Reclaimed Water Use in Agriculture: Effects on Soil Chemical and Biological Properties in a Long-Term Irrigated Citrus Farm
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site and Treatments
2.2. Soil Sampling and Laboratory Analysis
2.3. Data Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jaramillo, M.F.; Restrepo, I. Wastewater Reuse in Agriculture: A Review about Its Limitations and Benefits. Sustainability 2017, 9, 1734. [Google Scholar] [CrossRef] [Green Version]
- Rusan, M.M.J.; Hinnawi, S.; Rousan, L. Long term effect of wastewater irrigation of forage crops on soil and plant quality parameters. Desalination 2007, 215, 143–152. [Google Scholar] [CrossRef]
- Garcia-Delgado, C.; Eymar, E.; Contreras, J.I.; Segura, M.L. Effects of fertigation with purified urban wastewater on soil and pepper plant (Capsicum annuum L.) production, fruit quality and pollutant contents. Span. J. Agric. Res. 2012, 10, 209–221. [Google Scholar] [CrossRef] [Green Version]
- Bedbabis, S.; Triguia, D.; Ben Ahmeda, C.; Clodoveo, M.L.; Camposeo, S.; Vivaldi, G.A.; Rouina, B.B. Long-terms effects of irrigation with treated municipal wastewater on soil, yield and olive oil quality. Agric. Water Manag. 2015, 160, 14–21. [Google Scholar] [CrossRef]
- Schacht, K.; Marschner, B. Treated wastewater irrigation effects on soil hydraulic conductivity and aggregate stability of loamy soils in Israel. J. Hydrol. Hydromech. 2015, 63, 47–54. [Google Scholar] [CrossRef] [Green Version]
- Pedrero, F.; Grattan, S.R.; Ben-Gal, A.; Vivaldi, G.A. Opportunities for expanding the use of wastewaters for irrigation of olives. Agric. Water Manag. 2020, 241, 106333. [Google Scholar] [CrossRef]
- Tarchouna, L.G.; Merdy, P.; Raynaud, M.; Pfeifer, H.R.; Lucas, Y. Effects of long-term irrigation with treated wastewater. Part I: Evolution of soil physico-chemical properties. Appl. Geochem. 2010, 25, 1703–1710. [Google Scholar] [CrossRef]
- Abegunrin, T.P.; Awe, G.O.; Idowu, D.O.; Adejumobi, M.A. Impact of wastewater irrigation on soil physico-chemical properties, growth and water use pattern of two indigenous vegetables in southwest Nigeria. Catena 2016, 139, 167–178. [Google Scholar] [CrossRef]
- Feder, F. Irrigation with treated wastewater in humid regions: Effects on Nitisols, sugarcane yield and quality. Agric. Water Manag. 2021, 247, 106733. [Google Scholar] [CrossRef]
- Cook, B.D.; Allan, D.L. Dissolved organic matter in old field soils: Total amounts as a measure of available resources for soil mineralization. Soil Biol. Biochem. 1992, 24, 585–594. [Google Scholar] [CrossRef]
- Zhang, M.; He, Z.; Zhao, A.; Zhang, H.; Endale, D.M.; Schomberg, H.H. Water-Extractable soil organic carbon and nitrogen affected by tillage and manure application. Soil Sci. 2011, 176, 307–312. [Google Scholar] [CrossRef]
- Bongiovanni, M.D.; Lobartini, J.C. Particulate organic matter, carbohydrate, humic acid contents in soil macro- and microaggregates as affected by cultivation. Geoderma 2006, 136, 660–665. [Google Scholar] [CrossRef]
- Heitkamp, F.; Raupp, J.; Ludwig, B. Impact of fertilizer type and rate on carbon and nitrogen pools in a sandy cambisol. Plant Soil 2009, 319, 259–275. [Google Scholar] [CrossRef]
- Lee, S.B.; Lee, C.H.; Jung, K.Y.; Do Park, K.; Lee, D.; Kim, P.J. Changes of soil organic carbon and its fractions in relation to soil physical properties in a long-term fertilized paddy. Soil Tillage Res. 2009, 104, 227–232. [Google Scholar] [CrossRef]
- Stellacci, A.M.; Castellini, M.; Diacono, M.; Rossi, R.; Gattullo, C.E. Assessment of soil quality under different soil management strategies: Combined use of statistical approaches to select the most informative soil physico-chemical indicators. Appl. Sci. 2021, 11, 5099. [Google Scholar] [CrossRef]
- Garcia, C.; Hernandez, T.; Costa, F.; Ceccanti, B. Biochemical parameters in soil regenerated by the addition of organic wastes. Waste Manag. Res. 1994, 12, 457–466. [Google Scholar] [CrossRef]
