass and Storage of Potato Tubers in a Field Experiment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nanocomposites (NCs)
2.1.1. Se/AG NC
2.1.2. Se/CAR NC
2.1.3. Se/ST NC
2.2. Potato Material and Experiments
2.3. Biochemical Analysis
3. Results
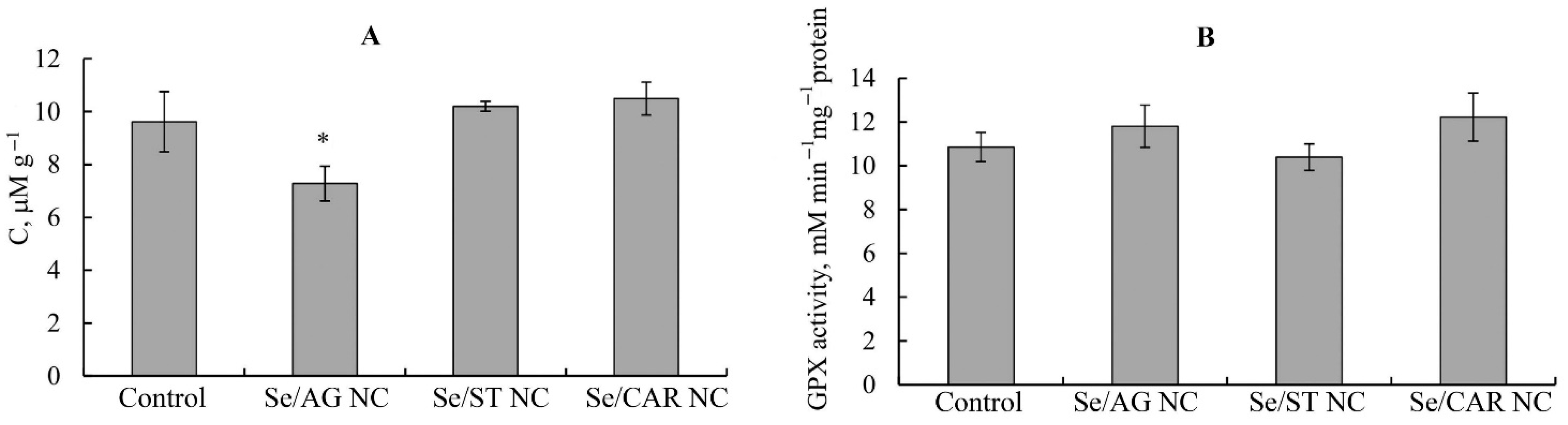
3.1. Biochemical Analysis
3.2. Field Experiment (1st Generation)
3.3. Tuber Storage Analysis
3.4. Field Experiment (2nd Generation)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Oliveira, T.B.; de Lucas, R.C.; Scarcella, A.S.A.; Contato, A.G.; Pasin, T.M.; Martinez, C.A.; Polizeli, M.L.T.M. Fungal communities differentially respond to warming and drought in tropical grassland soil. Mol. Ecol. 2020, 29, 1550–1559. [Google Scholar] [CrossRef] [PubMed]
- Yazid, S.N.E.; Jinap, S.; Ismail, S.I.; Magan, N.; Samsudin, N.I.P. Phytopathogenic organisms and mycotoxigenic fungi: Why do we control one and neglect the other? A biological control perspective in Malaysia. Compr. Rev. Food Sci. Food Saf. 2020, 19, 643–669. [Google Scholar] [CrossRef] [PubMed]
- Lykogianni, M.; Bempelou, E.; Karamaouna, F.; Aliferis, K.A. Do pesticides promote or hinder sustainability in agriculture? The challenge of sustainable use of pesticides in modern agriculture. Sci. Total Environ. 2021, 795, 148625. [Google Scholar] [CrossRef] [PubMed]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.Q. Heavy metals and pesticides toxicity in agricultural soil and plants: Ecological risks and human health implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Yang, H. Metabolism and detoxification of pesticides in plants. Sci. Total Environ. 2021, 790, 148034. [Google Scholar] [CrossRef]
- Freitas, L.M.; Paranaíba, J.; Peréz, A.; Machado, M.; Lima, F.C. Toxicity of pesticides in lizards. Hum. Exp. Toxicol. 2020, 39, 596–604. [Google Scholar] [CrossRef]
- Serrão, J.E.; Plata-Rueda, A.; Martínez, L.C.; Zanuncio, J.C. Side-effects of pesticides on non-target insects in agriculture: A mini-review. Naturwissenschaften 2022, 109, 17. [Google Scholar] [CrossRef]
- Oliveira, J.M.; Destro, A.L.F.; Freitas, M.B.; Oliveira, L.L. How do pesticides affect bats?—A brief review of recent publications. Braz. J. Biol. 2021, 81, 499–507. [Google Scholar] [CrossRef]
- Schmitt, F.; Babylon, L.; Dieter, F.; Eckert, G.P. Effects of pesticides on longevity and bioenergetics in invertebrates-the impact of polyphenolic metabolites. Int. J. Mol. Sci. 2021, 22, 13478. [Google Scholar] [CrossRef]
- Čadež, T.; Kolić, D.; Šinko, G.; Kovarik, Z. Assessment of four organophosphorus pesticides as inhibitors of human acetylcholinesterase and butyrylcholinesterase. Sci. Rep. 2021, 11, 21486. [Google Scholar] [CrossRef]
- Wan, E.T.; Darssan, D.; Karatela, S.; Reid, S.A.; Osborne, N.J. Association of pesticides and kidney function among adults in the US population 2001–2010. Int. J. Environ. Res. Public Health 2021, 18, 10249. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.; Thongkum, W.; Tudpor, K.; Turnbull, N.; Yukalang, N.; Sychareun, V.; Van Vo, T.; Win, L.L.; Watkins, A.; Jordan, S. Pesticides use and health impacts on farmers in Thailand, Vietnam, and Lao PDR: Protocol for a survey of knowledge, behaviours and blood acetyl cholinesterase concentrations. PLoS ONE 2021, 16, e0258134. [Google Scholar] [CrossRef] [PubMed]
- Bapat, M.S.; Singh, H.; Shukla, S.K.; Singh, P.P.; Vo, D.N.; Yadav, A.; Goyal, A.; Sharma, A.; Kumar, D. Evaluating green silver nanoparticles as prospective biopesticides: An environmental standpoint. Chemosphere 2022, 286, 131761. [Google Scholar] [CrossRef]
