A Case Study in Desertified Area: Soybean Growth Responses to Soil Structure and Biochar Addition Integrating Ridge Regression Models
Abstract
:1. Introduction
2. Materials and Methods
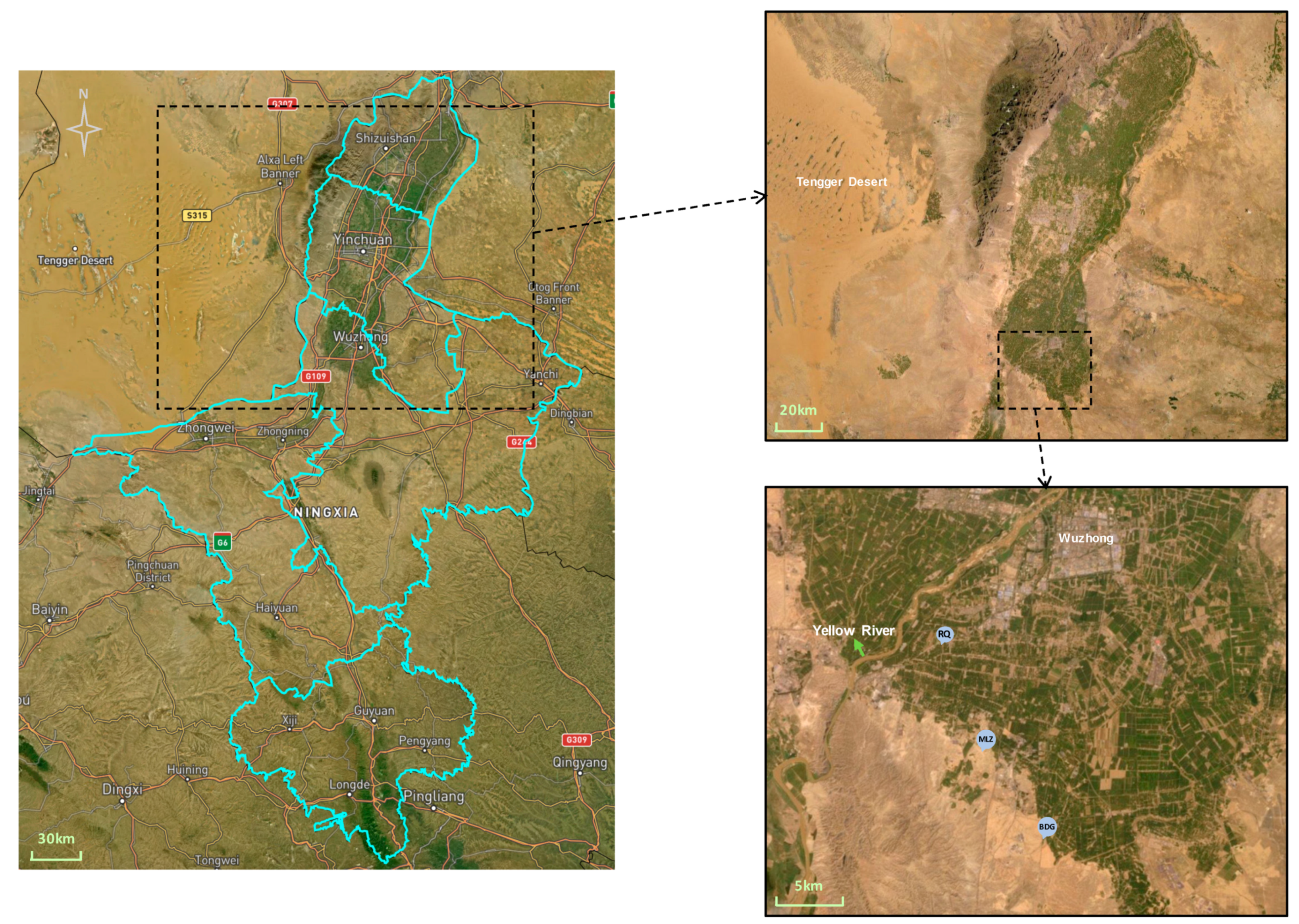
2.1. Study Site and Field Design
2.2. Plant Sampling and Analysis
2.3. Nodule Sampling and Leghemoglobin Content Analysis
2.4. Soil Sampling and Physicochemical Properties Analysis
2.5. Ridge Regression Model
2.6. Statistical Analysis
3. Results
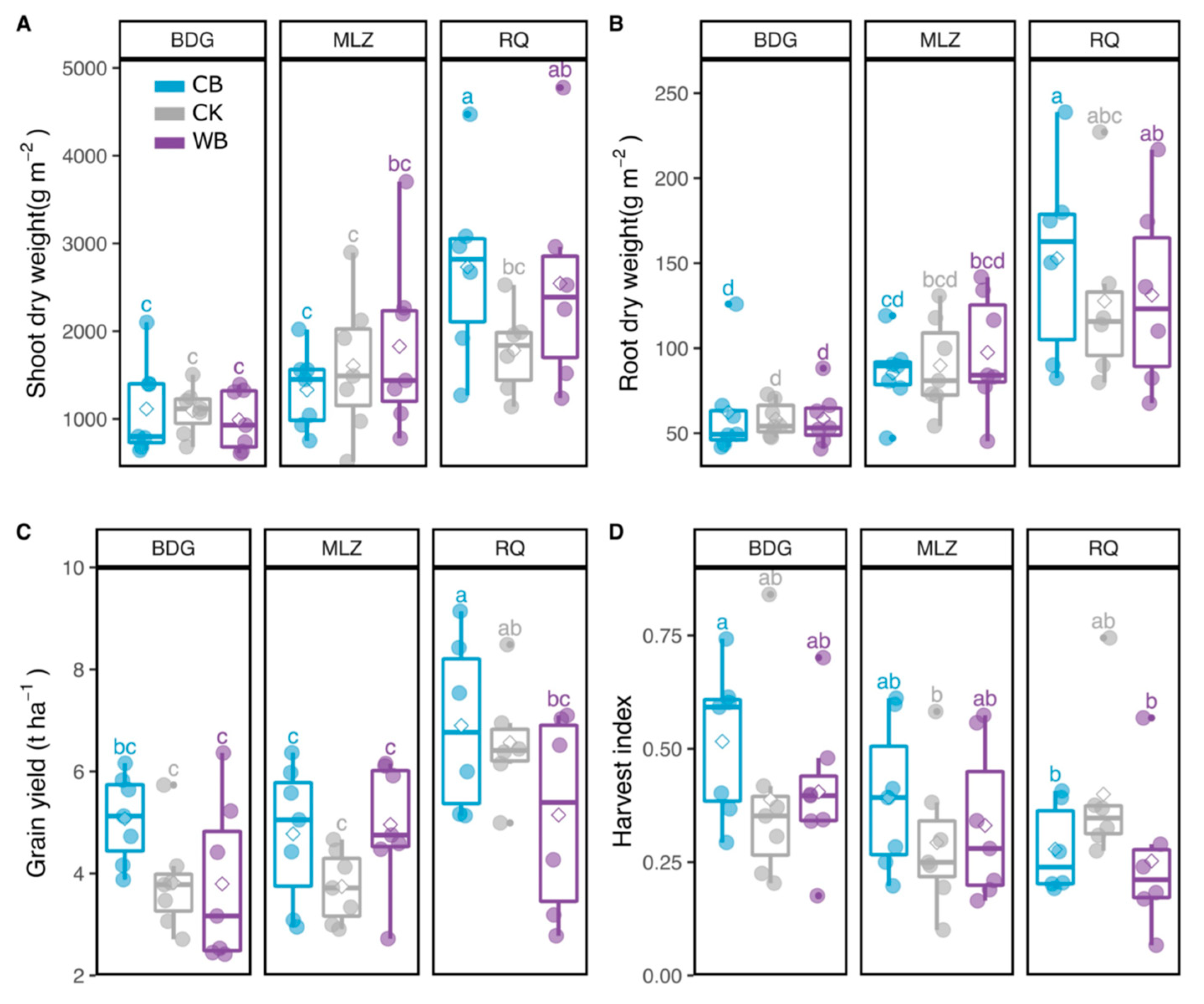
3.1. Biochar Effects on Plant Growth Indicators and Plant Nutrients Content
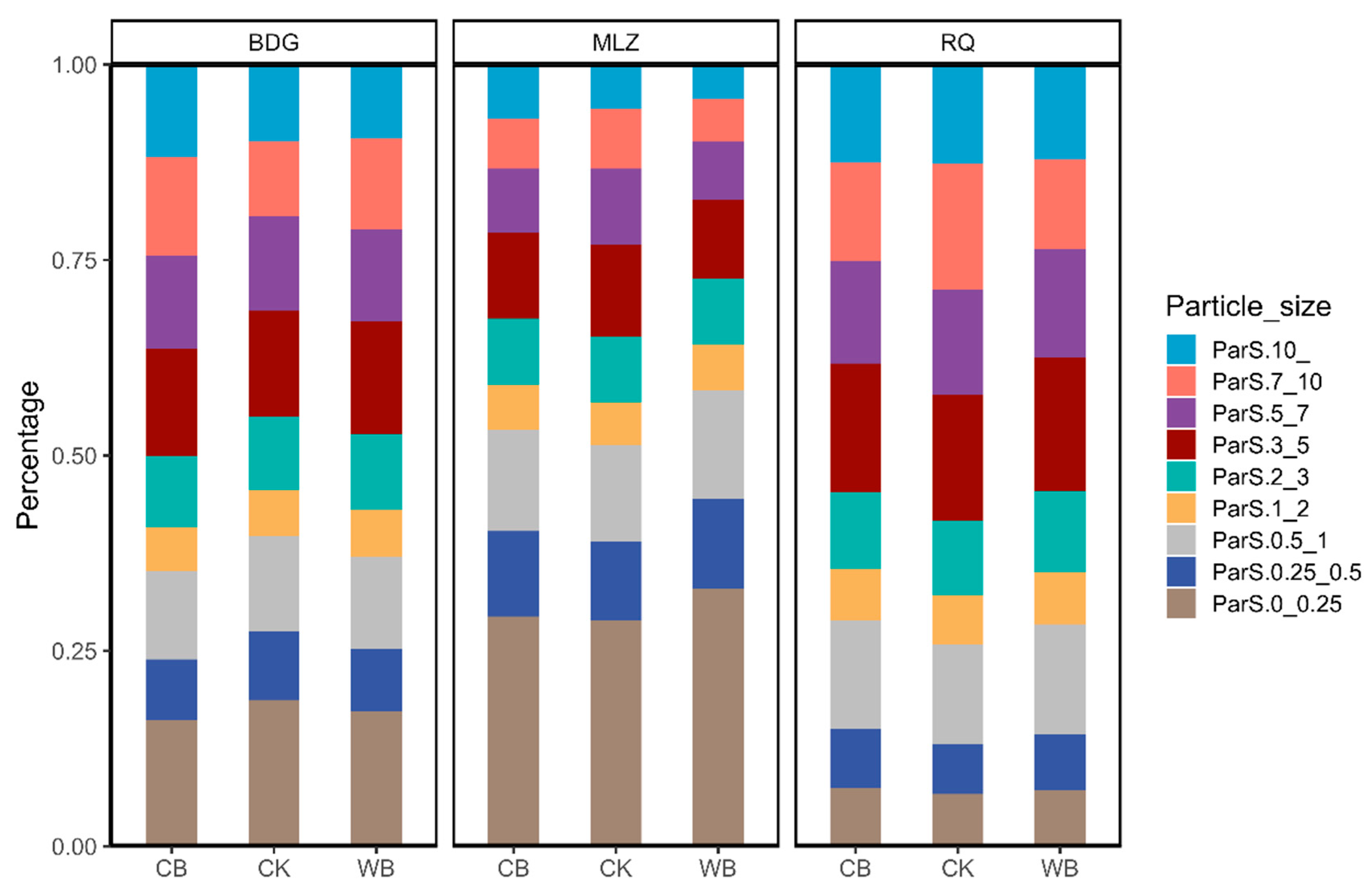
3.2. Biochar Effects on Lb Content and Soil Physicochemical Properties
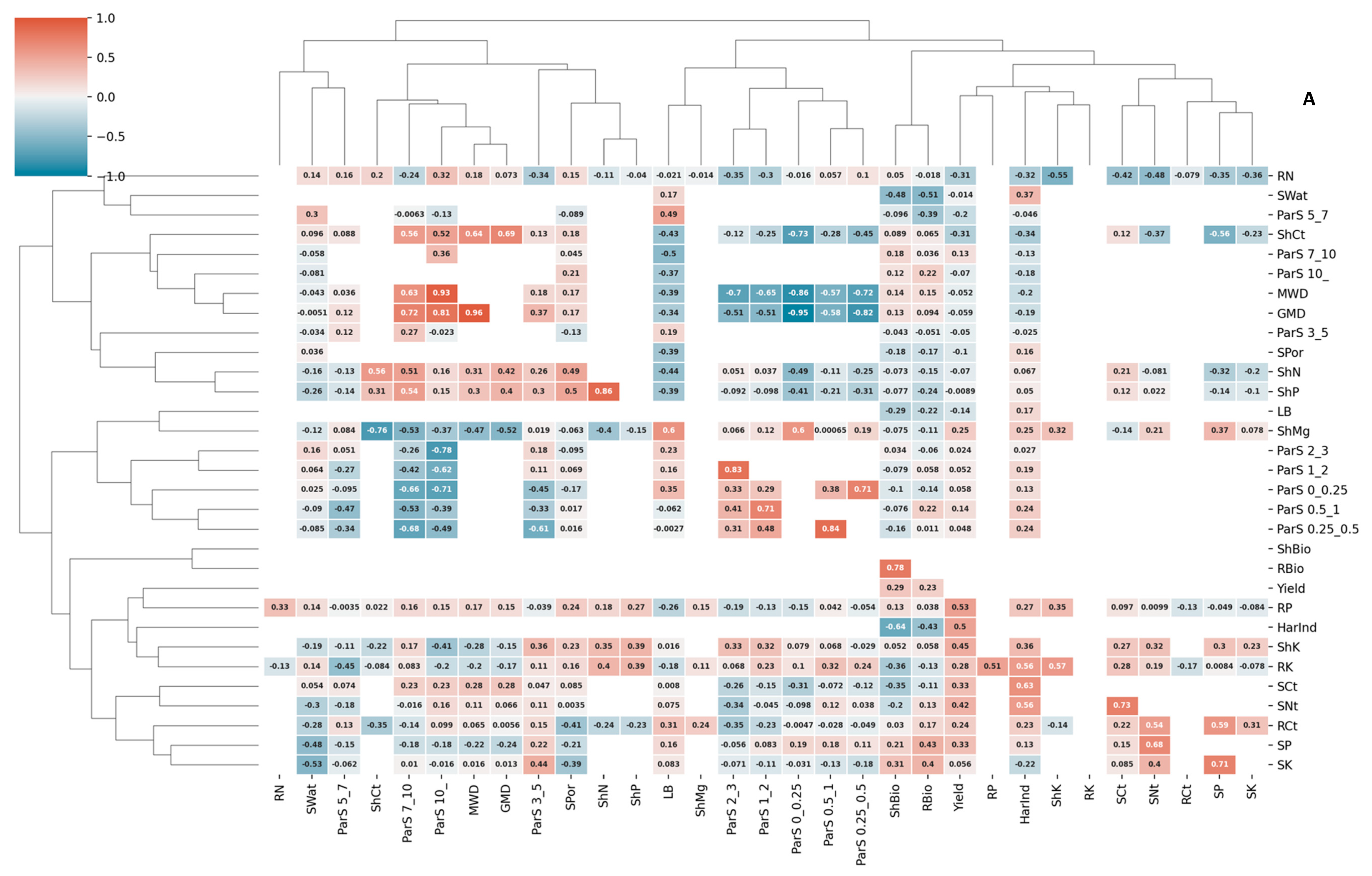
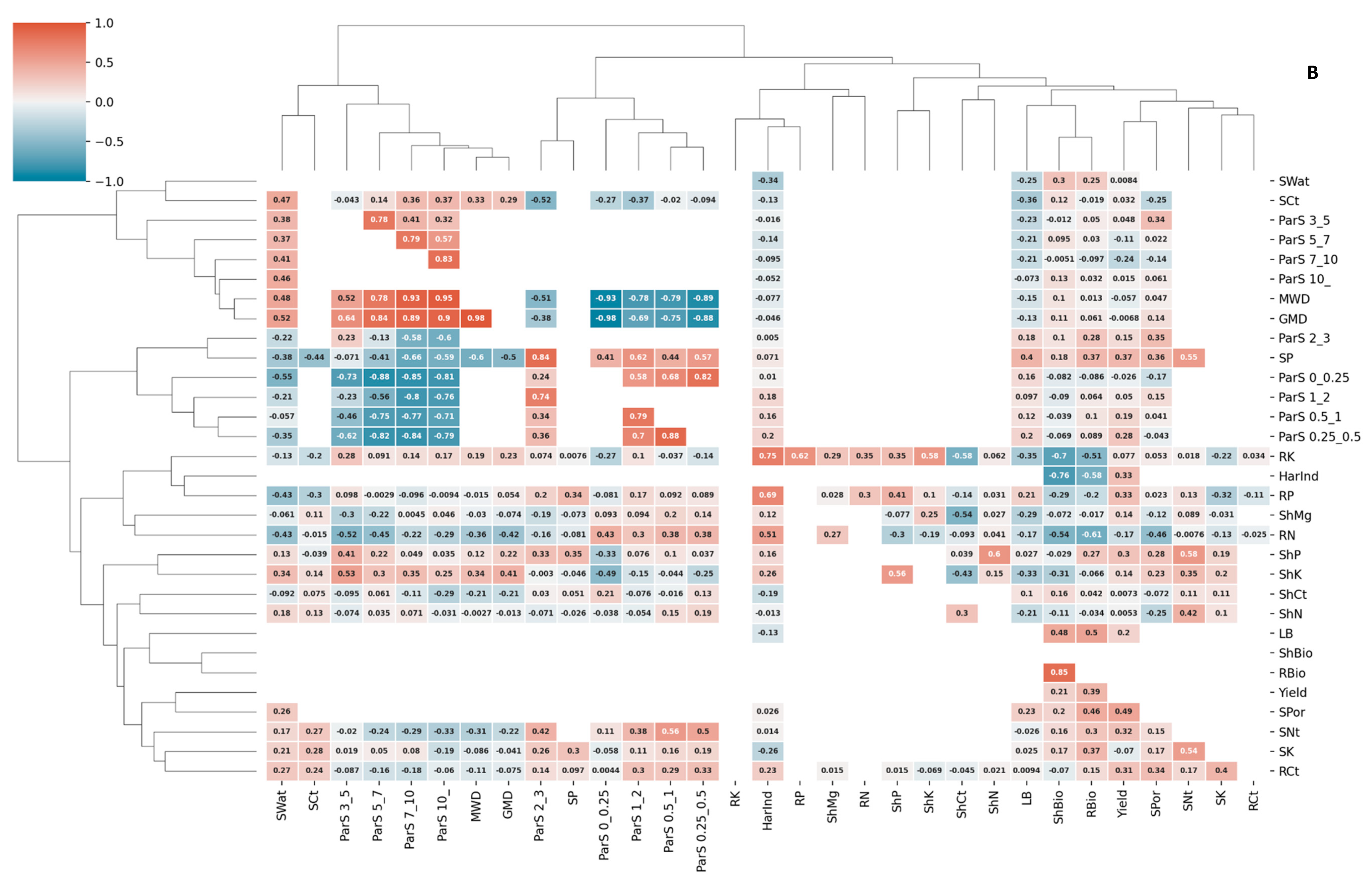
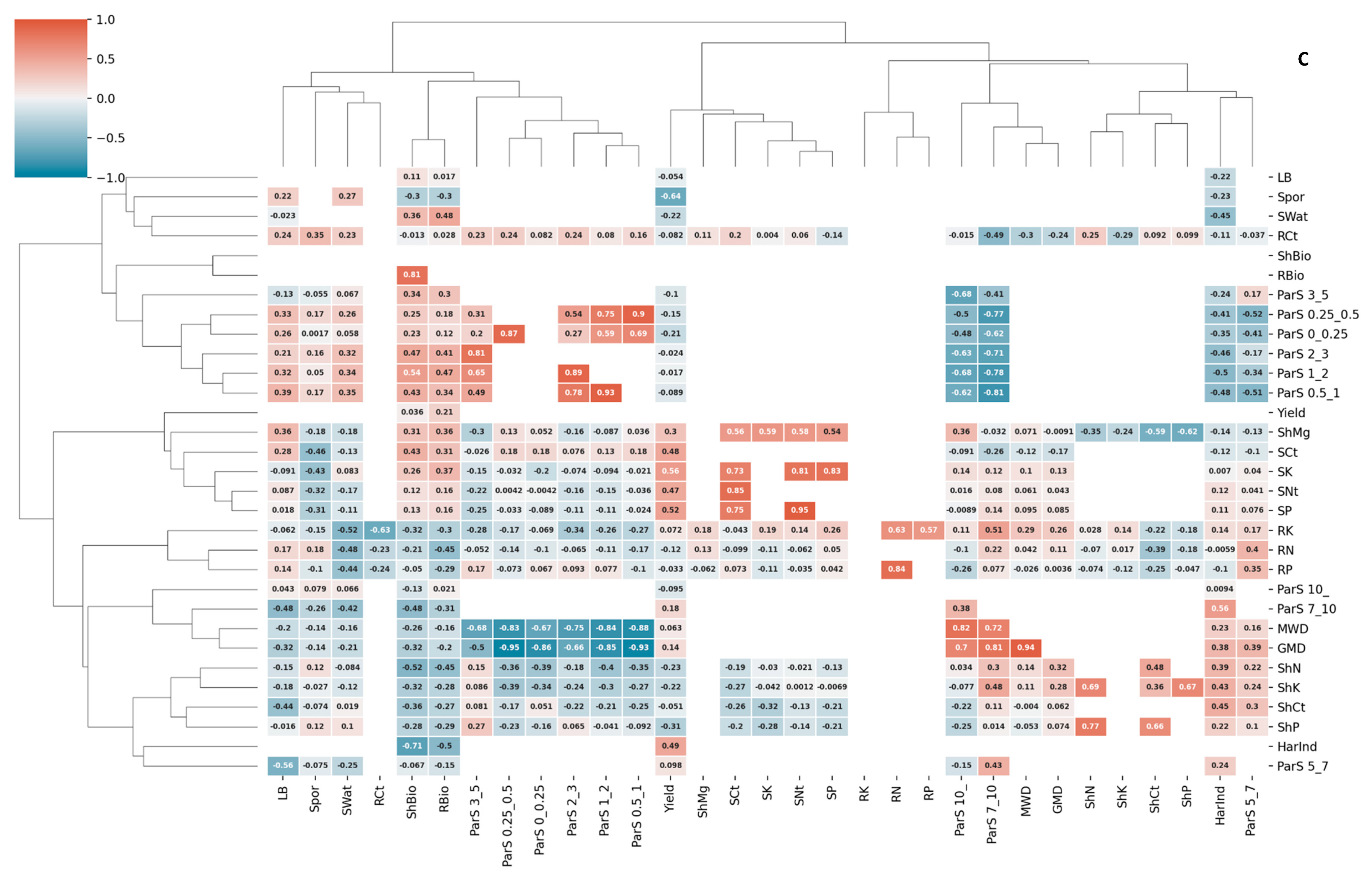
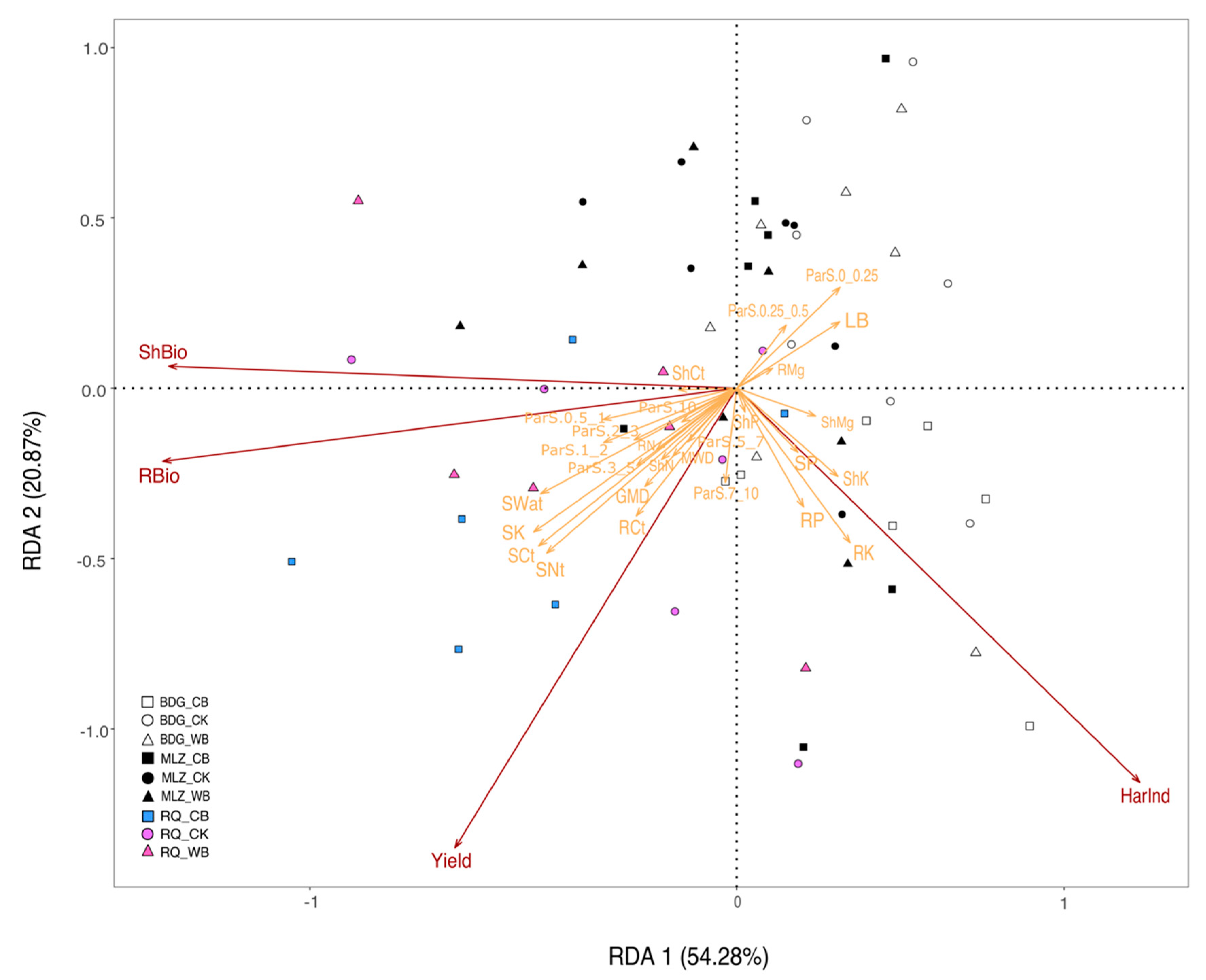
3.3. Correlations and Redundancy Analysis
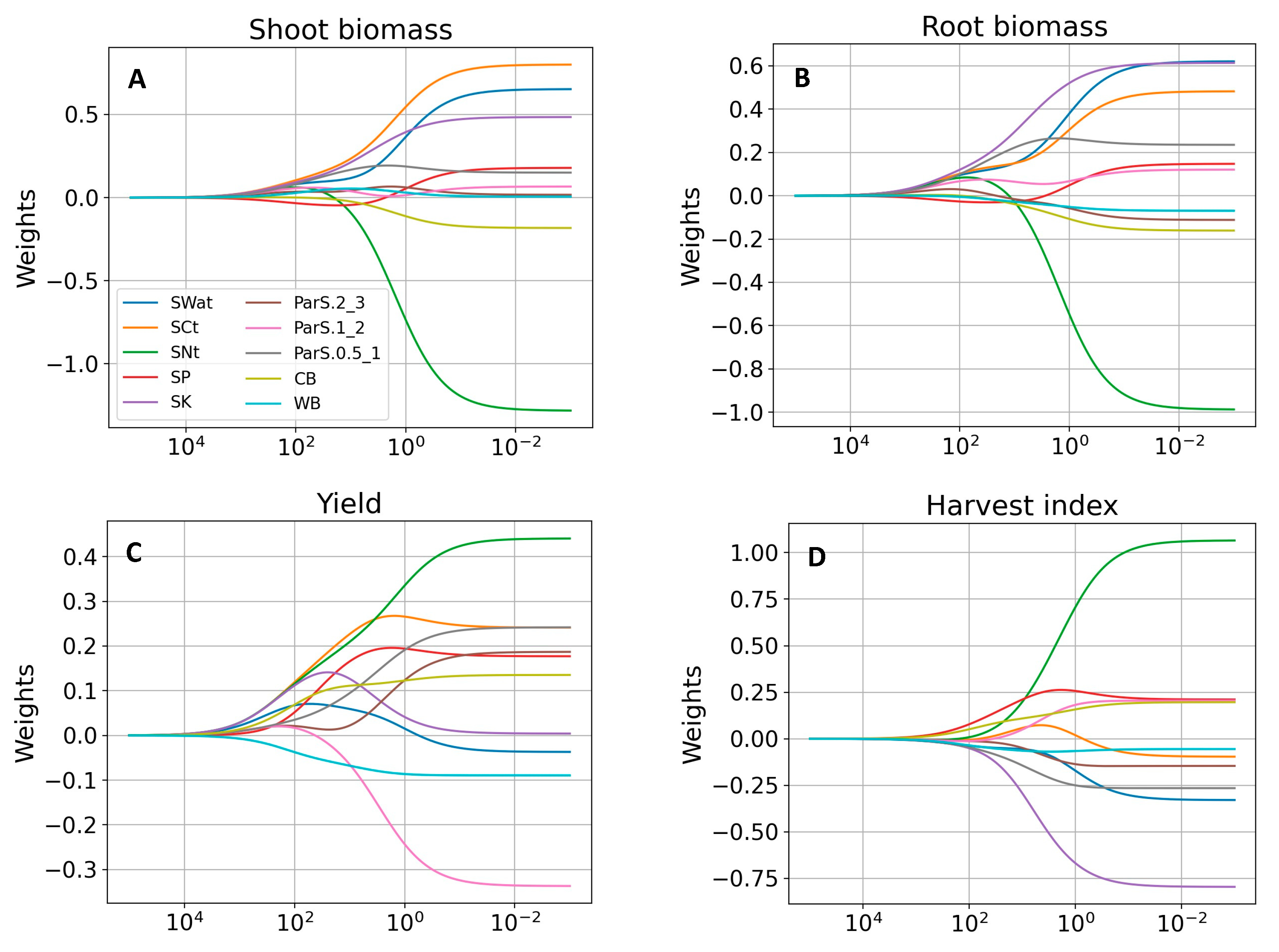
3.4. Ridge Regression
3.4.1. Determination of Regularization Parameter
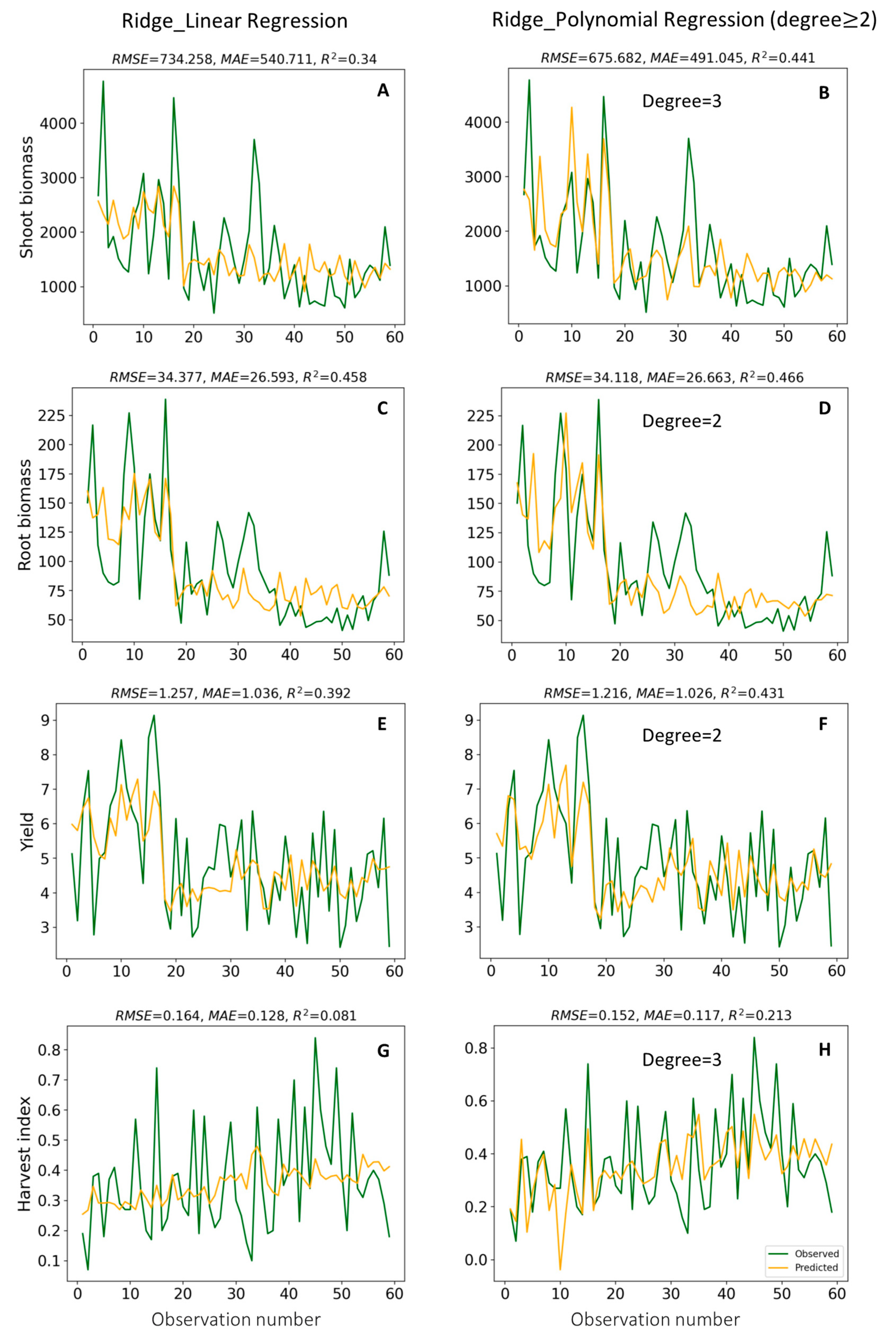
3.4.2. Prediction of Soybean Growth Indicators
4. Discussion
4.1. Plant Growth
4.2. Soil Aggregation
4.3. Nitrogen Fixation Ability
4.4. Modelling of Plant Growth Indicators
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Longjun, C. UN convention to combat desertification. In Encyclopedia of Environmental Health; Elsevier: Amsterdam, The Netherlands, 2019; pp. 238–251. [Google Scholar]
- UNCCD. United Nations Convention to Combat Desertification. 2022. Available online: https://www.unccd.int/our-work/land-life-programme (accessed on 30 May 2022).
- Qin, L.; Jiang, H.; Tian, J.; Zhao, J.; Liao, H. Rhizobia: Enhance acquisition of phosphorus from different sources by soybean plants. Plant Soil 2011, 349, 25–36. [Google Scholar] [CrossRef]
- Dos Santos Silva, F.C.; Sediyama, T.; de Cássia Teixeira Oliveira, R.; Borém, A.; da Silva, F.L.; Bezerra, A.R.G.; da Silva, A.F. Economic Importance and Evolution of Breeding. In Soybean Breeding; Da Silva, F.L., Gomes Bezerra, A.R., da Silva, A.F., Eds.; Springer: Cham, Switzerland, 2017; p. 3. [Google Scholar]
- Salvagiotti, F.; Cassman, K.G.; Weiss, A.; Dobermann, A. Nitrogen Uptake, Fixation and Response to N Fertilizer in Soybeans. Field Crops Res. 2008, 108, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Jiang, S.; Jardinaud, M.F.; Gao, J.; Pecrix, Y.; Wen, J.; Mysore, K.; Xu, P.; Sanchez-Canizares, C.; Ruan, Y.; Li, Q.; et al. NIN-like protein transcription factors regulate leghemoglobin genes in legume nodules. Science 2021, 374, 625–628. [Google Scholar] [CrossRef]
