Increasing Amino Acids Content of White Wines with Enzymes Treatments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
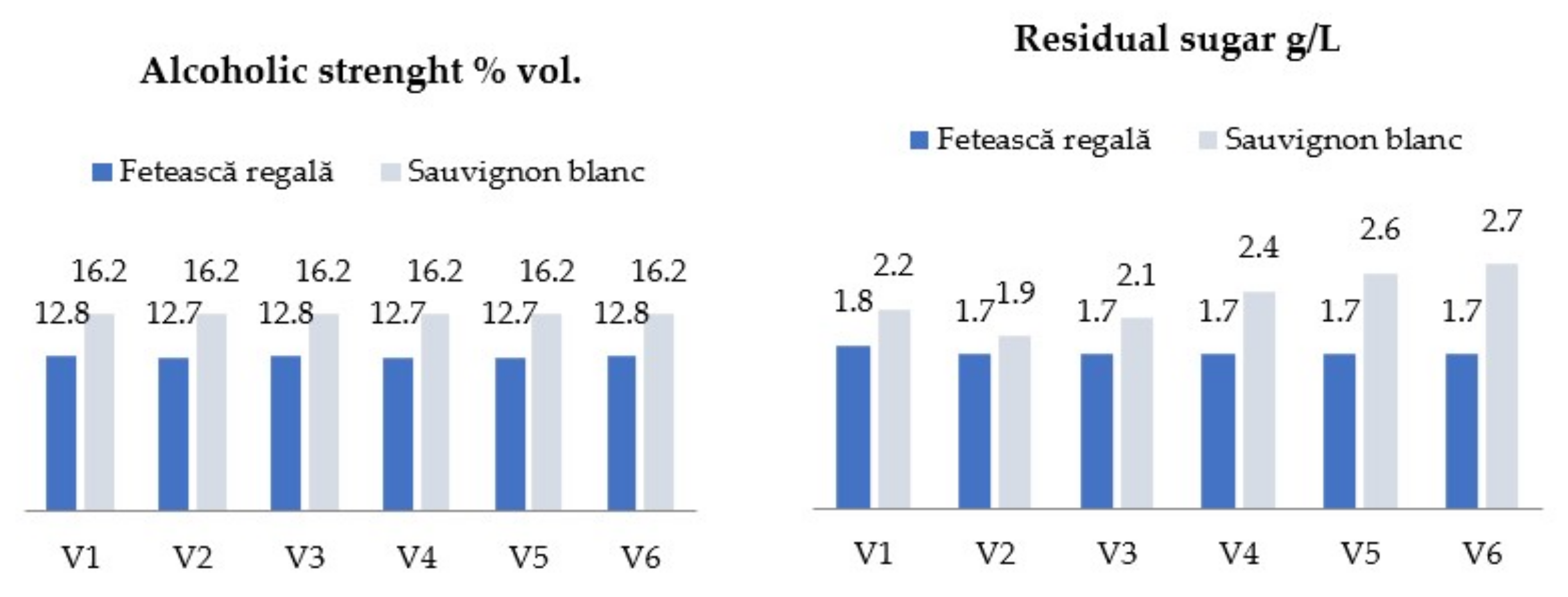
2.2. Grapes and Winemaking Procedures
2.3. Amino Acid Identification and Quantification
2.4. Sensory Evaluation
2.5. Statistical Analysis
3. Results
3.1. The Influence of Enzymes on Amino Acid Concentration
3.2. The Impact of Enzymes on Wines Sensory Perception
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Mojsov, K.; Andronikov, D.; Janevski, A.; Jordeva, S.; Zezova, S. Enzymes and wine: The enhanced quality and yield. Savrem. Tehnol. 2015, 4, 94–100. [Google Scholar] [CrossRef] [Green Version]
- Cotea, D.V.; Zănoagă, C.; Cotea, V.V. Tratat de Oenochimie; Romanian Academy Publishing: Bucharest, Romania, 2009. [Google Scholar]
- Ribéreau-Gayon, P.; Dubordieu, D.; Donèche, B.; Lonvaud, A. Handbook of Enology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006. [Google Scholar]
- McKinnon, A. The Impact of Amino Acids on Growth Performance and Major Volatile Compound Formation by Industrial Wine Yeast. 2013. Available online: http://scholar.sun.ac.za (accessed on 19 May 2022).