- Dick, R.P.; Breakwill, D.; Turco, R. Soil enzyme activities and biodiversity measurements as integrating biological indicators. In Handbook of Methods for Assessment of Soil Quality; Doran, J.W., Jones, A.J., Eds.; Soil Science Society of America: Madison, WI, USA, 1996; pp. 247–272. [Google Scholar]
- Masciandaro, G.; Ceccanti, B. Assessing soil quality in different agro-ecosystems through biochemical and chemico-structural properties of humic substances. Soil Tillage Res. 1999, 51, 129–137. [Google Scholar] [CrossRef]
- Staben, M.L.; Bezdicek, D.F.; Smith, J.L.; Fauci, M.F. Assessment of soil quality in conservation reserve program and wheat-fallow soils. Soil Sci. Soc. Am. J. 1997, 61, 124–130. [Google Scholar] [CrossRef]
- Chen, H.J. Phosphatase activity and P fractions in soils of an 18-year-old Chinese fir (Cunninghamia lanceolata) plantation. For. Ecol. Manag. 2003, 178, 301–310. [Google Scholar] [CrossRef]
- Floch, C.; Capowiez, Y.; Criquet, S. Enzyme activities in apple orchard agroecosystems: How are they affected by management strategy and soil properties. Soil Biol. Biochem. 2009, 41, 61–68. [Google Scholar] [CrossRef]
- Doran, J.W.; Parkin, T.B. Quantitative indicators of soil quality: A minimum data set. In Methods for Assessing Soil Quality; Doran, J.W., Jones, A.J., Eds.; Soil Science Society of America Special Publication: Madison, WI, USA, 1996; Volume 49, pp. 25–37. [Google Scholar]
- Anderson, T.H.; Domsch, K.H. Application of eco-physiological quotients on microbial biomasses from soils of different cropping histories. Soil Biol. Biochem. 1990, 22, 251–255. [Google Scholar] [CrossRef]
- Brookes, P.C. The use of microbial parameters in monitoring soil pollution by heavy metals. Biol. Fertil. Soils 1995, 19, 269–279. [Google Scholar] [CrossRef]
- Kubat, J.; Novakova, J.; Mikanova, O.; Apfelather, R. Organic carbon cycle, incidence of microorganisms and respiration activity in long-term field experiment. Rostl. Vyrob.-UZPI 1999, 9, 389–395. [Google Scholar]
- Brzezińska, M. Impact of treated wastewater on biological activity and accompanying processes in organic soils. Acta Agrophys. 2006, 131, 1–164, (In Polish with English summary). [Google Scholar]
- Fang, C.; Moncrieff, J.B. The dependence of soil CO2 efflux on temperature. Soil Biol. Biochem. 2001, 33, 155–165. [Google Scholar] [CrossRef]
- Vanhala, P. Seasonal variation in the soil respiration rate in coniferous forest soils. Soil Biol. Biochem. 2002, 34, 1375–1379. [Google Scholar] [CrossRef]
- Hong, S.L.; Ling, H.L.; Xing, G.H.; Huang, J.H.; Sun, J.X.; Wang, H.Y. Respiratory substrate availability plays a crucial role in the response of soil respiration to environmental factors. Appl. Soil Ecol. 2006, 32, 284–292. [Google Scholar]
- Chen, X.; Tang, J.; Jiang, L.; Li, B.; Chen, J.; Fang, C. Evaluating the impacts of incubation procedures on estimated Q10 values of soil respiration. Soil Biol. Biochem. 2010, 42, 2282–2288. [Google Scholar] [CrossRef]
- Balogh, J.; Pintér, K.; Fóti, S.; Cserhalmi, D.; Papp, M.; Nagy, Z. Dependence of soil respiration on soil moisture, clay content, soil organic matter, and CO2 uptake in dry grasslands. Soil Biol. Biochem. 2011, 43, 1006–1013. [Google Scholar] [CrossRef]
- Wang, B.; Zha, T.S.; Jia, X.; Wu, B.; Zhang, Q.; Qin, S.G. Soil moisture modifies the response of soil respiration to temperature in dessert shrub ecosystems. Biogeosciences 2014, 11, 259–268. [Google Scholar] [CrossRef] [Green Version]
- Leogrande, R.; Vitti, C. Use of organic amendments to reclaim saline and sodic soils: A review. Arid. Land Res. Manag. 2019, 33, 1–21. [Google Scholar] [CrossRef]
- Abadía, J.; Bastida, F.; Romero-Trigueros, C.; Bayona, J.M.; Vera, A.; García, C.; Alarcon, J.J.; Nicolas, E. Interactions between soil microbial communities and agronomic behavior in a mandarin crop subjected to water deficit and irrigated with reclaimed water. Agric. Water Manag. 2021, 247, 106749. [Google Scholar] [CrossRef]