- Pirmoradian, N.; Sepaskhah, A.R.; Hajabbasi, M.A. Application of fractal theory to quantify soil aggregate stability as influence by tillage treatments. Biosyst. Eng. 2005, 90, 227–234. [Google Scholar] [CrossRef]
- Wei, C.; Gao, M.; Shao, J.; Xie, D.; Pan, G. Soil aggregate and its response to land management practices. China Particuology 2006, 4, 211–219. [Google Scholar] [CrossRef]
- Barthès, B.; Roose, E. Aggregate stability as an indicator of soil susceptibility to runoff and erosion; validation at several levels. Catena 2022, 47, 133–149. [Google Scholar] [CrossRef] [Green Version]
- Cantón, Y.; Solé-Benet, A.; Asensio, C.; Chamizo, S.; Puigdefábregas, J. Aggregate stability in range sandy loam soils relationships with runoff and erosion. Catena 2009, 77, 192–199. [Google Scholar] [CrossRef] [Green Version]
- Ciric, V.; Manojlovic, M.; Nesic, L.; Belic, M. Soil dry aggregate size distribution: Effects of soil type and land use. J. Soil Sci. Plant Nutr. 2012, 12, 689–703. [Google Scholar] [CrossRef] [Green Version]
- Bünemann, E.K.; Bongiorno, G.; Bai, Z.; Creamer, R.E.; De Deyn, G.; de Goede, R.; Fleskens, L.; Geissen, V.; Kuyper, T.W.; Mader, P.; et al. Soil Quality—A Critical Review. Soil Biol. Biochem. 2018, 120, 105–125. [Google Scholar] [CrossRef]
- Smirnova, L.G.; Novykh, L.L.; Pelekhotse, E.A. Physical properties of chernozems on slopes in the landscape farming system. Eurasian Soil Sci. 2006, 39, 278–282. [Google Scholar] [CrossRef]
- Shahabinejad, N.; Mahmoodabadi, M.; Jalalian, A.; Chavoshi, E. The fractionation of soil aggregates associated with primary particles influencing wind erosion rates in arid to semiarid environments. Geoderma 2019, 356, 113936. [Google Scholar] [CrossRef]
- McKenzie, B.M.; Tisdall, J.M.; Vance, W.H. Soil Physical Quality. In Encyclopedia of Agrophysics; Encyclopedia of Earth Sciences Series; Gliński, J., Horabik, J., Lipiec, J., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 770–777. [Google Scholar]
- Magdoff, F. Soil Quality and Management. In Agroecology; CRC Press: Boca Raton, FL, USA, 2018; pp. 349–364. [Google Scholar]
- Atkinson, C.J.; Fitzgerald, J.D.; Hipps, N.A. Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: A review. Plant Soil 2010, 337, 1–18. [Google Scholar] [CrossRef]
- Biederman, L.A.; Harpole, W.S. Biochar and its effects on plant productivity and nutrient cycling: A meta-analysis. GCB Bioenergy 2013, 5, 202–214. [Google Scholar] [CrossRef]
- Brodowski, S.; John, B.; Flessa, H.; Amelung, W. Aggregate occluded black carbon in soil. Eur. J. Soil Sci. 2006, 57, 539–546. [Google Scholar] [CrossRef]
- Liang, B.; Lehmann, J.; Solomon, D.; Sohi, S.; Thies, J.E.; Skjemstad, J.O.; Luizão, F.J.; Engelhard, M.H.; Neves, E.G.; Wirick, S. Stability of biomass-derived black carbon in soils. Geochim. Cosmochim. Acta 2008, 72, 6069–6078. [Google Scholar] [CrossRef]