- Claus, H.; Mojsov, K. Enzymes for wine fermentation: Current and perspective applications. Fermentation 2018, 4, 52. [Google Scholar] [CrossRef] [Green Version]
- Kurbanoglu, S.; Erkmen, C.; Uslu, B. Frontiers in electrochemical enzyme based biosensors for food and drug analysis. Trends Anal. Chem. 2020, 124, 115809. [Google Scholar] [CrossRef]
- Ottone, C.; Romero, O.; Aburto, C.; Illanes, A.; Wilson, L. Biocatalysis in the winemaking industry: Challenges and opportunities for immobilized enzymes. Compr. Rev. Food Sci. 2020, 19, 595–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirás-Avalos, J.M.; Bouzas-Cid, Y.; Trigo-Córdoba, E.; Orriols, I.; Falqué, E. Amino acid profiles to differentiate white wines from three autochtonous Galician varieties. Foods 2020, 9, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soufleros, E.; Bouloumpasi, E.; Tsarchopoulos, C.; Biliaderis, C. Primary amino acid profiles of Greek white wines and their use in classification according to variety, origin and vintage. Food Chem. 2003, 80, 261–273. [Google Scholar] [CrossRef]
- Pereira, V.; Pereira, A.C.; Pérez Trujillo, J.P.; Cacho, J.; Marques, J.C. Amino acids and biogenic amines evolution during the estufagem of fortified wines. J. Chem. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Scutarașu, E.C.; Luchian, C.E.; Vlase, L.; Colibaba, L.C.; Gheldiu, A.M.; Cotea, V.V. Evolution of phenolic profile of white wines treated with enzymes. Food Chem. 2020, 340, 127910. [Google Scholar] [CrossRef]
- Scutarașu, E.C. Studies on Enzymes Impact on White Wines Technology from Iași Vineyard. Ph.D. Thesis, Iasi University of Life Sciences, Iasi, Romania, 2021. [Google Scholar]
- ISO 3591:1977; Sensory Analysis. Apparatus. Wine-Tasting Glass. ISO: Geneva, Switzerland, 2022.
- ISO 8589:2007; Sensory Analysis. General Guidance for the Design of Test Room. ISO: Geneva, Switzerland, 2017.
- OIV. Review Document on Sensory Analysis of Wine. Paris. 2015. Available online: https://www.oiv.int/public/medias/3307/review-on-sensory-analysis-of-wine.pdf (accessed on 18 May 2022).
- Zhou, W.; Fang, R.; Chen, Q. Effect of gallic and protocatechuic acids on the metabolism of ethyl carbamate in Chinese yellow rice wine brewing. Food Chem. 2017, 233, 174–181. [Google Scholar] [CrossRef]
- Mandl, K.; Silhavy-Richter, K.; Korntheuer, K.; Prinz, M.; Patzl-Fischerleitner, E.; Eder, R. Influence of different yeasts on the amino acid pattern of rosé wine. BIO Web Conf. 2017, 9, 02014. [Google Scholar] [CrossRef] [Green Version]
- Van Loon, L.J. Leucine as a pharmaconutrient in health and disease. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 71–77. [Google Scholar] [CrossRef]
- Hazelwood, L.A.; Daran, J.M.; van Maris, A.J.A.; Pronk, J.T.; Dickinson, J.R. The Ehrlich pathway for fusel alcohol production: A century of research on Saccharomyces cerevisiae metabolism. Appl. Environ. Microbiol. 2008, 74, 3920. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.Q.; Ye, D.Q.; Liu, P.T.; Duan, L.L.; Duan, C.Q.; Yan, G.L. Synergistic effects of branched-chain amino acids and phenylalanine addition on major volatile compounds in wine during alcoholic fermentation. S. Afr. J. Enol. Vitic. 2016, 37, 169–175. [Google Scholar] [CrossRef] [Green Version]