- Nile, A.S.; Kwon, Y.D.; Nile, S.H. Horticultural oils: Possible alternatives to chemical pesticides and insecticides. Environ. Sci. Pollut. Res. Int. 2019, 26, 21127–21139. [Google Scholar] [CrossRef] [PubMed]
- Mishra, J.; Dutta, V.; Arora, N.K. Biopesticides in India: Technology and sustainability linkages. 3 Biotech. 2020, 10, 210. [Google Scholar] [CrossRef]
- Kumar, J.; Ramlal, A.; Mallick, D.; Mishra, V. An overview of some biopesticides and their importance in plant protection for commercial acceptance. Plants 2021, 10, 1185. [Google Scholar] [CrossRef]
- Souto, A.L.; Sylvestre, M.; Tölke, E.D.; Tavares, J.F.; Barbosa-Filho, J.M.; Cebrián-Torrejón, G. Plant-derived pesticides as an alternative to pest management and sustainable agricultural production: Prospects, applications and challenges. Molecules 2021, 26, 4835. [Google Scholar] [CrossRef]
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The history of nanoscience and nanotechnology: From chemical-physical applications to nanomedicine. Molecules 2019, 25, 112. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Deng, H.; Hwang, H.M. The current application of nanotechnology in food and agriculture. J. Food Drug Anal. 2019, 27, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Manouchehri, M.; Seidi, S.; Abdullah, F.O. Application of magnetic nanomaterials in magnetic-chromatography: A review. Talanta 2021, 229, 122273. [Google Scholar] [CrossRef]
- Bognár, S.; Putnik, P.; Šojić Merkulov, D. Sustainable green nanotechnologies for innovative purifications of water: Synthesis of the nanoparticles from renewable sources. Nanomaterials 2022, 12, 263. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, R.T.; Salvadori, M.R.; Rafatullah, M.; Siddiqui, M.R.; Khan, M.A.; Alshareef, S.A. Exploration of microbial factories for synthesis of nanoparticles—A sustainable approach for bioremediation of environmental contaminants. Front. Microbiol. 2021, 12, 658294. [Google Scholar] [CrossRef] [PubMed]
- Harish, V.; Tewari, D.; Gaur, M.; Yadav, A.B.; Swaroop, S.; Bechelany, M.; Barhoum, A. Review on nanoparticles and nanostructured materials: Bioimaging, biosensing, drug delivery, tissue engineering, antimicrobial, and agro-food applications. Nanomaterials 2022, 12, 457. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Liu, H.; Zhao, D.; Pan, C.; Wang, C. Pesticide residue behavior and risk assessment in celery after Se nanoparticles application. Foods 2021, 10, 1987. [Google Scholar] [CrossRef]
- Bhatia, R.; Gulati, D.; Sethi, G. Biofilms and nanoparticles: Applications in agriculture. Folia Microbiol. 2021, 66, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Shende, S.; Rajput, V.D.; Gade, A.; Minkina, T.; Fedorov, Y.; Sushkova, S.; Mandzhieva, S.; Burachevskaya, M.; Boldyreva, V. Metal-based green synthesized nanoparticles: Boon for sustainable agriculture and food security. IEEE Trans Nanobiosci. 2022, 21, 44–54. [Google Scholar] [CrossRef]
- Dhiman, S.; Yadav, A.; Debnath, N.; Das, S. Application of core/shell nanoparticles in smart farming: A paradigm shift for making the agriculture sector more sustainable. J. Agric. Food Chem. 2021, 69, 3267–3283. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Ashraf, M. Nanoparticles potentially mediate salt stress tolerance in plants. Plant Physiol. Biochem. 2021, 160, 257–268. [Google Scholar] [CrossRef]
- Chaud, M.; Souto, E.B.; Zielinska, A.; Severino, P.; Batain, F.; Oliveira-Junior, J.; Alves, T. Nanopesticides in agriculture: Benefits and challenge in agricultural productivity, toxicological risks to human health and environment. Toxics 2021, 9, 131. [Google Scholar] [CrossRef]