- Purakayastha, T.J.; Kumari, S.; Sasmal, S.; Pathak, H. Biochar carbon sequestration in soil-A myth or reality? Int. J. Bio Resour. Stress Manag. 2015, 6, 623–630. [Google Scholar] [CrossRef]
- Kumari, K.G.I.D.; Moldrup, P.; Paradelo, M.; Elsgaard, L.; de Jonge, L.W. Soil Properties Control Glyphosate Sorption in Soils Amended with Birch Wood Biochar. Water Air Soil Pollut. 2016, 227, 174. [Google Scholar] [CrossRef]
- Busscher, W.J.; Novak, J.M.; Evans, D.E.; Watts, D.W.; Niandou, M.A.S.; Ahmedna, M. Influence of pecan biochar on physical properties of a Norfolk loamy sand. Soil Sci. 2010, 175, 10–14. [Google Scholar] [CrossRef] [Green Version]
- Peng, X.; Ye, L.L.; Wang, C.H.; Zhou, H.; Sun, B. Temperature- and duration-dependent rice straw-derived biochar: Characteristics and its effects on soil properties of an Ultisol in southern China. Soil Tillage Res. 2011, 112, 159–166. [Google Scholar] [CrossRef]
- Hardie, M.; Clothier, B.; Bound, S.; Oliver, G.; Close, D. Does biochar influence soil physical properties and soil water availability? Plant Soil 2014, 376, 347–361. [Google Scholar] [CrossRef]
- Sohi, S.; Lopez-Capel, E.; Krull, E.; Bol, R. Biochar, climate change and soil: A review to guide future research. Capel 2009, 5, 17–31. [Google Scholar]
- Ma, H.; Shurigin, V.; Jabborova, D.; Dela Cruz, J.A.; Dela Cruz, T.E.; Wirth, S.; Bellingrath-Kimura, S.D.; Egamberdieva, D. The Integrated Effect of Microbial Inoculants and Biochar Types on Soil Biological Properties, and Plant Growth of Lettuce (Lactuca sativa L.). Plants 2022, 11, 423. [Google Scholar] [CrossRef] [PubMed]
- Egamberdieva, D.; Li, L.; Ma, H.; Wirth, S.; Bellingrath-Kimura, S.D. Soil amendments with different maize biochars, to varying degrees, improve chickpea growth under drought by improving symbiotic performance with Mesorhizobium ciceri and soil bio-chemical properties. Front. Microbiol. 2019, 10, 2423. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Shurigin, V.; Alaylar, B.; Ma, H.; Müller, M.E.H.; Wirth, S.; Reckling, M.; Bellingrath-Kimura, S.D. The effect of biochars and endophytic bacteria on growth and root rot disease incidence of Fusarium infested narrow-leafed lupin (Lupinus angustifolius L.). Microorganisms 2020, 8, 496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salazar, J.J.; Garland, L.; Ochoa, J.; Pyrcz, M.J. Fair train-test split in machine learning: Mitigating spatial autocorrelation for improved prediction accuracy. J. Pet. Sci. Eng. 2022, 209, 109885. [Google Scholar] [CrossRef]
