GmDNAJC7 from Soybean Is Involved in Plant Tolerance to Alkaline-Salt, Salt, and Drought Stresses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
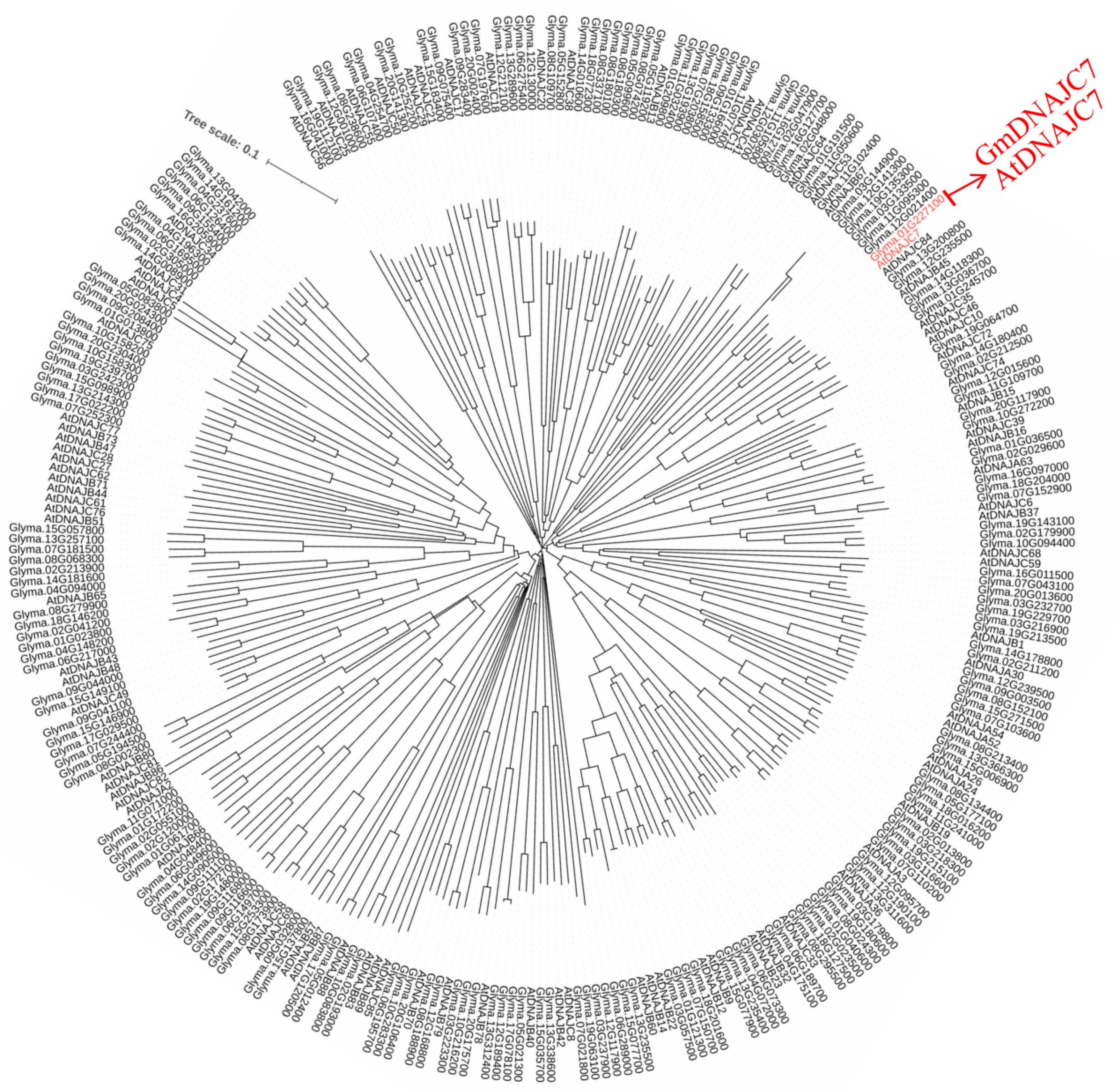
2.2. Phylogenetic Analysis of Arabidopsis and Soybean DNAJ Superfamily
2.3. RNA Extraction and Gene Expression Analysis
2.4. Subcellular Localization of GmDNAJC7
2.5. Obtaining and Analysis of Transgenic Soybean Composite Plants
2.6. Ectopic Expression of GmDNAJC7 in Arabidopsis and Abiotic Stress Treatment
2.7. Statistical Analysis
3. Results
3.1. Identification and Characterization of Soybean DNAJ Superfamily
3.2. Expression Profiling of Soybean DNAJ Genes in Response to Alkaline Salt, Salt and Drought Stresses
3.3. The Expression of GmDNAJC7 Is Upregulated in Response to NaHCO3, NaCl, and PEG Stresses
3.4. GmDNAJC7 Is Localized to the Nucleus and Cytoplasm
3.5. Overexpression of GmDNAJC7 Improved Soybean and Arabidopsis Tolerance to NaHCO3 Stress
3.6. Ectopic Expression of GmDNAJC7 in Arabidopsis Enhanced Salt Tolerance
3.7. Ectopic Expression of GmDNAJC7 in Arabidopsis Enhanced Drought Tolerance
4. Discussion
4.1. GmDNAJC7 Overexpression Can Improve the Tolerance of Soybeans and Arabidopsis to NaHCO3, NaCl, and Mannitol Stresses
4.2. GmDNAJC7 Mediates Plant Tolerance to Abiotic Stresses Probably through Endoplasmic Reticulum Stress Response, Hydrogen Peroxide Response, and Cellulose Biosynthesis Pathways
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CDS | Coding sequence |
| ER | Endoplasmic reticulum |
| GO | Gene Ontology |
| GFP | Green fluorescent protein |
| H2O2 | Hydrogen peroxide |
| LRWC | Leaf relative water content |
| MS | Murashige and Skoog |
| ML | Maximum likelihood |
| OE | Overexpression |
| SPAD | Soil and plant analysis development |
| PEG | Polyethylene glycol |
| ROS | Reactive oxygen species |
| WT | Wild type |
| YEB | Yeast extract broth |
References
- Liu, Y.; Du, H.; Li, P.; Shen, Y.; Tian, Z. Pan-Genome of wild and cultivated soybeans. Cell 2020, 182, 162–176. [Google Scholar] [CrossRef] [PubMed]
- Araújo, S.; Beebe, S.; Crespi, M.; Delbreil, B.; González, E.; Gruber, V.; Lejeune-Henaut, I.; Link, W.; Monteros, M.J.; Prats, E. Abiotic stress responses in legumes: Strategies used to cope with environmental challenges. Crit. Rev. Plant Sci. 2015, 34, 237–280. [Google Scholar] [CrossRef]
- Gray, S.B.; Brady, S.M. Plant developmental responses to climate change. Dev. Biol. 2016, 419, 64–77. [Google Scholar] [CrossRef] [Green Version]
- Carrera, C.S.; Solís, S.M. Leaf structure and ultrastructure changes induced by heat stress and drought during seed filling in field-grown soybean and their relationship with grain yield. An. Acad. Bras. Cienc. 2021, 93, e20191388. [Google Scholar] [CrossRef] [PubMed]