- Pedrero, F.; Mounzer, O.; Alarcon, J.J.; Bayona, J.M.; Nicolas, E. The viability of irrigating mandarin trees with saline reclaimed water in a semi-arid Mediterranean region: A preliminary assessment. Irrig. Sci. 2013, 31, 759–768. [Google Scholar] [CrossRef]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop evapotranspiration guidelines for computing crop water requirements. In Irrigation and Drainage; FAO: Rome, Italy, 1998; Volume 56, pp. 15–27. [Google Scholar]
- Castel, J.R.; Bautista, I.; Ramos, C.; Cruz, G. Evapotranspiration and irrigation efficiency of mature orange orchards in Valencia (Spain). Irrig. Drain. Syst. 1987, 3, 205–217. [Google Scholar] [CrossRef]
- Vitti, C.; Stellacci, A.M.; Leogrande, R.; Mastrangelo, M.; Cazzato, E.; Ventrella, D. Assessment of organic carbon in soils: A comparison between Springer-Klee wet digestion and the dry combustion methods in Mediterranean soils (Southern Italy). Catena 2016, 137, 113–119. [Google Scholar] [CrossRef]
- Page, A.L.; Miller, R.H.; Keeny, D.R. Methods of Soil Analysis, Part II, 2nd ed.; American Society of Agronomy: Madison, WI, USA, 1982. [Google Scholar]
- Sonmez, S.; Buyuktas, D.; Okturen, F.; Citak, S. Assessment of different soil to water ratios (1:1, 1:2.5, 1:5) in soil salinity studies. Geoderma 2008, 144, 361–369. [Google Scholar] [CrossRef]
- Rietz, D.N.; Hayne, R.J. Effects of irrigation-induced salinity and sodicity on soil microbial activity. Soil Biol. Biochem. 2003, 35, 845–854. [Google Scholar] [CrossRef]
- Haynes, R.J. Labile organic matter as an indicator of organic matter quality in arable and pastoral soils in New Zealand. Soil Biol. Biochem. 2000, 32, 211–219. [Google Scholar] [CrossRef]
- Rees, R.M.; Parker, J.P. Filtration increases the correlation between water extractable organic carbon and soil microbial activity. Soil Biol. Biochem. 2005, 37, 2240–2248. [Google Scholar] [CrossRef]
- Armenise, E.; Redmile-Gordon, M.A.; Stellacci, A.M.; Ciccarese, A.; Rubino, P. Developing a soil quality index to compare soil fitness for agricultural use under different managements in the Mediterranean environment. Soil Tillage Res. 2013, 130, 91–98. [Google Scholar] [CrossRef]
- Anderson, J.P.E.; Page, A.L.; Miller, R.H.; Keeney, D.R. Soil Respiration. In Methods of Soil Analysis, 2nd ed.; Page, A.L., Ed.; ASA and SSSA: Madison, WI, USA, 1982; pp. 831–837. [Google Scholar]
- Clark, G.J.; Dodgshun, N.; Sale, P.W.G.; Tang, C. Changes in chemical and biological properties of a sodic clay subsoil with addition of organic amendments. Soil Biol. Biochem. 2007, 39, 2806–2817. [Google Scholar] [CrossRef]
- Ferrara, R.M.; Mazza, G.; Muschitiello, C.; Castellini, M.; Stellacci, A.M.; Navarro, A.; Lagomarsino, A.; Vitti, C.; Rossi, R.; Rana, G. Short-term effects of conversion to no-tillage on respiration and chemical-physical properties of the soil: A case study in a wheat cropping system in semi-dry environment. Ital. J. Agrometeorol. 2017, 1, 47–58. [Google Scholar]
- Masciandaro, G.; Ceccanti, B.; Ronchi, V.; Bauer, C. Kinetic parameters of dehydrogenase in the assessment of the response of soil to vermicompost and inorganic fertilisers. Biol. Fertil. Soils 2000, 32, 479–483. [Google Scholar] [CrossRef]
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Mulvaney, R.L. Nitrogen-Inorganic forms. In Methods of Soil Analysis. Chemical Methods. Part 3; Sparks, D.L., Ed.; SSSA Book Series No. 5; Soil Science Society of America, Inc. and American Society of Agronomy; Inc.: Madison, WI, USA, 1996; pp. 1123–1184. [Google Scholar]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenol phosphate in assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Tabatabai, M.A. Soil Enzymes. In Methods of Soil Analysis; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; ASA, SSSA: Madison, WI, USA, 1982; pp. 903–947. [Google Scholar]
- SAS/STAT Software Release 9.2; Statistical Analysis System (SAS) Institute Inc.: Cary, NC, USA, 2010.