- Bedoui, A.; Lazar, N.A. Bayesian empirical likelihood for ridge and lasso regressions. Comput. Stat. Data Anal. 2020, 145, 106917. [Google Scholar] [CrossRef]
- Panzone, L.; Ulph, A.; Areal, F.; Grippo, V. A ridge regression approach to estimate the relationship between landfill taxation and waste collection and disposal in England. Waste Manag. 2021, 129, 95–110. [Google Scholar] [CrossRef]
- Lipovetsky, S. Two-parameter ridge regression and its convergence to the eventual pairwise model. Math. Comput. Model. 2006, 44, 304–318. [Google Scholar] [CrossRef]
- Ma, H.; Egamberdieva, D.; Wirth, S.; Bellingrath-Kimura, S.D. Effect of biochar and irrigation on soybean-rhizobium symbiotic performance and soil enzymatic activity in field rhizosphere. Agronomy 2019, 9, 626. [Google Scholar] [CrossRef] [Green Version]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon and organic matter. In Methods of Soil Analysis; Part 2. Agronomy Monographs, 9; Page, A.L., Ed.; ASA and SSSA: Madison, WI, USA, 1982; pp. 539–579. [Google Scholar]
- Hillel, D. Introduction to Environmental Soil Physics; Elsevier: Amsterdam, The Netherlands, 2004; 494p. [Google Scholar]
- Friendly, M. The Generalized Ridge Trace Plot: Visualizing Bias and Precision. J. Comput. Graph. Stat. 2013, 22, 50–68. [Google Scholar] [CrossRef] [Green Version]
- Rondon, M.A.; Lehmann, J.; Ramírez, J.; Hurtado, M. Biological nitrogen fixation by common beans (Phaseolus vulgaris L.) increases with bio-char additions. Biol. Fertil. Soils 2007, 43, 699–708. [Google Scholar] [CrossRef]
- Oram, N.J.; van de Voorde, T.F.J.; Ouwehand, G.-J.; Bezemer, T.M.; Mommer, L.; Jeffery, S.; Van Groenigen, J.W. Soil amendment with biochar increases the competitive ability of legumes via increased potassium availability. Agric. Ecosyst. Environ. 2014, 191, 92–98. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.; Egamberdieva, D.; Wirth, S.; Li, Q.; Omari, R.A.; Hou, M.; Bellingrath-Kimura, S.D. Effect of biochar and irrigation on the interrelationships among soybean growth, root nodulation, plant P uptake, and soil nutrients in a sandy field. Sustainability 2019, 11, 6542. [Google Scholar] [CrossRef] [Green Version]
- Egamberdieva, D.; Reckling, M.; Wirth, S. Biochar-based inoculum of Bradyrhizobium sp. improves plant growth and yield of lupin (Lupinus albus L.) under drought stress. Eur. J. Soil Biol. 2017, 78, 38–42. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Zoghi, Z.; Nazarov, K.; Wirth, S.; Bellingrath-Kimura, S.D. Plant growth response of broad bean (Vicia faba, L.) to biochar amendment of loamy sand soil under irrigated and drought conditions. Environ. Sustain. 2020, 3, 319–324. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.; Behrendt, U.; Abd_Allah, E.F.; Berg, G. Biochar treatment resulted in a combined effect on soybean growth promotion and a shift in plant growth promoting rhizobacteria. Front. Microbiol. 2016, 7, 209. [Google Scholar] [CrossRef] [Green Version]