- Crépin, L.; Sanchez, I.; Nidelet, T.; Dequin, S.; Camarasa, C. Efficient ammonium uptake and mobilization of vacuolar arginine by Saccharomyces cerevisiae wine strains during wine fermentation. Microb. Cell Factories 2014, 13, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Jimenez-Lorenzo, R.; Bloem, A.; Farines, V.; Sablayrolles, J.M.; Camarasa, C. How to modulate the formation of negative volatile sulfur compounds during wine fermentation? FEMS Yeast Res. 2021, 21, foab038. [Google Scholar] [CrossRef]
- Hornsey, I. The Chemistry and Biology of Winemaking; RSC Publishing: London, UK, 2007. [Google Scholar]
- Scott, W.T.; van Mastrigt, O.; Block, D.E.; Notebaart, R.A.; Smid, E.J. Nitrogenous compound utilization and production of volatile organic compounds among commercial wine yeasts highlight strain-specific metabolic diversity. Microbiol. Spectr. 2021, 9, e00485-21. [Google Scholar] [CrossRef]
- Meléndez-Hevia, E.; de Paz-Lugo, P.; Cornish-Bowden, A.; Cárdenas, M.L. A weak link in metabolism: The metabolic capacity for glycine biosynthesis does not satisfy the need for collagen synthesis. J. Biosci. 2009, 34, 853–872. [Google Scholar] [CrossRef]
- Krogerus, K.; Gibson, B.R. Influence of valine and other amino acids on total diacetyl and 2,3-pentanedione levels during fermentation of brewer’s wort. Appl. Microbiol. Biotechnol. 2013, 97, 6919–6930. [Google Scholar] [CrossRef] [Green Version]
- Galili, G.; Amir, R.; Fernie, A.R. The regulation of essential amino acid synthesis and accumulation in plants. Annu. Rev. Plant. Biol. 2016, 67, 153–178. [Google Scholar] [CrossRef]
- López-Rituerto, E.; Avenoza, A.; Busto, J.H.; Peregrina, J.M. NMR Study of histidine metabolism during alcoholic and malolactic fermentations of wine and their influence on histamine production. J. Agric. Food Chem 2013, 61, 9464–9469. [Google Scholar] [CrossRef]
- Agustini, B.C.; Lima, D.B.D.; Bonfim, T.M.B. Composition of amino acids and bioactive amines in common wines of Brazil. Acta Sci.-Health Sci. 2014, 36, 225. [Google Scholar] [CrossRef] [Green Version]
- Cosme, F.; Gonçalves, B.; Inês, A.; Jordão, A.M.; Vilela, A. Grape and wine metabolites: Biotechnological approaches to improve wine quality, grape and wine biotechnology. In Grape and Wine Biotechnology; Morata, A., Loira, I., Eds.; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef] [Green Version]
- Álvarez-Fernández, M.A.; Carafa, I.; Vrhovsek, U.; Arapitsas, P. Modulating Wine Aromatic Amino Acid Catabolites by Using Torulaspora delbrueckii in Sequentially Inoculated Fermentations or Saccharomyces cerevisiae Alone. Microorganisms 2020, 8, 1349. [Google Scholar] [CrossRef] [PubMed]
- Valero, E.; Millán, C.; Ortega, J.M.; Mauricio, J.C. Concentration of amino acids in wine after the end of fermentation by Saccharomyces cerevisiae strains. J. Sci. Food Agric. 2003, 83, 830–835. [Google Scholar] [CrossRef]
- Nishimura, A.; Tanikawa, T.; Takagi, H. Inhibitory effect of arginine on proline utilization in Saccharomyces cerevisiae. Yeast 2020, 37, 531–540. [Google Scholar] [CrossRef]
- Castor, J.G.B. The free amino acids of musts and wines. The fate of amino acids of must during alcoholic fermentation. J. Food Sci. 1952, 18, 146–151. [Google Scholar] [CrossRef]
- Pogorzelski, E.; Laskowska, J.; Czyżowska, A. Degradation products of nucleic acids in wines fermented with dried autolysate of sedimented wine yeast. Pol. J. Food Nutr. Sci. 2006, 56, 177–181. [Google Scholar]