- Jueschke, E.; Marschner, B.; Tarchitzky, J.; Chen, Y. Effects of treated wastewater irrigation on the dissolved and soil organic carbon in Israeli soils. Water Sci. Technol. 2008, 57, 727–733. [Google Scholar] [CrossRef]
- Jemai, I.; Ben Aissa, N.; Gallali, T.; Chenini, F. Effects of municipal reclaimed wastewater irrigation on organic and inorganic composition of soil and groundwater in Souhil Wadi area (Nabeul, Tunisia). Hydrol. Curr. Res. 2013, 4, 160. [Google Scholar]
- Mounzer, O.; Pedrero, F.; Bayona, J.M.; Nicolás, E.; Alarcón, J.J. Transient soil salinity under the combined effect of reclaimed water and regulated deficit drip irrigation of Mandarin trees. Agric. Water Manag. 2013, 120, 23–29. [Google Scholar] [CrossRef]
- Pedrero, F.; Maestre-Valero, J.F.; Mounzer, O.; Alarcón, J.J.; Nicolás, E. Physiological and agronomic mandarin trees performance under saline reclaimed water combined with regulated deficit irrigation. Agric. Water Manag. 2014, 146, 228–237. [Google Scholar] [CrossRef]
- Nicolas, E.; Alarcon, J.J.; Mounzer, O.; Pedrero, F.; Nortes, P.A.; Alcobendas, R.; Romero-Trigueros, C.; Bayona, J.M.; Maestre-Valero, J.F. Long-term physiological and agronomic responses of mandarin trees to irrigation with saline reclaimed water. Agric. Water Manag. 2016, 166, 1–8. [Google Scholar] [CrossRef]
- Erel, R.; Eppel, A.; Yermiyahu, U.; Ben-Gal, A.; Levy, G.; Zipori, I.; Schaumann, G.E.; Mayer, O.; Dag, A. Long-term irrigation with reclaimed wastewater: Implications on nutrient management, soil chemistry and olive (Olea europaea L.) performance. Agric. Water Manag. 2019, 213, 324–335. [Google Scholar] [CrossRef]
- Levy, G.J.; Mamedov, A.I. High-Energy-Moisture-Characteristic Aggregate Stability as a Predictor for Seal Formation. Soil Sci. Soc. Am. J. 2002, 66, 1603–1609. [Google Scholar] [CrossRef]