- Santos, T.S.; Silva, T.M.; Cardoso, J.C.; Albuquerque-Júnior, R.L.C.; Zielinska, A.; Souto, E.B.; Severino, P.; Mendonça, M.D.C. Biosynthesis of silver nanoparticles mediated by entomopathogenic fungi: Antimicrobial resistance, nanopesticides, and toxicity. Antibiotics 2021, 10, 852. [Google Scholar] [CrossRef]
- de Oliveira, J.L. Nano-biopesticides: Present concepts and future perspectives in integrated pest management. In Advances in Nano-Fertilizers and Nano-Pesticides in Agriculture; Jogaiah, S., Singh, H.B., Fraceto, L.F., Lima, R.D., Eds.; Woodhead Publishing: Sawston, UK, 2021; pp. 1–27. [Google Scholar] [CrossRef]
- Lade, B.D.; Gogle, D.P.; Nandeshwar, S.B. Nano bio pesticide to constraint plant destructive pests. J. Nanomed. Res. 2017, 6, 00158. [Google Scholar] [CrossRef] [Green Version]
- Perfileva, A.I.; Nozhkina, O.A.; Graskova, I.A.; Sidorov, A.V.; Lesnichaya, M.V.; Aleksandrova, G.P.; Dolmaa, G.; Klimenkov, I.V.; Sukhov, B.G. Synthesis of selenium and silver nanobiocomposites and their influence on phytopathogenic bacterium Clavibacter michiganensis subsp. sepedonicus. Russ. Chem. Bull. 2018, 67, 157–163. [Google Scholar] [CrossRef]
- Perfileva, A.I.; Nozhkina, O.A.; Ganenko, T.V.; Sukhov, B.G.; Artem’ev, A.V.; Trofimov, B.A.; Krutovsky, K.V. Selenium nanocomposites in natural matrices as potato recovery agent. Int. J. Mol. Sci. 2021, 22, 4576. [Google Scholar] [CrossRef] [PubMed]
- Perfileva, A.I.; Tsivileva, O.M.; Nozhkina, O.A.; Karepova, M.S.; Graskova, I.A.; Ganenko, T.V.; Sukhov, B.G.; Krutovsky, K.V. Effect of natural polysaccharide matrix-based selenium nanocomposites on Phytophthora cactorum and rhizospheric microorganisms. Nanomaterials 2021, 11, 2274. [Google Scholar] [CrossRef] [PubMed]
- Perfileva, A.I.; Nozhkina, O.A.; Tretyakova, M.S.; Graskova, I.A.; Klimenkov, I.V.; Sudakov, N.P.; Alexandrova, G.P.; Sukhov, B.G. Biological activity and environmental safety of selenium nanoparticles encapsulated in starch macromolecules. Nanotechnol. Russ. 2020, 15, 96–104. [Google Scholar] [CrossRef]
- Nozhkina, O.A.; Perfileva, A.I.; Graskova, I.A.; Dyakova, A.V.; Nurminsky, V.N.; Klimenkov, I.V.; Ganenko, T.V.; Borodina, T.N.; Aleksandrova, G.P.; Sukhov, B.G.; et al. The biological activity of a selenium nanocomposite encapsulated in carrageenan macromolecules with respect to ring rot pathogenesis of potato plants. Nanotechnol. Russ. 2019, 14, 255–262. [Google Scholar] [CrossRef]
- Graskova, I.A.; Perfilieva, A.I.; Nozhkina, O.A.; Sukhov, B.G.; Aleksandrova, G.P.; Trofimov, B.A. Silver-containing nanocomposites of humic substances, agents for healing of potatoes from the ring rot. Dokl. Biol. Sci. 2018, 483, 239–242. [Google Scholar] [CrossRef]
- Perfileva, A.I.; Nozhkina, O.A.; Aleksandrova, G.P.; Karepova, M.S.; Zabanova, N.S.; Graskova, I.A.; Sukhov, B.G.; Trofimov, B.A. The effects of humic substances and humic substance-based silver nanocomposites on the viability of rhizospheric microorganisms. Nanobiotechnol. Rep. 2021, 16, 525–531. [Google Scholar] [CrossRef]
- Khutsishvili, S.S.; Perfileva, A.I.; Nozhkina, O.A.; Karepova, M.S.; Ganenko, T.V.; Sukhov, B.G.; Krutovsky, K.V. Novel nanobiocomposites based on natural polysaccharides as universal trophic low-dose micronutrients. Int. J. Mol. Sci. 2021, 22, 12006. [Google Scholar] [CrossRef]
- Nurminsky, V.N.; Perfileva, A.I.; Kapustina, I.S.; Graskova, I.A.; Sukhov, B.G.; Trofimov, B.A. Growth-stimulating activity of natural polymer-based nanocomposites of selenium during the germination of cultivated plant seeds. Dokl. Biochem. Biophys. 2020, 495, 296–299. [Google Scholar] [CrossRef]