- Mete, F.Z.; Mia, S.; Dijkstra, F.A.; Abuyusuf, M.; Hossain, A.; Agronomy, D.O.; University, S.T.; Science, D.; Sydney, T.U.O. Synergistic effects of biochar and NPK fertilizer on soybean yield in an alkaline soil. Pedosphere 2015, 25, 713–719. [Google Scholar] [CrossRef]
- Jin, T.; Sun, Y.; Shan, Z.; He, J.; Wang, N.; Gai, J.; Li, Y. Natural variation in the promoter of GsERD15B affects salt tolerance in soybean. Plant Biotechnol. J. 2021, 19, 1155–1169. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Mcfarlane, H.E.; Persson, S. The impact of abiotic factors on cellulose synthesis. J. Exp. Bot. 2016, 67, 543–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Georgopoulos, C.P.; Lam, B.; Lundquist-Heil, A.; Rudolph, C.F.; Yochem, J.; Feiss, M. Identification of the E. coli dnaK (groPC756) gene product. Mol. Gen. Genet. 1979, 172, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Cai, G.; Xu, N.; Zhang, L.; Sun, X.; Guan, J.; Meng, Q. Novel DnaJ protein facilitates thermotolerance of transgenic tomatoes. Int. J. Mol. Sci. 2019, 20, 367. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Cai, G.; Kong, F.; Deng, Y.; Ma, N.; Meng, Q. Overexpression of tomato chloroplast-targeted DnaJ protein enhances tolerance to drought stress and resistance to Pseudomonas solanacearum in transgenic tobacco. Plant Physiol. Biochem. 2014, 82, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Qiu, G. Overexpression of seagrass DnaJ gene ZjDjB1 enhances the thermotolerance of transgenic Arabidopsis thaliana. Physiol. Mol. Biol. Plants 2021, 27, 2043–2055. [Google Scholar] [CrossRef]
- Yang, Y.; Qin, Y.; Xie, C.; Zhao, F.; Zhao, J.; Liu, D.; Chen, S.; Fuglsang, A.T.; Palmgren, M.G.; Schumaker, K.S.; et al. The Arabidopsis chaperone J3 regulates the plasma membrane H+-ATPase through interaction with the PKS5 kinase. Plant Cell 2010, 22, 1313–1332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salas-Muñoz, S.; Rodríguez-Hernández, A.A.; Ortega-Amaro, M.A.; Salazar-Badillo, F.B.; Jiménez-Bremont, J.F. Arabidopsis AtDjA3 null mutant shows increased sensitivity to abscisic acid, salt, and osmotic stress in germination and post-germination stages. Front. Plant Sci. 2016, 7, 220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, F.; Wang, Q.; Fazal, A.; Wang, L.J.; Song, S.; Kong, M.J. The DnaJ-like zinc finger protein ORANGE promotes proline biosynthesis in drought-stressed Arabidopsis seedlings. Int. J. Mol. Sci. 2022, 23, 3907. [Google Scholar] [CrossRef] [PubMed]