- Beltran, G.; Novo, M.; Rozes, N.; Mas, A.; Guillamon, J. Nitrogen catabolite repression in during wine fermentations. FEMS Yeast Res. 2004, 4, 625–632. [Google Scholar] [CrossRef] [Green Version]
- Guitard, A.; Hernándes-Orte, P.; Cacho, J. Effects of maceration on the amino acid content of Chardonnay musts and wines. Vitis 1997, 36, 43–47. [Google Scholar] [CrossRef]
- Bell, S.J.; Henschke, P.A. Implications of nitrogen nutrition for grapes, fermentation and wine. Aust. J. Grape Wine Res. 2005, 11, 242–295. [Google Scholar] [CrossRef]
- Klosse, P. Umami in wine. Tour. Hosp. Manag. 2013, 2, 25–28. [Google Scholar] [CrossRef]
- Bakker, J.; Bellworthy, S.J.; Reader, H.P.; Watkins, S.J. Effects of enzymes during vinification on color and sensory properties of port wines. Am. J. Enol. Vitic. 1999, 50, 271–276. [Google Scholar]
- Carrau, F.M.; Medina, K.; Farina, L.; Boido, E.; Henschke, P.A.; Dellacassa, E. Production of fermentation aroma compounds by Saccharomyces cerevisiae wine yeasts: Effects of yeast assimilable nitrogen on two model strains. FEMS Yeast Res. 2008, 8, 1196–1207. [Google Scholar] [CrossRef] [Green Version]
- Pinu, F.R.; Edwards, P.J.; Gardner, R.C.; Villas-Boas, S.G. Nitrogen and carbon assimilation by Saccharomyces cerevisiae during Sauvignon blanc juice fermentation. FEMS Yeast Res. 2014, 14, 1206–1222. [Google Scholar] [CrossRef] [Green Version]
- Ugliano, M.; Travis, B.; Francis, I.L.; Henschke, P.A. Volatile composition and sensory properties of Shiraz wines as affected by nitrogen supplementation and yeast species: Rationalizing nitrogen modulation of wine aroma. J. Agric. Food Chem. 2010, 58, 12417–12425. [Google Scholar] [CrossRef]
- Hernández-Orte, P.; Ibarz, M.J.; Cacho, J.; Ferreira, V. Effect of the addition of ammonium and amino acids to musts of Airen variety on aromatic composition and sensory properties of the obtained wine. Food Chem. 2005, 89, 163–174. [Google Scholar] [CrossRef]



| C | V1 | V2 | V3 | V4 | V5 | V6 | p | C | V1 | V2 | V3 | V4 | V5 | V6 | p |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arg | 400.27 0.28 * | 191.83 1.08 * | 180.50 0.22 * | 252.95 0.83 * | 245.29 0.28 * | 282.29 1.29 * | 0.0000 | Leu | 10.98 0.00 * | 7.27 0.01 * | 7.91 0.00 * | 9.89 0.01 * | 8.40 0.00 * | 11.66 0.01 * | 0.0000 |
| Lys | 13.76 0.00 * | 8.88 0.05 * | 11.36 0.01 * | 14.93 0.06 * | 7.33 0.01 * | 9.65 0.03 * | 0.0000 | Thr | 8.20 0.00 * | 8.71 0.01 * | 8.53 0.00 * | 9.72 0.01 * | 9.23 0.00 * | 9.68 0.01 * | 0.0000 |
| His | 55.71 0.19 * | 7.79 0.04 * | 6.85 0.02 * | 12.89 0.05 * | 9.38 0.00 * | 13.14 0.08 * | 0.0000 | Trp | 1.70 0.00 * | 0.88 0.00 * | 0.82 0.00 * | 1.51 0.00 * | 0.99 0.00 * | 1.66 0.00 * | 0.0000 |
| Cys | 0.11 0.00b | 0.04 0.00 * | 0.11 0.00 b | 0.09 0.00 a | 0.11 0.00 b | 0.10 0.00 * | 0.0000 | Ile | 13.09 0.00 * | 7.70 0.03 * | 5.89 0.00 * | 7.41 0.01 * | 6.28 0.00 * | 8.72 0.01 * | 0.0000 |
| Cystine | 0.13 0.00 * | 0.06 0.00 * | 0.08 0.00 * | 0.15 0.00 b | 0.12 0.00 a | 0.14 0.00 * | 0.0000 | Glu | 30.27 0.02 * | 21.68 0.05 * | 18.35 0.01 * | 22.99 0.03 * | 21.03 0.01 * | 24.37 0.01 * | 0.0000 |
| Gly | 13.60 0.01 * | 6.95 0.02 a | 4.06 0.02 * | 4.93 0.01 * | 3.74 0.00 * | 6.91 0.02 a | 0.0000 | Met | 0.98 0.00 * | 0.56 0.00 * | 0.46 0.00a | 0.66 0.00 * | 0.57 0.00 * | 0.73 0.00 * | 0.0000 |
| Asn | 36.41 0.00 * | 41.37 0.02 * | 47.48 0.01 * | 50.86 0.04 * | 48.43 0.00 * | 55.06 0.01 * | 0.0000 | Asp | 18.84 0.03 a | 22.84 0.07 * | 18.15 0.01 * | 22.73 0.03 * | 18.88 0.02 a | 29.43 0.05 * | 0.0000 |