- Papkina, A.V.; Perfileva, A.I.; Zhivet’yev, M.A.; Borovskii, G.B.; Graskova, I.A.; Klimenkov, I.V.; Lesnichaya, M.V.; Sukhov, B.G.; Trofimov, B.A. Complex effects of selenium-arabinogalactan nanocomposite on both phytopathogen Clavibacter michiganensis subsp. sepedonicus and potato plants. Nanotechnologies Russ. 2015, 10, 484–491. [Google Scholar] [CrossRef]
- Sukhov, B.G.; Pogodaeva, N.N.; Kuznetsov, S.V.; Kupriyanovich, Y.N.; Trofimov, B.A.; Yurinova, G.V.; Selivanova, D.S.; Pristavka, A.A.; Medvedeva, P.A.; Dzhioev, Y.P.; et al. Prebiotic effect of native noncovalent arabinogalactan-flavonoid conjugates on bifidobacteria. Russ. Chem. Bull. 2014, 63, 2189–2194. [Google Scholar] [CrossRef]
- Artem’ev, A.V.; Malysheva, S.F.; Gusarova, N.K.; Trofimov, B.A. Rapid and convenient one-pot method for the preparation of alkali metal phosphinodiselenoates. Synthesis 2010, 14, 2463–2467. [Google Scholar] [CrossRef]
- Faithfull, T. (Ed.) Methods in Agricultural Chemical Analysis: A Practical Handbook; CABI Publishing: Wallingford, UK, 2002; 304p. [Google Scholar] [CrossRef]
- Bolland, J.L.; Koch, H.P. The course of antioxidant reaction in polyisoprenes and allied compounds. Part IX. The primary thermal oxidation product of ethyl linoleate. J. Chem. Soc. 1945, 7, 445–447. [Google Scholar] [CrossRef]
- Revin, V.V.; Gromova, N.V.; Revina, E.S.; Samonova, A.Y.; Tychkov, A.Y.; Bochkareva, S.S.; Moskovkin, A.A.; Kuzmenko, T.P. The influence of oxidative stress and natural antioxidants on morphometric parameters of red blood cells, the hemoglobin oxygen binding capacity, and the activity of antioxidant enzyme. BioMed. Res. Int. 2019, 2019, 2109269. [Google Scholar] [CrossRef]
- Pagila, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar]
- Mhamdi, A.; Van Breusegem, F. Reactive oxygen species in plant development. Development 2018, 145, dev164376. [Google Scholar] [CrossRef] [Green Version]
- Castro, B.; Citterico, M.; Kimura, S.; Stevens, D.M.; Wrzaczek, M.; Coaker, G. Stress-induced reactive oxygen species compartmentalization, perception and signalling. Nat. Plants 2021, 7, 403–412. [Google Scholar] [CrossRef]
- Noctor, G.; Reichheld, J.-P.; Foyer, C.H. ROS-related redox regulation and signaling in plants. Semin. Cell Dev. Biol. 2018, 80, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Halliwell, B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef] [Green Version]
- Mani, F.; Bettaieb, T.; Doudech, N.; Hannachi, C. Physiological mechanisms for potato dormancy release and sprouting: A review. Afr. Crop Sci. J. 2014, 22, 155–174. [Google Scholar]
- Mani, F.; Hannachi, C. Physiology of potato sprouting. J. New Sci. Agric. Biotechnol. 2015, 17, 591–602. [Google Scholar]
- Sonnewald, S.; Sonnewald, U. Regulation of potato tuber sprouting. Planta 2014, 239, 27–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.Y. Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxid. Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef] [Green Version]
- Moldogazieva, N.T.; Mokhosoev, I.M.; Mel’nikova, T.I.; Porozov, Y.B.; Terentiev, A.A. Oxidative stress and advanced lipoxidation and glycation end products (ALEs and AGEs) in aging and age-related diseases. Oxid. Med. Cell. Longev. 2019, 2019, 3085756. [Google Scholar] [CrossRef] [Green Version]
- Kokila, K.; Elavarasan, N.; Sujatha, V. Diospyros montana leaf extract-mediated synthesis of selenium nanoparticles and their biological applications. New J. Chem. 2017, 41, 7481–7490. [Google Scholar] [CrossRef]