- Mete, F.Z.; Mia, S.; Dijkstra, F.A.; Abuyusuf, M.; Iqbal Hossain, A.S.M. Synergistic effects of biochar and NPK Fertilizer on soybean yield in an alkaline soil. Pedosphere 2015, 25, 713–719. [Google Scholar] [CrossRef]
- Yooyen, J.; Wijitkosum, S.; Sriburi, T. Increasing yield of soybean by adding biochar. J. Environ. Res. Dev. 2015, 9, 1066–1074. [Google Scholar]
- Steiner, C.; Teixeira, W.G.; Lehmann, J.; Nehls, T.; de Macedo, J.L.V.; Blum, W.E.H.; Zech, W. Long term effects of manure, charcoal and mineral fertilization on crop production and fertility on a highly weathered Central Amazonian upland soil. Plant Soil 2007, 291, 275. [Google Scholar] [CrossRef] [Green Version]
- Kimetu, J.M.; Lehmann, J.; Ngoze, S.O.; Mugendi, D.N.; Kinyangi, J.M.; Riha, S.; Verchot, L.; Recha, J.W.; Pell, A.N. Reversibility of soil productivity decline with organic matter of differing quality along a degradation gradient. Ecosystems 2008, 11, 726. [Google Scholar] [CrossRef]
- Van Zwieten, L.; Rose, T.; Herridge, D.; Kimber, S.; Rust, J.; Cowie, A.; Morris, S. Enhanced biological N2 fixation and yield of faba bean (Vicia faba L.) in an acid soil following biochar addition: Dissection of causal mechanisms. Plant Soil 2015, 395, 7–20. [Google Scholar] [CrossRef] [Green Version]
- Bonser, S.P.; Aarssen, L.W. Allometry and plasticity of meristem allocation throughout development in Arabidopsis thaliana. J. Ecol. 2001, 89, 72–79. [Google Scholar] [CrossRef]
- Caton, B.P.; Foin, T.C.; Hill, J.E. Phenotypic plasticity of Ammannia spp. in competition with rice. Weed Res. 1997, 37, 33–38. [Google Scholar] [CrossRef]
- Huhta, A.P.; Tuomi, J.; Rautio, P. Cost of apical dominance in two monocarpic herbs, Erysimum strictum and Rhinanthus minor. Can. J. Bot. 2000, 78, 591–599. [Google Scholar]
- Wang, P.; Weiner, J.; Cahill, J.F.; Zhou, D.W.; Bian, H.F.; Song, Y.T.; Sheng, L.X. Shoot competition, root competition and reproductive allocation in Chenopodium acuminatum. J. Ecol. 2015, 102, 1688–1696. [Google Scholar] [CrossRef]
- Imbufe, A.U.; Patti, A.F.; Burrow, D.; Surapaneni, A.; Jackson, W.R.; Milner, A.D. Effects of potassium humate on aggregate stability of two soils from Victoria, Australia. Geoderma 2005, 125, 321–330. [Google Scholar] [CrossRef]
- Zhao, H.L.; Yi, X.Y.; Zhou, R.L.; Zhao, X.Y.; Zhang, T.H.; Drake, S. Wind erosion and sand accumulation effects on soil properties in Horqin Sandy Farmland, Inner Mongolia. CATENA 2006, 65, 71–79. [Google Scholar] [CrossRef]