| Ala | 119.30 0.00 * | 60.35 0.13 * | 36.17 0.04 * | 44.96 0.03 * | 29.89 0.02 * | 42.84 0.01 * | 0.0000 | Tyr | 6.13 0.01 * | 3.50 0.01 * | 2.65 0.00 * | 3.63 0.01 * | 3.01 0.00 * | 4.18 0.00 * | 0.0000 |
| Gln | 20.78 0.02 * | 14.05 0.03 * | 13.29 0.00 * | 18.43 0.02 * | 12.43 0.00 * | 15.75 0.02 * | 0.0000 | Phe | 17.05 0.00 * | 11.27 0.03 * | 10.75 0.00 * | 13.41 0.02 * | 12.00 0.00 * | 12.89 0.01 * | 0.0000 |
| Ser | 15.04 0.00 * | 10.99 0.01 * | 13.32 0.01 * | 16.39 0.00 * | 13.94 0.01 * | 19.08 0.02 * | 0.0000 | Pro | 338.06 0.03 * | 429.08 1.17 * | 351.73 0.04 * | 424.47 0.65 * | 387.17 0.06 * | 372.46 0.56 * | 0.0000 |
| Val | 12.70 0.00 * | 9.35 0.02 * | 7.86 0.01 * | 9.17 0.02 * | 7.82 0.00 * | 9.97 0.01 * | 0.0000 | Hyp | 3.38 0.00 * | 2.97 0.01 * | 2.44 0.00 * | 2.92 0.01 * | 2.73 0.00 * | 2.88 0.01 * | 0.0000 |
| C | V1 | V2 | V3 | V4 | V5 | V6 | p | C | V1 | V2 | V3 | V4 | V5 | V6 | p |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arg | 14.67 0.01 a | 17.39 0.01 b | 15.07 0.03 a | 18.75 0.03 d | 18.32 0.01 cd | 17.76 0.05 bc | 0.0000 | Leu | 14.06 0.00 * | 19.23 0.03 * | 18.30 0.01 a | 18.33 0.00 a | 18.61 0.05 * | 13.51 0.01 * | 0.0000 |
| Lys | 5.83 0.00 * | 9.56 0.00 * | 10.03 0.00 * | 8.08 0.03 * | 9.99 0.01 * | 5.93 0.00 * | 0.0000 | Thr | 11.99 0.00 * | 14.93 0.01 * | 14.22 0.01 * | 13.74 0.00 * | 15.00 0.04 * | 11.47 0.01 * | 0.0000 |
| His | 12.53 0.01 a | 17.09 0.01 * | 12.49 0.04 a | 10.93 0.04 * | 15.30 0.03 * | 9.46 0.00 * | 0.0000 | Trp | 2.86 0.00 * | 6.12 0.00 * | 5.17 0.00 a | 5.06 0.00 * | 5.17 0.02 a | 3.70 0.00 * | 0.0000 |
| Cys | 0.08 0.00 d | 0.14 0.00 * | 0.04 0.00 b | 0.11 0.00 * | 0.04 0.00 b | 0.01 0.00 a | 0.0000 | Ile | 10.45 0.00 * | 14.22 0.02 * | 13.64 0.01 * | 13.56 0.00 * | 13.86 0.04 * | 10.18 0.01 * | 0.0000 |
| Cystine | 0.03 0.00 b | 0.07 0.00 * | 0.03 0.00 bc | 0.07 0.00 c | 0.06 0.00 * | 0.03 0.00 b | 0.0000 | Glu | 32.12 0.01 * | 39.63 0.04 * | 41.26 0.01 * | 39.18 0.01 * | 38.63 0.09 * | 30.29 0.01 * | 0.0000 |
| Gly | 10.51 0.00 * | 11.29 0.01 * | 12.88 0.01 * | 7.60 0.01 * | 11.10 0.02 * | 9.25 0.00 * | 0.0000 | Met | 1.03 0.00 * | 1.25 0.00 * | 1.33 0.00 b | 1.33 0.00 b | 1.29 0.00 * | 0.99 0.00 * | 0.0000 |
| Asn | 3.11 0.00 a | 4.36 0.00 * | 3.35 0.00 * | 3.17 0.00 a | 3.85 0.01 * | 2.97 0.00 * | 0.0000 | Asp | 32.83 0.03 * | 40.05 0.05 * | 44.76 0.01 * | 41.40 0.04 * | 45.38 0.10 * | 30.63 0.02 * | 0.0000 |
| Ala | 41.65 0.00 a | 48.77 0.03 * | 53.04 0.04 * | 41.84 0.00 * | 55.74 0.06 * | 45.55 0.02 * | 0.0000 | Tyr | 6.61 0.01 * | 7.60 0.01 b | 8.93 0.00 * | 8.60 0.01 * | 9.30 0.02 * | 6.78 0.00 * | 0.0000 |
| Gln | 11.07 0.00 * | 17.27 0.00 * | 16.10 0.00 * | 13.33 0.00 * | 17.77 0.04 * | 13.21 0.01 * | 0.0000 | Phe | 17.61 0.01 * | 27.67 0.03 * | 25.53 0.00 * | 23.46 0.02 * | 26.89 0.07 * | 20.06 0.02 * | 0.0000 |
| Ser | 23.60 0.02 a | 29.58 0.00 * | 30.37 0.01 * | 25.44 0.01 * | 30.07 0.06 * | 22.04 0.01 * | 0.0000 | Pro | 224.07 0.33 * | 321.85 0.13 * | 307.06 0.04 * | 260.30 0.37 * | 303.37 0.35 * | 250.75 0.11 * | 0.0000 |
| Val | 10.25 0.00 * | 13.98 0.01 * | 13.44 0.00 * | 12.79 0.00 * | 14.62 0.03 * | 11.27 0.02 * | 0.0000 | Hyp | 4.33 0.01 * | 5.86 0.00 * | 5.64 0.00 * | 5.36 0.01 * | 6.11 0.01 * | 4.74 0.00 * | 0.0000 |