- Chen, W.; Yue, L.; Jiang, Q.; Xia, W. Effect of chitosan with different molecular weight on the stability, antioxidant and anticancer activities of well-dispersed selenium nanoparticles. IET Nanobiotechnol. 2019, 13, 30–35. [Google Scholar] [CrossRef]
- Kondaparthi, P.; Flora, S.J.S.; Naqvi, S. Selenium nanoparticles: An insight on its Pro-oxidant and antioxidant properties. Front. Nanosci. Nanotechnol. 2019, 6, 1–5. [Google Scholar] [CrossRef]
- Rehman, A.; John, P.; Bhatti, A. Biogenic selenium nanoparticles: Potential solution to oxidative stress mediated inflammation in rheumatoid arthritis and associated complications. Nanomaterials 2021, 11, 2005. [Google Scholar] [CrossRef]
- Fararh, K.M.; Farid, A.S.; Abdalla, O.A.; Algharib, S.A. Antioxidant effect of selenium and its Nano form on oxidative stress induced by iron overload. Benha Vet. Med. J. 2016, 31, 96–102. [Google Scholar]
- Hernández-Hernández, H.; Quiterio-Gutiérrez, T.; Cadenas-Pliego, G.; Ortega-Ortiz, H.; Hernández-Fuentes, A.D.; Cabrera de la Fuente, M.; Valdés-Reyna, J.; Juárez-Maldonado, A. Impact of selenium and copper nanoparticles on yield, antioxidant system, and fruit quality of tomato plants. Plants 2019, 8, 355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubrovina, V.I.; Medvedeva, A.S.; Vityazeva, S.A.; Kolesnikova, O.B.; Aleksandrova, G.P.; Gutsol, L.O.; Grischenko, L.A.; Chetveriakova, T.D. Structure and Immunomodulatory Effects of Arabinogalactan of Siberian Larch and Its Metal Intermediates; Asprint: Irkutsk, Russia, 2007; p. 145. [Google Scholar]
- Pawar, R.P.; Kushekar, B.A.; Jadhav, B.S.; Kharat, K.R.; Borade, R.M.; Domb, A.J. Arabinogalactan in clinical use. In Biodegradable Polymers in Clinical Use and Clinical Development; Domb, A.J., Kumar, N., Ezra, A., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; Chapter 7; pp. 217–245. [Google Scholar] [CrossRef]
- Pangestuti, R.; Kim, S.-K. Biological activities of carrageenan. Adv. Food Nutr. Res. 2014, 72, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Hu, Y. New definition of resistant starch types from the gut microbiota perspectives—A review. Crit. Rev. Food Sci. Nutr. 2022. Online ahead of print. [Google Scholar] [CrossRef]
- Shurygina, I.A.; Sukhov, B.G.; Fadeeva, T.V.; Umanets, V.A.; Shurygin, M.G.; Ganenko, T.V.; Kostyro, Y.A.; Grigoriev, E.G.; Trofimov, B.A. Bactericidal action of Ag(0)-antithrombotic sulfated arabinogalactan nanocomposite: Coevolution of initial nanocomposite and living microbial cell to a novel nonliving nanocomposite. Nanomed. NBM 2011, 7, 827–833. [Google Scholar] [CrossRef]
- Reintjes, G.; Arnosti, C.; Fuchs, B.M.; Amann, R. An alternative polysaccharide uptake mechanism of marine bacteria. ISME J. 2017, 11, 1640–1650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shukla, P.S.; Borza, T.; Critchley, A.T.; Prithiviraj, B. Carrageenans from red seaweeds as promoters of growth and elicitors of defense response in plants. Front. Mar. Sci. 2016, 3, 81. [Google Scholar] [CrossRef]
- Pacheco, D.; Cotas, J.; Rocha, C.P.; Araújo, G.S.; Figueirinha, A.; Gonçalves, A.M.M.; Bahcevandziev, K.; Pereira, L. Seaweeds’ carbohydrate polymers as plant growth promoters. Carbohydr. Polym. Technol. Appl. 2021, 2, 100097. [Google Scholar] [CrossRef]
- Garza-García, J.J.O.; Hernández-Díaz, J.A.; Zamudio-Ojeda, A.; León-Morales, J.M.; Guerrero-Guzmán, A.; Sánchez-Chiprés, D.R.; López-Velázquez, J.C.; García-Morales, S. The role of selenium nanoparticles in agriculture and food technology. Biol. Trace Elem. Res. 2022, 200, 2528–2548. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Basuini, M.F.E.; Yilmaz, S.; Abdel-Latif, H.M.R.; Kari, Z.A.; Abdul Razab, M.K.A.; Ahmed, H.A.; Alagawany, M.; Gewaily, M.S. Selenium nanoparticles as a natural antioxidant and metabolic regulator in aquaculture: A review. Antioxidants 2021, 10, 1364. [Google Scholar] [CrossRef]