- Miernyk, J.A. The J-domain proteins of Arabidopsis thaliana: An unexpectedly large and diverse family of chaperones. Cell Stress Chaperones 2001, 6, 209–218. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; Mcgettigan, P.A.; Mcwilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [Green Version]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, Y.; Li, Y.; Zhu, Y.M.; Bai, X.; Lv, D.K.; Guo, D.; Ji, W.; Cai, H. Global transcriptome profiling of wild soybean (Glycine soja) roots under NaHCO3 treatment. BMC Plant Biol. 2010, 10, 153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Y.; Ji, D.; Li, S.; Wang, P.; Li, Q.; Xiang, F. The dynamic changes of DNA methylation and histone modifications of salt responsive transcription factor genes in soybean. PLoS ONE 2012, 7, e41274. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, H.H.; Brustolini, O.J.; Pimenta, M.R.; Mendes, G.C.; Gouveia, B.C.; Silva, P.A.; Silva, J.C.; Mota, C.S.; Soares-Ramos, J.R.; Fontes, E.P. The molecular chaperone binding protein BiP prevents leaf dehydration-induced cellular homeostasis disruption. PLoS ONE 2014, 9, e86661. [Google Scholar] [CrossRef] [Green Version]
- Guan, R.; Qu, Y.; Guo, Y.; Yu, L.; Liu, Y.; Jiang, J.; Chen, J.; Ren, Y.; Liu, G.; Tian, L.; et al. Salinity tolerance in soybean is modulated by natural variation in GmSALT3. Plant J. 2014, 80, 937–950. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−△△CT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Kereszt, A.; Li, D.; Indrasumunar, A.; Nguyen, C.D.; Nontachaiyapoom, S.; Kinkema, M.; Gresshoff, P.M. Agrobacterium rhizogenes-mediated transformation of soybean to study root biology. Nat. Protoc. 2007, 2, 948–952. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, J.; Jiang, W.; Liu, J.; Yang, S.; Gai, J.; Li, Y. Identification and analysis of NaHCO3 stress responsive genes in wild soybean (Glycine soja) roots by RNA-seq. Front. Plant Sci. 2016, 7, 1842. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Patricio, M.; Camas-Anzueto, J.L.; Sanchez-Alegría, A.; Aguilar-González, A.; Gutiérrez-Miceli, F. Optical method for estimating the Chlorophyll contents in plant leaves. Sensors 2018, 18, 650. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Zhang, J. Bulk analysis by resequencing and RNA-seq identifies candidate genes for maintaining leaf water content under water deficit in maize. Physiol. Plant. 2021, 173, 1935–1945. [Google Scholar] [CrossRef] [PubMed]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ariyarathne, M.A.; Wone, B.W.M. Overexpression of the Selaginella lepidophylla bHLH transcription factor enhances water-use efficiency, growth, and development in Arabidopsis. Plant Sci. 2022, 315, 111129. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Duan, X.; Ding, X.; Chen, C.; Zhu, D.; Yin, K.; Cao, L.; Song, X.; Zhu, P.; Li, Q.; et al. A novel AP2/ERF family transcription factor from Glycine soja, GsERF71, is a DNA binding protein that positively regulates alkaline stress tolerance in Arabidopsis. Plant Mol. Biol. 2017, 94, 509–530. [Google Scholar] [CrossRef]
- Li, X.; Tang, Y.; Zhou, C.; Zhang, L.; Lv, J. A wheat WRKY transcription factor TaWRKY46 enhances tolerance to osmotic stress in transgenic Arabidopsis plants. Int. J. Mol. Sci. 2020, 21, 1321. [Google Scholar] [CrossRef] [Green Version]