| Arg | Lys | His | Cys | Cystine | Gly | Asn | Ala | Gln | Ser | Val | Leu | Thr | Trp | Ile | Glu | Met | Asp | Tyr | Phe | Pro | Hyp | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arg | 1 | 0.4299 | 0.9279 | 0.4226 | 0.6283 | 0.8449 | −0.3806 | 0.7935 | 0.8163 | 0.4627 | 0.8817 | 0.7812 | −0.1941 | 0.8298 | 0.9175 | 0.9603 | 0.9781 | 0.0061 | 0.9410 | 0.9749 | −0.5101 | 0.8262 |
| Lys | 0.4299 | 1 | 0.5063 | 0.2042 | 0.4422 | 0.3961 | −0.1842 | 0.4746 | 0.8283 | 0.2915 | 0.4863 | 0.4204 | −0.0752 | 0.5527 | 0.4433 | 0.4474 | 0.4722 | −0.1219 | 0.4627 | 0.5869 | −0.1298 | 0.3847 |
| His | 0.9279 | 0.5063 | 1 | 0.3218 | 0.3492 | 0.9330 | −0.6637 | 0.9401 | 0.818 | 0.1646 | 0.9132 | 0.5591 | −0.5218 | 0.6393 | 0.9488 | 0.9186 | 0.9197 | −0.2447 | 0.9433 | 0.9499 | −0.5722 | 0.8245 |
| Cys | 0.4226 | 0.2042 | 0.3218 | 1 | 0.8245 | 0.0313 | 0.2143 | 0.0014 | 0.1565 | 0.5059 | 0.0417 | 0.4637 | 0.0031 | 0.3167 | 0.1227 | 0.1635 | 0.2535 | −0.2861 | 0.1517 | 0.335 | −0.7583 | −0.1527 |
| Cystine | 0.6283 | 0.4422 | 0.3492 | 0.8245 | 1 | 0.1704 | 0.3969 | 0.09481 | 0.5772 | 0.8588 | 0.3295 | 0.8312 | 0.5706 | 0.8332 | 0.3385 | 0.5132 | 0.5747 | 0.3099 | 0.4178 | 0.5969 | −0.1351 | 0.3401 |
| Gly | 0.8449 | 0.3961 | 0.933 | 0.0313 | 0.1704 | 1 | −0.6954 | 0.9749 | 0.7761 | 0.1037 | 0.9782 | 0.5237 | −0.5371 | 0.6123 | 0.9847 | 0.9242 | 0.9016 | −0.02481 | 0.9657 | 0.8643 | −0.4464 | 0.8899 |
| Asn | −0.3806 | −0.1842 | −0.6637 | 0.2143 | 0.3969 | −0.6954 | 1 | −0.809 | −0.3608 | 0.6199 | −0.561 | 0.2213 | 0.8767 | 0.0785 | 0.5907 | −0.4665 | −0.4075 | 0.5925 | −0.5346 | −0.4642 | 0.2273 | −0.5855 |
| Ala | 0.7935 | 0.4746 | 0.9401 | 0.0014 | 0.0948 | 0.9749 | -0.809 | 1 | 0.784 | −0.0516 | 0.9354 | 0.3832 | −0.6337 | 0.5083 | 0.9449 | 0.8733 | 0.8441 | −0.207 | 0.9202 | 0.8514 | −0.3997 | 0.8738 |
| Gln | 0.8163 | 0.8283 | 0.8180 | 0.1565 | 0.5772 | 0.7761 | −0.3608 | 0.784 | 1 | 0.4113 | 0.8632 | 0.6953 | −0.119 | 0.8364 | 0.8372 | 0.8658 | 0.8728 | 0.06532 | 0.8610 | 0.9091 | −0.2157 | 0.8106 |
| Ser | 0.4627 | 0.2915 | 0.1646 | 0.5059 | 0.8588 | 0.1037 | 0.6199 | −0.0516 | 0.4113 | 1 | 0.2695 | 0.9002 | 0.6276 | 0.8073 | 0.2522 | 0.3783 | 0.3783 | 0.6335 | 0.3182 | 0.3810 | −0.2376 | 0.1455 |
| Val | 0.8817 | 0.4863 | 0.9132 | 0.0417 | 0.3295 | 0.9782 | −0.561 | 0.9354 | 0.8632 | 0.2695 | 1 | 0.6561 | −0.363 | 0.7524 | 0.9921 | 0.9655 | 0.9505 | 0.1189 | 0.9888 | 0.9062 | −0.3732 | 0.9207 |
| Leu | 0.7812 | 0.4204 | 0.5591 | 0.4637 | 0.8312 | 0.5237 | 0.2213 | 0.3832 | 0.6953 | 0.9002 | 0.6561 | 1 | 0.3092 | 0.9628 | 0.6481 | 0.7367 | 0.7877 | 0.5096 | 0.6985 | 0.7192 | −0.3945 | 0.5236 |
| Thr | −0.1941 | −0.07526 | −0.5218 | 0.0031 | 0.5706 | −0.5371 | 0.8767 | −0.6337 | −0.119 | 0.6276 | −0.363 | 0.3092 | 1 | 0.2775 | −0.4064 | −0.2188 | −0.1855 | 0.6693 | −0.3311 | −0.2367 | 0.5187 | −0.2325 |
| Trp | 0.8298 | 0.5527 | 0.6393 | 0.3167 | 0.8332 | 0.6123 | 0.0785 | 0.5083 | 0.8364 | 0.8073 | 0.7524 | 0.9628 | 0.2775 | 1 | 0.7322 | 0.8292 | 0.8628 | 0.4595 | 0.7838 | 0.8172 | −0.2438 | 0.6855 |
| Ile | 0.9175 | 0.4433 | 0.9488 | 0.1227 | 0.3385 | 0.9847 | 0.5907 | 0.9449 | 0.8372 | 0.2522 | 0.9921 | 0.6481 | −0.4064 | 0.7322 | 1 | 0.9742 | 0.9629 | 0.0409 | 0.9960 | 0.9280 | −0.4448 | 0.9126 |
| Glu | 0.9603 | 0.4474 | 0.9186 | 0.1635 | 0.5132 | 0.9242 | −0.4665 | 0.8733 | 0.8658 | 0.3783 | 0.9655 | 0.7367 | −0.2188 | 0.8292 | 0.9742 | 1 | 0.9949 | 0.1191 | 0.9896 | 0.9632 | −0.3519 | 0.9379 |