- Abedi, S.; Iranbakhsh, A.; Ardebili, Z.O.; Ebadi, M. Nitric oxide and selenium nanoparticles confer changes in growth, metabolism, antioxidant machinery, gene expression, and flowering in chicory (Cichorium intybus L.): Potential benefits and risk assessment. Environ. Sci. Pollut. Res. 2020, 265, 114727. [Google Scholar] [CrossRef]
- Jiang, C.; Zu, C.; Shen, J.; Shao, F.; Li, T. Effects of selenium on the growth and photosynthetic characteristics of flue-cured tobacco (Nicotiana tabacum L.). Acta. Soc. Bot. Pol. 2015, 84, 71. [Google Scholar] [CrossRef] [Green Version]
- Hussein, H.A.; Darwesh, O.M.; Mekki, B.B.; El-Hallouty, S.M. Evaluation of cytotoxicity, biochemical profile and yield components of groundnut plants treated with nano-selenium. Biotechnol. Rep. 2019, 12, e00377. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.A.; Blinov, A.V.; Serov, A.V.; Gvozdenko, A.A.; Kravtsov, A.A.; Nagdalian, A.A.; Raffa, V.V.; Maglakelidze, D.G.; Blinova, A.A.; Kobina, A.V. Effect of selenium nanoparticles on germination of hordéum vulgáre barley seeds. Coatings 2021, 11, 862. [Google Scholar] [CrossRef]
- Rady, M.M.; Desoky, E.M.; Ahmed, S.M.; Majrashi, A.; Ali, E.F.; Arnaout, S.M.A.I.; Selem, E. Foliar nourishment with nano-selenium dioxide promotes physiology, biochemistry, antioxidant defenses, and salt tolerance in Phaseolus vulgaris. Plants 2021, 10, 1189. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Chen, S.; Gao, D.; Liu, G.Q.; Bai, H.X.; Li, A.; Peng, L.X.; Ren, Z.Y. Selenium improves photosynthesis and protects photosystem II in pear (Pyrus bretschneideri), grape (Vitis vinifera), and peach (Prunus persica). Photosynthetica 2015, 53, 609. [Google Scholar] [CrossRef]
- Babajani, A.; Iranbakhsh, A.; Ardebili, Z.O.; Eslami, B. Differential growth, nutrition, physiology, and gene expression in Melissa officinalis mediated by zinc oxide and elemental selenium nanoparticles. Environ. Sci. Pollut. Res Int. 2019, 26, 24430. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, T.; Liu, H.; Zhou, F.; Zhang, J.; Zhang, M.; Liu, X.; Shi, W.; Cao, T. Nano-selenium controlled cadmium accumulation and improved photosynthesis in indica rice cultivated in lead and cadmium combined paddy soils. J. Environ. Sci. 2021, 103, 336–346. [Google Scholar] [CrossRef]
- Morales-Espinoza, M.C.; Cadenas-Pliego, G.; Pérez-Alvarez, M.; Hernández-Fuentes, A.D.; Cabrera de la Fuente, M.; Benavides-Mendoza, A.; Valdés-Reyna, J.; Juárez-Maldonado, A. Se nanoparticles induce changes in the growth, antioxidant responses, and fruit quality of tomato developed under NaCl stress. Molecules 2019, 24, 3030. [Google Scholar] [CrossRef] [Green Version]
- Quiterio-Gutiérrez, T.; Ortega-Ortiz, H.; Cadenas-Pliego, G.; Hernández-Fuentes, A.D.; Sandoval-Rangel, A.; Benavides-Mendoza, A.; Cabrera-de la Fuente, M.; Juárez-Maldonado, A. The application of selenium and copper nanoparticles modifies the biochemical responses of tomato plants under stress by Alternaria solani. Int. J. Mol. Sci. 2019, 20, 1950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haghighi, M.; Abolghasemi, R. The effect of metallic selenium and nano on the physiological characteristics of tomato plants. J. Soil Plant Interact. 2018, 9, 63–78. [Google Scholar] [CrossRef]