- Cucci, G.; Lacolla, G.; Pagliai, M.; Vignozzi, N. Effect of reclamation on the structure of silty-clay soils irrigated with saline-sodic waters. Int. Agrophys. 2015, 29, 23–30. [Google Scholar] [CrossRef]
- Levy, G.J.; Assouline, S.; Fine, P.; Bar-Tal, A. Physical aspects. In Use of Treated Wastewater in Agriculture: Impacts on the Soil Environment and Crops; Levy, G.J., Ed.; Wiley-Blackwell Publishing: Oxford, UK, 2011; p. 306. [Google Scholar]
- Assouline, S.; Narkis, K. Effects of long-term irrigation with treated wastewater on the hydraulic properties of a clayey soil. Water Resour. Res. 2011, 47, W08530. [Google Scholar] [CrossRef]
- Assouline, S.; Narkis, K.; Gherabli, R.; Sposito, G. Combined effect of sodicity and organic matter on soil properties under long-term irrigation with treated wastewater. Vadose Zone J. 2016, 15, 1–10. [Google Scholar] [CrossRef]
- Usman, A.R.A.; Kuzyakov, Y.; Stahr, K. Dynamics of organic C mineralization and the mobile fraction of heavy metals in a calcareous soil incubated with organic wastes. Water Air Soil Pollut. 2004, 158, 401–418. [Google Scholar] [CrossRef] [Green Version]
- Usman, A.R.A.; Almaroai, Y.A.; Ahmad, M.; Vithanage, M.; Ok, Y.S. Toxicity of synthetic chelators and metal availability in poultry manure amended Cd, Pb and as contaminated agricultural soil. J. Hazard. Mater. 2013, 262, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xiao, F.; He, T.; Wang, S. Responses of labile soil organic carbon and enzyme activity in mineral soils to forest conversion in the subtropics. Ann. Sci. 2013, 70, 579–587. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, J.; Hu, R.; Feng, M.; Lin, S.; Malghani, S.; Ali, I.M. Microbial biomass, and dissolved organic carbon and nitrogen strongly affect soil respiration in different land uses: A case study at Three Gorges Reservoir Area, South China. Agric. Ecosyst. Environ. 2010, 137, 294–307. [Google Scholar] [CrossRef]