- Zobeck, T.M.; Baddock, M.; Van Pelt, R.S.; Tatarko, J.; Acosta-Martinez, V. Soil property effects on wind erosion of organic soils. Aeolian Res. 2013, 10, 43–51. [Google Scholar] [CrossRef]
- Gurmessa, B.; Demissie, A.; Lemma, B.; Biyensa, G. Susceptibility of soil to wind erosion in arid area of the Central Rift Valley of Ethiopia. Environ. Syst. Res. 2015, 4, 101. [Google Scholar] [CrossRef] [Green Version]
- Gong, H.; Meng, D.; Li, X.; Zhu, F. Soil Degradation and Food Security Coupled with Global Climate Change in Northeastern China. Chin. Geogr. Sci. 2013, 23, 562–573. [Google Scholar] [CrossRef] [Green Version]
- Massah, J.; Azadegan, B. Effect of Chemical Fertilizers on Soil Compaction and Degradation. Agric. Mech. Asia Afr. Lat. Am. 2016, 47, 44–50. [Google Scholar]
- Camacho, F.; Fuster, B.; Li, W.; Weiss, M.; Ganguly, S.; Lacaze, R.; Baret, F. Crop specific algorithms trained over ground measurements provide the best performance for GAI and fAPAR estimates from Landsat-8 observations. Remote Sens. Environ. 2021, 260, 112453. [Google Scholar] [CrossRef]

| Biochar Feedstock | Total (g kg−1) | Available (mg kg−1) | C/N | ||
|---|---|---|---|---|---|
| C | N | K | P | ||
| Mixed wood | 712 | 7.53 | 2698 | 191 | 95 |
| Maize cob | 755 | 5.1 | 3802 | 65 | 148 |
| Plant Nutrient Concentration (g kg−1) | BDG | MLZ | RQ | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CB | CK | WB | CB | CK | WB | CB | CK | WB | |
| Shoot TC | 467.09 ± 2.7 a | 463.34 ± 3.58 a | 466.8 ± 1.12 a | 463.11 ± 2.88 a | 463.23 ± 3.7 a | 465.51 ± 1.7 a | 468.59 ± 3.08 a | 405.05 ± 67.55 a | 406.56 ± 67.77 a |
| Shoot N | 24.82 ± 0.64 a | 23.29 ± 1.32 a | 24.73 ± 1.24 a | 24.84 ± 1.21 a | 22.96 ± 0.78 a | 25.54 ± 1.19 a | 29.27 ± 1.69 a | 29.17 ± 1.23 a | 30.38 ± 1.61 a |
| Shoot P | 3.7 ± 0.16 a | 3.49 ± 0.18 a | 3.7 ± 0.22 a | 3.76 ± 0.21 a | 3.66 ± 0.2 a | 3.99 ± 0.26 a | 3.55 ± 0.21 a | 3.71 ± 0.17 a | 3.94 ± 0.23 a |
| Shoot K | 18.56 ± 0.44 a | 16.96 ± 0.37 a | 17.68 ± 0.69 a | 16.6 ± 0.72 a | 16.76 ± 1.14 a | 17.28 ± 0.39 a | 16.23 ± 0.56 a | 17.4 ± 0.9 a | 16.35 ± 0.91 a |
| Shoot Mg | 5.48 ± 0.19 a | 5.72 ± 0.3 a | 5.42 ± 0.12 a | 5.34 ± 0.14 a | 5.14 ± 0.3 a | 5.7 ± 0.2 a | 5.1 ± 0.24 a | 4.82 ± 0.15 a | 4.56 ± 0.16 a |
| Root TC | 467.49 ± 2.38 a | 472.9 ± 4.02 a | 468.26 ± 2.21 a | 478.14 ± 2.65 a | 463.87 ± 2.34 b | 465.77 ± 1.8 b | 477.71 ± 1.29 a | 475.14 ± 1.54 a | 480.19 ± 2.62 a |
| Root N | 7.25 ± 0.17 a | 8.17 ± 0.36 a | 7.46 ± 0.33 a | 7.12 ± 0.37 a | 6.89 ± 0.3 a | 7.08 ± 0.31 a | 10.66 ± 0.56 a | 10.08 ± 0.8 a | 9.63 ± 0.63 a |