- Neysanian, M.; Iranbakhsh, A.; Ahmadvand, R.; Ardebili, Z.O.; Ebadi, M. Comparative efficacy of selenate and selenium nanoparticles for improving growth, productivity, fruit quality, and postharvest longevity through modifying nutrition, metabolism, and gene expression in tomato; potential benefits and risk assessment. PLoS ONE 2020, 15, e0244207. [Google Scholar] [CrossRef]
- Soleymanzadeh, R.; Iranbakhsh, A.; Habibi, G.; Ardebili, Z.O. Selenium nanoparticle protected strawberry against salt stress through modifications in salicylic acid, ion homeostasis, antioxidant machinery, and photosynthesis performance. Acta Biol. Crac. Ser. Bot. 2020, 62, 33–42. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Abdelrahman, M.; Hosseini, M.S.; Hoveizehe, N.F.; PhanTran, L.-S. Alleviation of the effect of salinity on growth and yield of strawberry by foliar spray of selenium-nanoparticles. Environ. Pollut. 2019, 253, 246. [Google Scholar] [CrossRef] [PubMed]
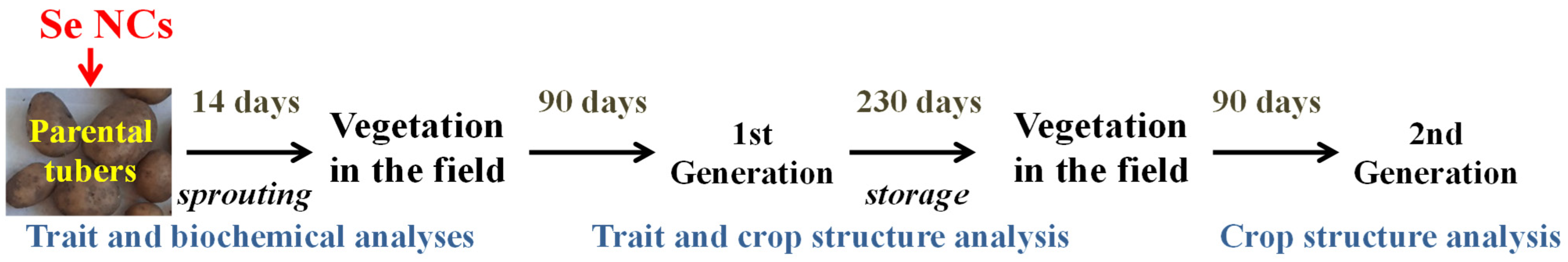




| Trait | Parental Tubers | First Generation | Second Generation | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | Se/AG | Se/ST | Se/CAR | C | Se/AG | Se/ST | Se/CAR | C | Se/AG | Se/ST | Se/CAR | |
| Mean length and weight of shoots measured after 14 days following treatment and in control | 20 (84) | 20 (87) | 20 (96) | 20 (66) | 10 | 10 | 10 | 10 | ||||
| Content of LPO and DC | 9 | 9 | 9 | 9 | ||||||||
| Number of planted tubers | 10 | 10 | 10 | 10 | 20 | 20 | 20 | 20 | ||||
| Mean number and biomass of tubers per plant | 64 | 112 | 70 | 122 | ||||||||
| Crop structure and number of rotten tubers | 64 | 112 | 70 | 122 | 334 | 232 | 419 | 406 | ||||
| Analysis of sprouting and health condition after 230 days of storage | 64 | 112 | 70 | 122 | ||||||||
| Shoot Trait | Control (84) | Se/AG NC (87) | Se/ST NC (96) | Se/CAR NC (66) |
|---|---|---|---|---|
| length, cm | 2.04 ± 0.10 | 2.20 ± 0.11 | 1.90 ± 0.08 | 3.12 ± 0.15 * |
| weight, g | 0.27 ± 0.02 | 0.32 ± 0.02 | 0.26 ± 0.02 | 0.46 ± 0.04 * |
| Trait | Control | Se/AG NC | Se/ST NC | Se/CAR NC | |
|---|---|---|---|---|---|
| Total tuber weight, g (based on the total number of plants studied in the brackets) | 1st repeat | 3990 (90) | 8180 (112) | 4735 (70) | 8885 (122) |
| 2nd repeat | 17,765 (412) | 12,499 (296) | 17,463 (378) | 14,914 (325) | |
| Median tuber weight, g | 54.0 [29.5; 85.5] | 42.0 [22.5; 68.3] * | 41.5 [24.1; 69.8] | 56.0 [32.0; 90.0] | |
| Median number of shoots per tuber | 3 [3; 4] | 3 [2; 4] | 3 [2; 4] | 3 [2; 4] | |
| Median length of shoots, cm | 1.4 [0.8; 2.3] | 1.6 [0.2; 1.9] | 1.4 [0.7;1.9] | 1.4 [0.7;2.1] | |
| Median shoot weight, g | 0.16 [0.07; 0.27] | 0.13 [0.06; 0.19] * | 0.13 [0.04; 0.22] * | 0.13 [0.05; 0.22] | |
| Tuber Trait | Effect | Se/AG NC | Control | p * | Se/ST NC | Control | p * | Se/CAR NC | Control | p * |
|---|---|---|---|---|---|---|---|---|---|---|
| Plot 1 | ||||||||||
| scab | affected | 0 | 59 | 0.0000 | 35 | 59 | 0.0000 | 34 | 59 | 0.0000 |
| unaffected | 106 | 5 | 36 | 5 | 23 | 5 | ||||
| dry pitted rot | affected | 10 | 17 | 0.0042 | 6 | 17 | 0.0057 | 12 | 17 | 0.5244 |
| unaffected | 96 | 46 | 65 | 46 | 45 | 46 | ||||
| wireworm | affected | 0 | 62 | 0.0000 | 0 | 62 | 0.0000 | 0 | 62 | 0.0000 |
| unaffected | 96 | 1 | 71 | 1 | 57 | 1 | ||||
| color | green | 6 | 0 | 0.0819 | 0 | 0 | 1.0000 | 0 | 0 | 1.0000 |
| normal | 90 | 63 | 71 | 63 | 57 | 63 | ||||
| Plot 2 | ||||||||||
| scab | affected | 80 | 32 | 0.0106 | 76 | 32 | 0.0000 | 80 | 32 | 0.0001 |
| unaffected | 35 | 32 | 14 | 32 | 21 | 32 | ||||
| dry pitted rot | affected | 11 | 10 | 0.2355 | 63 | 10 | 0.0000 | 10 | 10 | 0.3297 |