| Met | 0.9781 | 0.4722 | 0.9197 | 0.2535 | 0.5747 | 0.9016 | −0.4075 | 0.8441 | 0.8728 | 0.3783 | 0.9505 | 0.7877 | −0.1855 | 0.8628 | 0.9629 | 0.9949 | 1 | 0.1228 | 0.9825 | 0.9731 | −0.408 | 0.9003 |
| Asp | 0.0061 | −0.1219 | −0.2447 | −0.2861 | 0.3099 | −0.0248 | 0.5925 | −0.207 | 0.0653 | 0.6335 | 0.1189 | 0.5096 | 0.6693 | 0.4595 | 0.0409 | 0.1191 | 0.1228 | 1 | 0.0772 | −0.06445 | 0.3031 | 0.1057 |
| Tyr | 0.9410 | 0.4627 | 0.9433 | 0.1517 | 0.4178 | 0.9657 | −0.5346 | 0.9202 | 0.8610 | 0.3182 | 0.9888 | 0.6985 | −0.3311 | 0.7838 | 0.9960 | 0.9896 | 0.9825 | 0.0772 | 1 | 0.9492 | −0.4217 | 0.9210 |
| Phe | 0.9749 | 0.5869 | 0.9499 | 0.335 | 0.5969 | 0.8643 | −0.4642 | 0.8514 | 0.9091 | 0.381 | 0.9062 | 0.7192 | −0.2367 | 0.8172 | 0.928 | 0.9632 | 0.9731 | −0.0644 | 0.9492 | 1 | −0.4191 | 0.8689 |
| Pro | −0.5101 | −0.1298 | −0.5722 | −0.7583 | −0.1351 | −0.4464 | 0.2273 | −0.3997 | −0.2157 | −0.2376 | −0.3732 | −0.3945 | 0.5187 | −0.2438 | −0.4448 | −0.3519 | −0.408 | 0.3031 | −0.4217 | −0.4191 | 1 | |
| Hyp | 0.8262 | 0.3847 | 0.8245 | −0.1527 | 0.3401 | 0.8899 | −0.5855 | 0.8738 | 0.8106 | 0.1455 | 0.9207 | 0.5236 | −0.2325 | 0.6855 | 0.9126 | 0.9379 | 0.9003 | 0.1057 | 0.9210 | 0.8689 | −0.0619 | 1 |
| Arg | Lys | His | Cys | Cystine | Gly | Asn | Ala | Gln | Ser | Val | Leu | Thr | Trp | Ile | Glu | Met | Asp | Tyr | Phe | Pro | Hyp | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arg | 1 | 0.1757 | 0.01649 | 0.1112 | 0.7199 | −0.6112 | 0.222 | 0.05874 | 0.3203 | −0.00094 | 0.4465 | 0.3242 | 0.2758 | 0.4372 | 0.3284 | 0.1306 | 0.2678 | 0.1887 | 0.3201 | 0.3869 | 0.208 | 0.4394 |
| Lys | 0.1757 | 1 | 0.6611 | 0.1966 | 0.4966 | 0.5425 | 0.708 | 0.7977 | 0.9113 | 0.9763 | 0.9527 | 0.9316 | 0.9593 | 0.8843 | 0.9407 | 0.9275 | 0.8868 | 0.9423 | 0.85 | 0.953 | 0.9307 | 0.9548 |
| His | 0.0164 | 0.6611 | 1 | 0.5205 | 0.5363 | 0.5406 | 0.9549 | 0.5231 | 0.7175 | 0.7515 | 0.6542 | 0.6542 | 0.7771 | 0.6225 | 0.6481 | 0.5365 | 0.4191 | 0.5066 | 0.2882 | 0.6949 | 0.7082 | 0.658 |
| Cys | 0.1112 | 0.1966 | 0.5205 | 1 | 0.7 | −0.1681 | 0.5277 | −0.3449 | 0.06784 | 0.2121 | 0.1827 | 0.4885 | 0.4077 | 0.4398 | 0.4616 | 0.4069 | 0.3465 | 0.1634 | −0.0415 | 0.2817 | 0.1939 | 0.1852 |
| Cystine | 0.7199 | 0.4966 | 0.5363 | 0.7 | 1 | −0.3206 | 0.6468 | 0.07136 | 0.4800 | 0.4032 | 0.6438 | 0.7375 | 0.6883 | 0.7261 | 0.7259 | 0.5611 | 0.6098 | 0.4947 | 0.4326 | 0.6458 | 0.4638 | 0.6411 |
| Gly | −0.6112 | 0.5425 | 0.5406 | −0.1681 | −0.3206 | 1 | 0.5394 | 0.7472 | 0.813 | 0.7472 | 0.7533 | 0.7203 | 0.8113 | 0.7755 | 0.576 | 0.576 | 0.4662 | 0.5104 | 0.3150 | 0.8100 | 0.8161 | 0.7563 |
| Asn | 0.222 | 0.708 | 0.9549 | 0.5277 | 0.6468 | 0.5394 | 1 | 0.5394 | 0.813 | 0.7472 | 0.6066 | 0.7203 | 0.8113 | 0.7755 | 0.7175 | 0.576 | 0.4662 | 0.5104 | 0.3150 | 0.8100 | 0.8161 | 0.7563 |
| Ala | 0.05874 | 0.7977 | 0.5231 | −0.3449 | 0.0713 | 0.7472 | 0.5394 | 1 | 0.8801 | 0.8117 | 0.7838 | 0.5407 | 0.675 | 0.5518 | 0.5633 | 0.5332 | 0.4902 | 0.6947 | 0.6783 | 0.6783 | 0.7994 | 0.7852 |
| Gln | 0.3203 | 0.9113 | 0.7175 | 0.06784 | 0.4800 | 0.813 | 0.8130 | 0.8801 | 1 | 0.8869 | 0.9532 | 0.7925 | 0.8758 | 0.8577 | 0.8069 | 0.7126 | 0.6577 | 0.7586 | 0.6829 | 0.9545 | 0.9653 | 0.9545 |
| Ser | −0.0009 | 0.9763 | 0.7515 | 0.2121 | 0.4032 | 0.7472 | 0.7472 | 0.8117 | 0.8869 | 1 | 0.8912 | 0.88 | 0.9402 | 0.8084 | 0.8873 | 0.8877 | 0.8139 | 0.9023 | 0.7692 | 0.9005 | 0.9108 | 0.8947 |