| Root P | 1.37 ± 0.09 a | 1.16 ± 0.08 a | 1.14 ± 0.16 a | 1.12 ± 0.15 a | 1.19 ± 0.1 a | 1.22 ± 0.13 a | 1.3 ± 0.09 a | 1.12 ± 0.15 a | 1.09 ± 0.12 a |
| Root K | 5.9 ± 0.24 a | 5.25 ± 0.36 a | 5.51 ± 0.36 a | 5.51 ± 0.54 a | 5.01 ± 0.54 a | 4.91 ± 0.48 a | 6.3 ± 0.3 a | 5.98 ± 0.38 a | 4.88 ± 0.39 b |
| Root Mg | 1.82 ± 0.12 b | 2.21 ± 0.06 a | 1.8 ± 0.06 b | 1.97 ± 0.15 a | 2.34 ± 0.08 a | 2.24 ± 0.14 a | 2.25 ± 0.18 a | 2.11 ± 0.22 a | 1.79 ± 0.19 a |
| Soil Physicochemical Property | BDG | MLZ | RQ | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CB | CK | WB | CB | CK | WB | CB | CK | WB | |
| >10 mm (%) | 11.79 ± 1.63 a | 9.76 ± 1.58 a | 9.4 ± 1.67 a | 6.94 ± 2.22 a | 5.65 ± 1.01 a | 4.4 ± 1.91 a | 12.16 ± 1.64 a | 12.18 ± 1.17 a | 13.34 ± 1.31 a |
| 7–10 mm (%) | 12.66 ± 1.12 a | 9.59 ± 1.01 b | 11.63 ± 0.45 ab | 6.28 ± 1.47 a | 7.63 ± 0.97 a | 5.37 ± 1.21 a | 12.56 ± 1.58 a | 15.12 ± 1.5 a | 11.2 ± 0.89 a |
| 5–7 mm (%) | 11.85 ± 0.7 a | 12.08 ± 0.88 a | 11.79 ± 0.48 a | 8.17 ± 0.98 a | 9.77 ± 0.92 a | 7.52 ± 1.21 a | 12.96 ± 0.5 a | 13.53 ± 0.68 a | 13.39 ± 0.45 a |
| 3–5 mm (%) | 13.8 ± 0.47 a | 13.54 ± 0.62 a | 14.48 ± 0.9 a | 10.93 ± 1.05 a | 11.65 ± 0.82 a | 10.03 ± 0.86 a | 16.38 ± 0.39 a | 15.96 ± 0.43 a | 16.85 ± 0.46 a |
| 2–3 mm (%) | 9.02 ± 0.15 a | 9.29 ± 0.5 a | 9.66 ± 0.32 a | 8.43 ± 0.5 a | 8.43 ± 0.27 a | 8.46 ± 0.37 a | 9.91 ± 0.29 a | 9.6 ± 0.35 a | 10.12 ± 0.28 a |
| 1–2 mm (%) | 5.63 ± 0.08 a | 5.91 ± 0.27 a | 5.92 ± 0.19 a | 5.69 ± 0.29 a | 5.47 ± 0.23 a | 5.82 ± 0.21 a | 6.6 ± 0.36 a | 6.33 ± 0.25 a | 6.48 ± 0.24 a |
| 0.5–1 mm (%) | 11.29 ± 0.36 a | 12.24 ± 0.58 a | 11.82 ± 0.4 a | 12.93 ± 0.62 a | 12.4 ± 0.44 a | 13.87 ± 0.57 a | 13.82 ± 0.9 a | 13.25 ± 0.75 a | 13.44 ± 0.54 a |
| 0.25–0.5 mm (%) | 7.74 ± 0.36 a | 8.69 ± 0.55 a | 7.99 ± 0.37 a | 10.88 ± 0.73 a | 10 ± 0.42 a | 11.47 ± 0.62 a | 7.54 ± 0.67 a | 6.78 ± 0.57 a | 6.83 ± 0.38 a |
| 0–0.25 mm (%) | 16.17 ± 1.84 a | 18.69 ± 1.27 a | 17.23 ± 1.31 a | 29.25 ± 2.98 a | 28.89 ± 2.2 a | 32.89 ± 3.46 a | 7.85 ± 0.83 a | 7.06 ± 0.64 a | 7.11 ± 0.37 a |
| Soil TC (g kg−1) | 26.46 ± 1.16 a | 21.74 ± 0.58 b | 22.68 ± 0.76 b | 25.61 ± 0.93 a | 23.59 ± 0.94 a | 25.44 ± 1.16 a | 36.31 ± 1.32 a | 31.13 ± 0.77 b | 33.6 ± 0.68 ab |
| Soil TN (g kg−1) | 1.21 ± 0.03 a | 1.12 ± 0.05 a | 1.13 ± 0.05 a | 1.03 ± 0.03 a | 1 ± 0.02 a | 1.07 ± 0.02 a | 2.01 ± 0.08 a | 1.87 ± 0.08 a | 1.91 ± 0.03 a |
| Soil P (mg kg−1) | 55.64 ± 2.84 a | 56.3 ± 4.78 a | 58.79 ± 4.86 a | 46.17 ± 9.49 a | 44.97 ± 5.42 a | 49.76 ± 5.56 a | 41.13 ± 4.87 a | 36.37 ± 4.91 a | 34.43 ± 1.6 a |
| Soil K (mg kg−1) | 129.96 ± 24.53 a | 134.48 ± 9.3 a | 166.21 ± 13.81 a | 124.49 ± 11.93 a | 122.58 ± 7.85 a | 114.09 ± 5.38 a | 402.35 ± 49.77 a | 287.2 ± 33.11 b | 272.86 ± 12.08 b |