| unaffected | 104 | 54 | 27 | 54 | 91 | 54 | ||||
| wireworm | affected | 0 | 22 | 0.0000 | 0 | 22 | 0.0000 | 0 | 22 | 0.0000 |
| unaffected | 115 | 42 | 90 | 42 | 101 | 42 | ||||
| color | green | 0 | 0 | 1.0000 | 0 | 0 | 1.0000 | 0 | 0 | 1.0000 |
| normal | 115 | 64 | 90 | 64 | 101 | 64 | ||||
| Plot 3 | ||||||||||
| scab | affected | 103 | 37 | 0.0055 | 65 | 37 | 0.0805 | 86 | 37 | 0.7049 |
| unaffected | 12 | 15 | 12 | 15 | 29 | 15 | ||||
| dry pitted rot | affected | 80 | 5 | 0.0000 | 46 | 5 | 0.0000 | 17 | 5 | 0.4625 |
| unaffected | 35 | 47 | 31 | 47 | 98 | 47 | ||||
| wireworm | affected | 0 | 35 | 0.0000 | 0 | 35 | 0.0000 | 0 | 35 | 0.0000 |
| unaffected | 115 | 17 | 77 | 17 | 115 | 17 | ||||
| color | green | 0 | 0 | 1.0000 | 0 | 0 | 1.0000 | 25 | 0 | 0.0000 |
| normal | 115 | 52 | 77 | 52 | 90 | 52 | ||||
| Trait | Control | Se/AG NC | Se/ST NC | Se/CAR NC |
|---|---|---|---|---|
| Total tuber weight, g (based on 20 plants) | 13,462 | 8815 | 17,956 | 15,035 |
| Total number of tubers | 334 | 232 | 419 | 406 |
| Median tuber weight, g | 38 [20; 59] | 37 [19; 50] | 40 [21; 63] | 41 [25; 64] |
| Trait | Se/AG NC | Se/ST NC | Se/CAR NC |
|---|---|---|---|
| Parental tubers | |||
| DC level | decreased (17%) | - | - |
| GPX activity | - | - | - |
| Shoot length | - | - | increased (53%) |
| Shoot weight | - | - | increased (70%) |
| First generation | |||
| Crop structure | - | increased the number of seedable tubers (27%) | increased the number of marketable tubers (44%) |
| Mass of tubers | increased (59%) | - | increased (83%) |
| Number of tubers | increased (120%) | - | increased (80%) |
| Number of rotten tubers | decreased | - | - |
| Tuber weight after storage | decreased (23%) | - | - |
| Number of tubers affected by scab | decreased (90%) | decreased (7%) | decreased (4%) |
| Number of tubers affected by dry pitted rot | decreased (18%) | decreased (20%) | - |
| Number of tubers affected by wireworm | decreased (67%) | decreased (67%) | decreased (67%) |
| Shoot weight after storage | decreased (19%) | decreased (19%) | - |
| Second generation | |||
| Crop structure | decreased the number of seedable tubers (32%) | increased the number of marketable tubers (140%) | increased the number of marketable tubers (160%) |
| Mass of tubers | - | - | increased (12%) |
| Number of tubers | - | increased (31%) | - |
| Number of rotten tubers | - | decreased (28%) | decreased (36%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perfileva, A.I.; Graskova, I.A.; Sukhov, B.G.; Krutovsky, K.V. Effect of Selenium Nanocomposites Based on Natural Polymer Matrices on the Biomass and Storage of Potato Tubers in a Field Experiment. Agronomy 2022, 12, 1281. https://doi.org/10.3390/agronomy12061281
Perfileva AI, Graskova IA, Sukhov BG, Krutovsky KV. Effect of Selenium Nanocomposites Based on Natural Polymer Matrices on the Biomass and Storage of Potato Tubers in a Field Experiment. Agronomy. 2022; 12(6):1281. https://doi.org/10.3390/agronomy12061281
Chicago/Turabian StylePerfileva, Alla I., Irina A. Graskova, Boris G. Sukhov, and Konstantin V. Krutovsky. 2022. "Effect of Selenium Nanocomposites Based on Natural Polymer Matrices on the Biomass and Storage of Potato Tubers in a Field Experiment" Agronomy 12, no. 6: 1281. https://doi.org/10.3390/agronomy12061281
APA StylePerfileva, A. I., Graskova, I. A., Sukhov, B. G., & Krutovsky, K. V. (2022). Effect of Selenium Nanocomposites Based on Natural Polymer Matrices on the Biomass and Storage of Potato Tubers in a Field Experiment. Agronomy, 12(6), 1281. https://doi.org/10.3390/agronomy12061281