- Fan, L.C.; Yang, M.Z.; Han, W.Y. Soil respiration under different land uses in Eastern China. PLoS ONE 2015, 10, e0124198. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Wu, L.; Frankenberger, W.T., Jr.; Chang, A.C. Soil enzyme activities of long-term reclaimed wastewater-irrigated soils. J. Environ. Qual 2008, 37, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Friedel, J.K.; Langer, T.; Siebe, C.; Stahr, K. Effects of long term wastewater irrigation on soil organic matter, soil microbial biomass and its activities in central Mexico. Biol. Fertil. Soils 2000, 31, 414–421. [Google Scholar] [CrossRef]
- Ros, M.; Hernandez, M.T.; Garcia, C. Soil microbial activity after restoration of a semiarid soil by organic amendments. Soil Biol. Biochem. 2003, 35, 463–469. [Google Scholar] [CrossRef]
- Yan, J.; Pan, G. Effects of pulp wastewater irrigation on soil enzyme activities and respiration from a managed wetland. Soil Sediment Contam. 2010, 19, 204–216. [Google Scholar] [CrossRef]
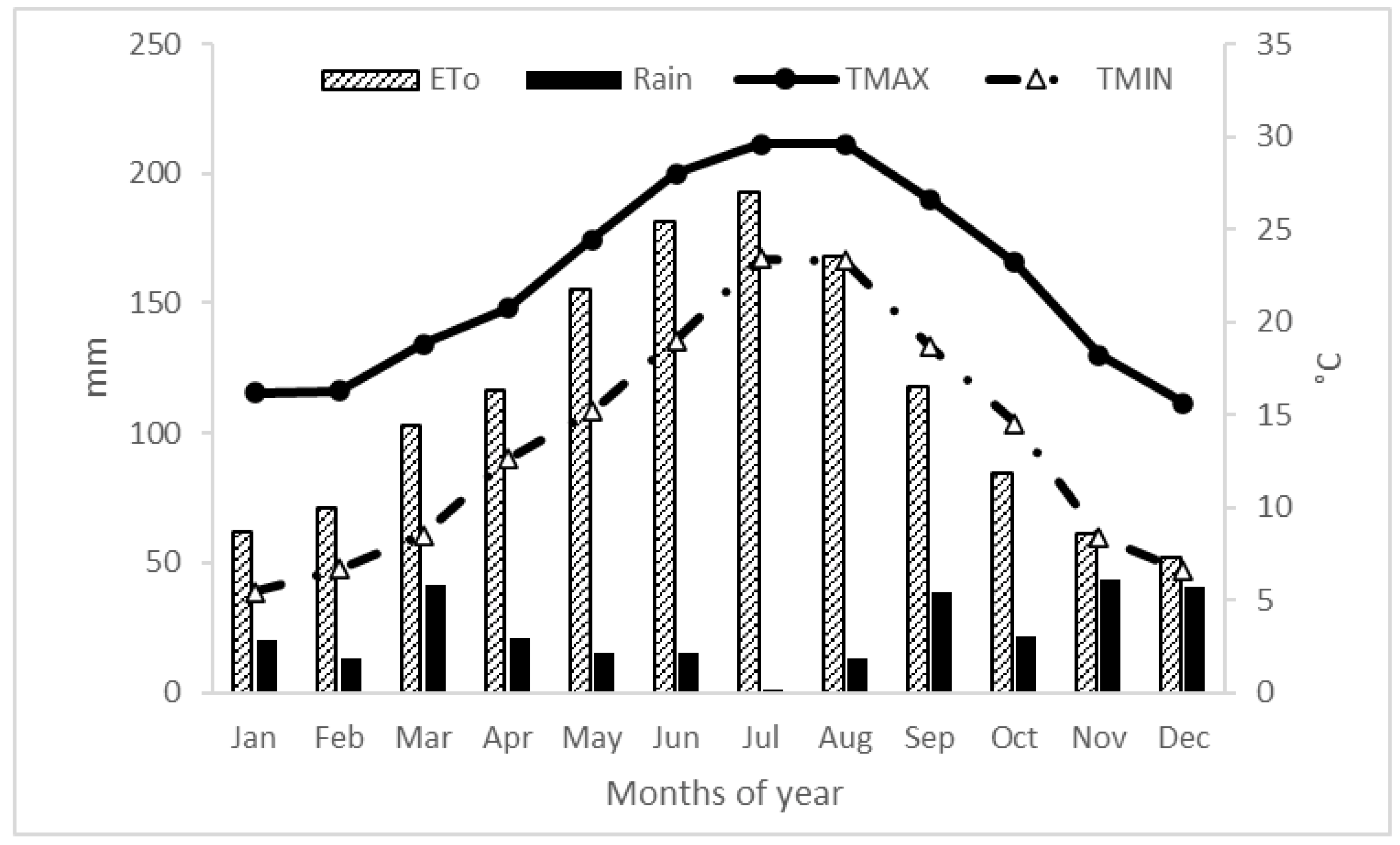
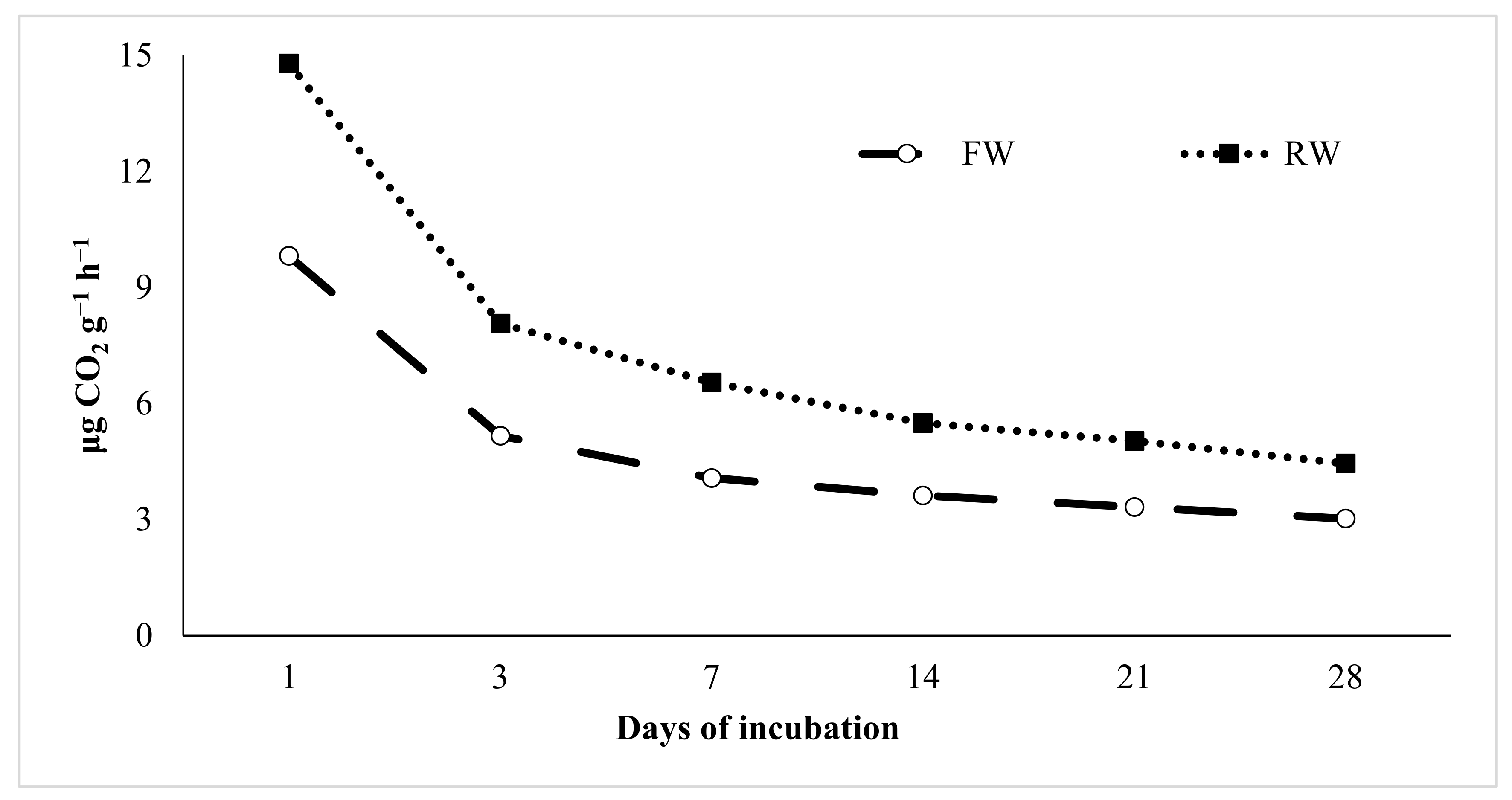
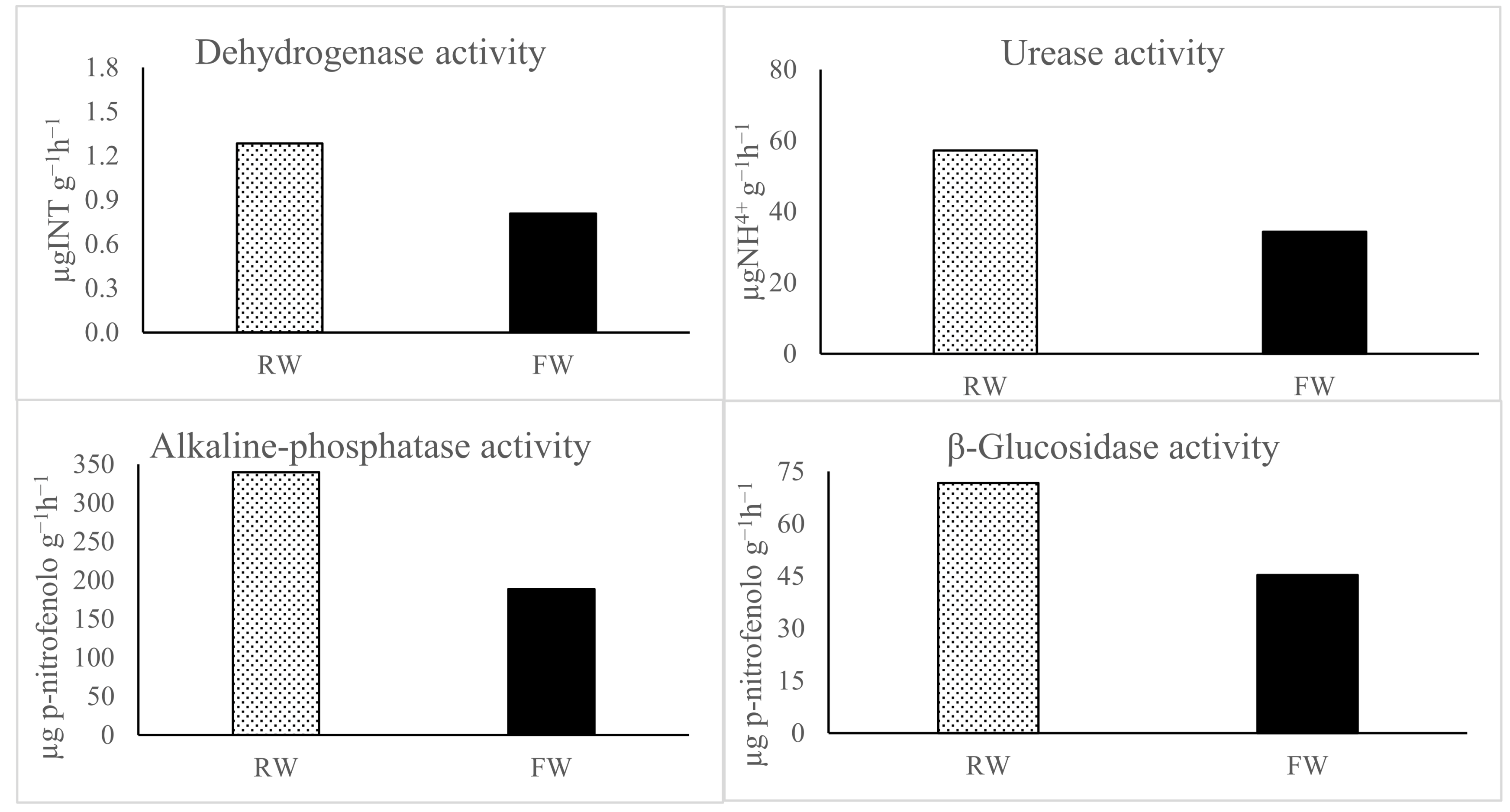
| Parameters | Units | FW | RW |
|---|---|---|---|
| ECw | dS m−1 | 1.2 | 3.4 |
| SARw | 2.2 | 6.4 | |
| pH | 8.2 | 7.8 | |
| Ca2+ | meq L−1 | 4.1 | 6.8 |
| Mg2+ | meq L−1 | 4.0 | 8.2 |
| K+ | mg L−1 | 9.1 | 36.1 |
| Na+ | meq L−1 | 4.6 | 16.8 |
| meq L−1 | 3.9 | 15.4 | |
| mg L−1 | 3.8 | 12.2 | |
| mg L−1 | 1.8 | 2.2 | |
| meq L−1 | 5.4 | 12.4 |
| Treatments | TOC | WEOC | pH | EC1:5 | Total N | Available P | Ca2+ | K+ | Mg2+ | Na+ | ESP |
|---|---|---|---|---|---|---|---|---|---|---|---|
| g kg−1 | mg kg−1 | dS m−1 | g kg−1 | mg kg−1 | mg kg−1 | mg kg−1 | mg kg−1 | mg kg−1 | % | ||
| FW | 17.004 | 14.302 | 8.462 | 0.481 | 0.904 | 154.9 | 2345.9 | 268.1 | 341.9 | 65.8 | 1.857 |
| RW | 21.770 | 22.514 | 8.486 | 0.717 | 1.024 | 241.5 | 2515.8 | 235.2 | 518.4 | 358.3 | 8.356 |
| n.s. | * | n.s. | ** | n.s. | ** | n.s. | n.s. | * | *** | *** |