| Soil porosity (%) | 42.19 ± 1.74 a | 41.10 ± 1.20 a | 40.64 ± 1.32 a | 47.67 ± 1.56 a | 46.59 ± 0.73 a | 45.40 ± 1.25 a | 45.07 ± 1.17 b | 47.30 ± 1.12 ab | 49.46 ± 1.22 a |
| Df | Variance | F | Pr (>F) | Signif. | ||
|---|---|---|---|---|---|---|
| Model | 28 | 3.14359 | 4.064 | 0.001 | *** | |
| Axis | RDA1 | 1 | 2.17135 | 139.448 | 0.001 | *** |
| RDA2 | 1 | 0.83498 | 53.624 | 0.042 | * | |
| RDA3 | 1 | 0.09565 | 6.143 | 1 | ||
| RDA4 | 1 | 0.04161 | 2.672 | 1 | ||
| Explanatory variables | LB | 1 | 0.25295 | 7.7685 | 0.002 | ** |
| SWat | 1 | 0.61597 | 18.9176 | 0.001 | *** | |
| SPor | 1 | 0.0523 | 1.6061 | 0.221 | ||
| ParS.10_ | 1 | 0.026 | 0.7985 | 0.451 | ||
| ParS.7_10 | 1 | 0.18183 | 5.5843 | 0.005 | ** | |
| ParS.5_7 | 1 | 0.03474 | 1.0669 | 0.321 | ||
| ParS.3_5 | 1 | 0.00306 | 0.0941 | 0.953 | ||
| ParS.2_3 | 1 | 0.00229 | 0.0703 | 0.966 | ||
| ParS.1_2 | 1 | 0.01708 | 0.5246 | 0.596 | ||
| ParS.0.5_1 | 1 | 0.03285 | 1.009 | 0.374 | ||
| ParS.0.25_0.5 | 1 | 0.04492 | 1.3795 | 0.254 | ||
| ParS.0_0.25 | 1 | 0.21643 | 6.6471 | 0.007 | ** | |
| MWD | 1 | 0.13205 | 4.0555 | 0.03 | * | |
| GMD | 1 | 0.05726 | 1.7586 | 0.174 | ||
| SCt | 1 | 0.19856 | 6.098 | 0.005 | ** | |
| SNt | 1 | 0.0222 | 0.6818 | 0.498 | ||
| SP | 1 | 0.04169 | 1.2803 | 0.26 | ||
| SK | 1 | 0.15023 | 4.6137 | 0.02 | * | |
| ShCt | 1 | 0.06954 | 2.1358 | 0.096 | ||
| ShN | 1 | 0.04044 | 1.2421 | 0.279 | ||
| ShP | 1 | 0.0867 | 2.6627 | 0.075 | ||
| ShK | 1 | 0.10648 | 3.2703 | 0.046 | * | |
| ShMg | 1 | 0.03127 | 0.9604 | 0.405 | ||
| RCt | 1 | 0.03391 | 1.0413 | 0.379 | ||
| RN | 1 | 0.06323 | 1.9418 | 0.143 | ||
| RP | 1 | 0.20455 | 6.2822 | 0.004 | ** | |
| RK | 1 | 0.26496 | 8.1373 | 0.002 | ** | |
| RMg | 1 | 0.00711 | 0.2184 | 0.83 | ||
| Residual | 31 | 1.00939 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, H.; Li, Q.; Egamberdieva, D.; Bellingrath-Kimura, S.D. A Case Study in Desertified Area: Soybean Growth Responses to Soil Structure and Biochar Addition Integrating Ridge Regression Models. Agronomy 2022, 12, 1341. https://doi.org/10.3390/agronomy12061341
Ma H, Li Q, Egamberdieva D, Bellingrath-Kimura SD. A Case Study in Desertified Area: Soybean Growth Responses to Soil Structure and Biochar Addition Integrating Ridge Regression Models. Agronomy. 2022; 12(6):1341. https://doi.org/10.3390/agronomy12061341
Chicago/Turabian StyleMa, Hua, Qirui Li, Dilfuza Egamberdieva, and Sonoko Dorothea Bellingrath-Kimura. 2022. "A Case Study in Desertified Area: Soybean Growth Responses to Soil Structure and Biochar Addition Integrating Ridge Regression Models" Agronomy 12, no. 6: 1341. https://doi.org/10.3390/agronomy12061341