| Val | 0.4465 | 0.9527 | 0.6542 | 0.1827 | 0.6438 | 0.7533 | 0.6066 | 0.7838 | 0.9532 | 0.8912 | 1 | 0.9127 | 0.9474 | 0.9177 | 0.9232 | 0.837 | 0.8301 | 0.8805 | 0.829 | 0.9824 | 0.9232 | 1.0000 |
| Leu | 0.3242 | 0.9316 | 0.6542 | 0.4885 | 0.7375 | 0.7203 | 0.7203 | 0.5407 | 0.7925 | 0.8800 | 0.9127 | 1 | 0.9710 | 0.9338 | 0.9995 | 0.9636 | 0.9471 | 0.9112 | 0.8036 | 0.9273 | 0.8388 | 0.9139 |
| Thr | 0.2758 | 0.9593 | 0.7771 | 0.4077 | 0.6883 | 0.8113 | 0.8113 | 0.675 | 0.8758 | 0.9402 | 0.9474 | 0.971 | 1 | 0.9006 | 0.9729 | 0.914 | 0.8818 | 0.9124 | 0.7931 | 0.9483 | 0.8825 | 0.9489 |
| Trp | 0.4372 | 0.8843 | 0.6225 | 0.4398 | 0.7261 | 0.7755 | 0.7755 | 0.5518 | 0.8577 | 0.8084 | 0.9177 | 0.9338 | 0.9006 | 1 | 0.9376 | 0.8645 | 0.8231 | 0.7667 | 0.6646 | 0.9657 | 0.9171 | 0.919 |
| Ile | 0.3284 | 0.9407 | 0.6481 | 0.4616 | 0.7259 | 0.5760 | 0.7175 | 0.5633 | 0.8069 | 0.8873 | 0.9232 | 0.9995 | 0.9729 | 0.9376 | 1 | 0.9643 | 0.9487 | 0.9181 | 0.8154 | 0.9357 | 0.8501 | 0.9244 |
| Glu | 0.1306 | 0.9275 | 0.5365 | 0.4069 | 0.5611 | 0.576 | 0.576 | 0.5332 | 0.7126 | 0.8877 | 0.837 | 0.9636 | 0.914 | 0.8645 | 0.9643 | 1 | 0.9743 | 0.9362 | 0.8304 | 0.8574 | 0.7962 | 0.8394 |
| Met | 0.2678 | 0.8868 | 0.4191 | 0.3465 | 0.6098 | 0.4662 | 0.4662 | 0.4902 | 0.6577 | 0.8139 | 0.8301 | 0.9471 | 0.8818 | 0.8231 | 0.9487 | 0.9743 | 1 | 0.9544 | 0.9042 | 0.8169 | 0.7086 | 0.8305 |
| Asp | 0.1887 | 0.9423 | 0.5066 | 0.1634 | 0.4947 | 0.5104 | 0.5104 | 0.6947 | 0.7586 | 0.9023 | 0.8805 | 0.9112 | 0.9124 | 0.7667 | 0.9181 | 0.9362 | 0.9544 | 1 | 0.957 | 0.8384 | 0.7576 | 0.881 |
| Tyr | 0.3201 | 0.85 | 0.2882 | −0.04157 | 0.4326 | 0.3150 | 0.3150 | 0.6783 | 0.6829 | 0.7692 | 0.829 | 0.8036 | 0.7931 | 0.6646 | 0.8154 | 0.8304 | 0.9042 | 0.957 | 1 | 0.748 | 0.6401 | 0.8273 |
| Phe | 0.3869 | 0.9530 | 0.6949 | 0.2817 | 0.6458 | 0.8100 | 0.8100 | 0.6783 | 0.9545 | 0.9005 | 0.9824 | 0.9273 | 0.9483 | 0.9657 | 0.9357 | 0.8574 | 0.8169 | 0.8384 | 0.748 | 1 | 0.9662 | 0.9836 |
| Pro | 0.208 | 0.9307 | 0.7082 | 0.1939 | 0.4638 | 0.8161 | 0.8161 | 0.7994 | 0.9653 | 0.9108 | 0.9232 | 0.8388 | 0.8825 | 0.9171 | 0.8501 | 0.7962 | 0.7086 | 0.7576 | 0.6401 | 0.9662 | 1 | 0.9263 |
| Hyp | 0.4394 | 0.9548 | 0.658 | 0.1852 | 0.6411 | 0.7563 | 0.7563 | 0.7852 | 0.9545 | 0.8947 | 1 | 0.9139 | 0.9489 | 0.919 | 0.9244 | 0.8394 | 0.8305 | 0.8810 | 0.8273 | 0.9836 | 0.9263 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scutarașu, E.C.; Luchian, C.E.; Cioroiu, I.B.; Trincă, L.C.; Cotea, V.V. Increasing Amino Acids Content of White Wines with Enzymes Treatments. Agronomy 2022, 12, 1406. https://doi.org/10.3390/agronomy12061406
Scutarașu EC, Luchian CE, Cioroiu IB, Trincă LC, Cotea VV. Increasing Amino Acids Content of White Wines with Enzymes Treatments. Agronomy. 2022; 12(6):1406. https://doi.org/10.3390/agronomy12061406
Chicago/Turabian StyleScutarașu, Elena Cristina, Camelia Elena Luchian, Ionel Bogdan Cioroiu, Lucia Carmen Trincă, and Valeriu V. Cotea. 2022. "Increasing Amino Acids Content of White Wines with Enzymes Treatments" Agronomy 12, no. 6: 1406. https://doi.org/10.3390/agronomy12061406
APA StyleScutarașu, E. C., Luchian, C. E., Cioroiu, I. B., Trincă, L. C., & Cotea, V. V. (2022). Increasing Amino Acids Content of White Wines with Enzymes Treatments. Agronomy, 12(6), 1406. https://doi.org/10.3390/agronomy12061406