| Soil Respiration | Enzyme Activities | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 Day (34) | 3 Days (36) | 7 Days (36) | 10 Days (36) | 14 Days (36) | 21 Days (36) | 28 Days (36) | Urease (36) | Dehydr (36) | βGluc (36) | Phosph (36) | |
| TOC | 0.45978 | 0.59437 | 0.61466 | 0.64113 | 0.61961 | 0.62516 | 0.67392 | 0.23013 | 0.51759 | 0.41802 | −0.01207 |
| 0.063 | 0.0119 | 0.0086 | 0.0055 | 0.008 | 0.0073 | 0.003 | 0.3742 | 0.0333 | 0.095 | 0.9633 | |
| WEOC | 0.69787 | 0.78623 | 0.85926 | 0.83792 | 0.89064 | 0.9052 | 0.88855 | 0.69849 | 0.77609 | 0.4382 | 0.4159 |
| 0.0018 | 0.0002 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0018 | 0.0002 | 0.0785 | 0.0968 | |
| pH | −0.3959 | −0.32745 | −0.30456 | −0.26701 | −0.23229 | −0.27723 | −0.31231 | −0.39116 | 0.08072 | −0.13862 | −0.14404 |
| 0.1157 | 0.1995 | 0.2346 | 0.3002 | 0.3696 | 0.2814 | 0.2223 | 0.1205 | 0.7581 | 0.5957 | 0.5813 | |
| EC1:5 | 0.48219 | 0.56918 | 0.55966 | 0.59199 | 0.53791 | 0.57089 | 0.58783 | 0.43388 | 0.31287 | 0.2756 | 0.31576 |
| 0.05 | 0.0171 | 0.0195 | 0.0123 | 0.0259 | 0.0167 | 0.0131 | 0.818 | 0.2214 | 0.2843 | 0.217 | |
| Available P | 0.1986 | 0.24264 | 0.34859 | 0.38744 | 0.35139 | 0.35608 | 0.38532 | 0.32302 | 0.47847 | 0.20872 | 0.06369 |
| 0.4448 | 0.3481 | 0.1703 | 0.1244 | 0.1666 | 0.1607 | 0.1267 | 0.206 | 0.052 | 0.4214 | 0.8081 | |
| Total N | 0.71939 | 0.79253 | 0.8092 | 0.8236 | 0.76879 | 0.75451 | 0.78736 | 0.3378 | 0.67907 | 0.80982 | 0.16781 |
| 0.0011 | 0.0001 | <0.0001 | <0.0001 | 0.0003 | 0.0005 | 0.0002 | 0.1848 | 0.0027 | <0.0001 | 0.5197 | |
| Ca2+ | 0.3419 | 0.44895 | 0.47784 | 0.4816 | 0.46576 | 0.48694 | 0.54279 | 0.17266 | 0.31619 | 0.23773 | −0.09645 |
| 0.1792 | 0.0706 | 0.0524 | 0.0503 | 0.0595 | 0.0474 | 0.0244 | 0.5075 | 0.2163 | 0.3582 | 0.7127 | |
| K+ | 0.1597 | 0.10658 | 0.0958 | 0.03214 | 0.02285 | 0.06141 | 0.11375 | 0.07441 | −0.18581 | −0.06115 | −0.10484 |
| 0.5404 | 0.6839 | 0.7145 | 0.9025 | 0.9306 | 0.8149 | 0.6638 | 0.7765 | 0.4752 | 0.8157 | 0.6888 | |
| Mg2+ | 0.59715 | 0.70762 | 0.65355 | 0.68948 | 0.65998 | 0.6585 | 0.64061 | 0.29127 | 0.50528 | 0.36482 | 0.29656 |
| 0.0114 | 0.0015 | 0.0044 | 0.0022 | 0.0039 | 0.004 | 0.0056 | 0.2567 | 0.0386 | 0.1499 | 0.2477 | |
| Na+ | 0.48417 | 0.59072 | 0.55174 | 0.61125 | 0.57504 | 0.5743 | 0.53763 | 0.29294 | 0.53597 | 0.30568 | 0.37555 |
| 0.0489 | 0.0125 | 0.0217 | 0.0091 | 0.0157 | 0.0159 | 0.026 | 0.2538 | 0.0266 | 0.2328 | 0.1374 | |
| ESP | 0.3802 | 0.46019 | 0.42087 | 0.4885 | 0.45315 | 0.44308 | 0.38901 | 0.26508 | 0.47294 | 0.25072 | 0.4113 |
| 0.1322 | 0.0631 | 0.0925 | 0.0466 | 0.0677 | 0.0749 | 0.1228 | 0.3038 | 0.0552 | 0.3317 | 0.101 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leogrande, R.; Pedrero, F.; Nicolas, E.; Vitti, C.; Lacolla, G.; Stellacci, A.M. Reclaimed Water Use in Agriculture: Effects on Soil Chemical and Biological Properties in a Long-Term Irrigated Citrus Farm. Agronomy 2022, 12, 1317. https://doi.org/10.3390/agronomy12061317
Leogrande R, Pedrero F, Nicolas E, Vitti C, Lacolla G, Stellacci AM. Reclaimed Water Use in Agriculture: Effects on Soil Chemical and Biological Properties in a Long-Term Irrigated Citrus Farm. Agronomy. 2022; 12(6):1317. https://doi.org/10.3390/agronomy12061317
Chicago/Turabian StyleLeogrande, Rita, Francisco Pedrero, Emilio Nicolas, Carolina Vitti, Giovanni Lacolla, and Anna Maria Stellacci. 2022. "Reclaimed Water Use in Agriculture: Effects on Soil Chemical and Biological Properties in a Long-Term Irrigated Citrus Farm" Agronomy 12, no. 6: 1317. https://doi.org/10.3390/agronomy12061317
APA StyleLeogrande, R., Pedrero, F., Nicolas, E., Vitti, C., Lacolla, G., & Stellacci, A. M. (2022). Reclaimed Water Use in Agriculture: Effects on Soil Chemical and Biological Properties in a Long-Term Irrigated Citrus Farm. Agronomy, 12(6), 1317. https://doi.org/10.3390/agronomy12061317