- Harbaoui, M.; Ben Romdhane, W.; Ben Hsouna, A.; Brini, F.; Ben Saad, R. The durum wheat annexin, TdAnn6, improves salt and osmotic stress tolerance in Arabidopsis via modulation of antioxidant machinery. Protoplasma 2021, 258, 1047–1059. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, X.; Ye, X.; Guo, P.; Hu, Z.; Qi, G.; Cui, F.; Liu, S. An S-ribonuclease binding protein EBS1 and brassinolide signaling are specifically required for Arabidopsis tolerance to bicarbonate. J. Exp. Bot. 2021, 72, 1449–1459. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Rueden, C.T.; Hiner, M.C.; Eliceiri, K.W. The ImageJ ecosystem: An open platform for biomedical image analysis. Mol. Reprod. Dev. 2015, 82, 518–529. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Yang, J.Y.; Zheng, W.; Tian, Y.; Wu, Y.; Zhou, D.W. Effects of various mixed salt-alkaline stresses on growth, photosynthesis, and photosynthetic pigment concentrations of Medicago ruthenica seedlings. Photosynthetica 2011, 49, 275–284. [Google Scholar] [CrossRef]
- Arya, H.; Singh, M.B.; Bhalla, P.L. Towards developing drought-smart soybeans. Front. Plant Sci. 2021, 12, 750664. [Google Scholar] [CrossRef] [PubMed]
- Van Zelm, E.; Zhang, Y.; Testerink, C. Salt tolerance mechanisms of plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Yang, H.; Fang, Y.; Guo, W.; Chen, H.; Zhang, X.; Dai, W.; Chen, S.; Hao, Q.; Yuan, S.; et al. Overexpression of GmMYB14 improves high-density yield and drought tolerance of soybean through regulating plant architecture mediated by the brassinosteroid pathway. Plant Biotechnol. J. 2021, 19, 702–716. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.H.; Li, W.; Niu, C.F.; Wei, W.; Hu, Y.; Han, J.Q.; Lu, X.; Tao, J.J.; Jin, M.; Qin, H.; et al. A class B heat shock factor selected for during soybean domestication contributes to salt tolerance by promoting flavonoid biosynthesis. New Phytol. 2020, 225, 268–283. [Google Scholar] [CrossRef] [Green Version]
- Yu, T.F.; Liu, Y.; Fu, J.D.; Ma, J.; Fang, Z.W.; Chen, J.; Zheng, L.; Lu, Z.W.; Zhou, Y.B.; Chen, M.; et al. The NF-Y-PYR module integrates the abscisic acid signal pathway to regulate plant stress tolerance. Plant Biotechnol. J. 2021, 19, 2589–2605. [Google Scholar] [CrossRef]
- Ketehouli, T.; Zhou, Y.G.; Dai, S.Y.; Carther, K.F.I.; Sun, D.Q.; Li, Y.; Nguyen, Q.V.H.; Xu, H.; Wang, F.W.; Liu, W.C.; et al. A soybean calcineurin B-like protein-interacting protein kinase, GmPKS4, regulates plant responses to salt and alkali stresses. J. Plant Physiol. 2021, 256, 153331. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.B.; Lohani, N.; Bhalla, P.L. The role of endoplasmic reticulum stress response in pollen development and heat stress tolerance. Front. Plant Sci. 2021, 12, 661062. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Jiang, L. Endoplasmic reticulum (ER) stress and the unfolded protein response (UPR) in plants. Protoplasma 2016, 253, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Cakır Aydemir, B.; Yüksel zmen, C.; Kibar, U.; Mutaf, F.; Büyük, P.B.; Bakır, M.; Ergül, A. Salt stress induces endoplasmic reticulum stress-responsive genes in a grapevine rootstock. PLoS ONE 2020, 15, e0236424. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Sun, X.; Gao, S.; Qin, F.; Dai, M. Deletion of an endoplasmic reticulum stress response element in a ZmPP2C-A gene facilitates drought tolerance of maize seedlings. Mol. Plant 2017, 10, 456–469. [Google Scholar] [CrossRef] [Green Version]
- Sandhu, J.; Irvin, L.; Liu, K.; Staswick, P.; Zhang, C.; Walia, H. Endoplasmic reticulum stress pathway mediates the early heat stress response of developing rice seeds. Plant Cell Environ. 2021, 44, 2604–2624. [Google Scholar] [CrossRef]
- Qureshi, M.K.; Gawroński, P.; Munir, S.; Jindal, S.; Kerchev, P. Hydrogen peroxide-induced stress acclimation in plants. Cell Mol. Life Sci. 2022, 79, 129. [Google Scholar] [CrossRef]
- Neill, S.; Desikan, R.; Hancock, J. Hydrogen peroxide signalling. Curr. Opin. Plant Biol. 2002, 5, 388–395. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Raihan, M.R.H. Regulation of reactive oxygen species and antioxidant defense in plants under salinity. Int. J. Mol. Sci. 2021, 22, 9326. [Google Scholar] [CrossRef]
- Wu, W.W.; He, S.S.; An, Y.Y. Hydrogen peroxide as a mediator of 5-aminolevulinic acid-induced Na+ retention in roots for improving salt tolerance of strawberries. Physiol. Plant. 2019, 167, 5–20. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Wang, P.; Lu, Y.; Bai, Y.; Wei, Y.; Liu, G.; Shi, H. MeRAV5 promotes drought stress resistance in cassava by modulating hydrogen peroxide and lignin accumulation. Plant J. 2021, 107, 847–860. [Google Scholar] [CrossRef] [PubMed]
- Endler, A.; Kesten, C.; Schneider, R.; Zhang, Y.; Ivakov, A.; Froehlich, A.; Funke, N.; Persson, S. A mechanism for sustained cellulose synthesis during salt stress. Cell 2015, 162, 1353–1364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shafi, A.; Gill, T.; Zahoor, I.; Ahuja, P.S.; Sreenivasulu, Y.; Kumar, S.; Singh, A.K. Ectopic expression of SOD and APX genes in Arabidopsis alters metabolic pools and genes related to secondary cell wall cellulose biosynthesis and improve salt tolerance. Mol. Biol. Rep. 2019, 46, 1985–2002. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Liu, X.; Lin, J.; Liu, X.; Wang, Z.; Xin, M.; Yao, Y.; Peng, H.; Zhou, D.X.; Ni, Z.; et al. Histone acetyltransferase GCN5 contributes to cell wall integrity and salt stress tolerance by altering the expression of cellulose synthesis genes. Plant J. 2019, 97, 587–602. [Google Scholar] [PubMed]
- Moore, J.P.; Vicré-Gibouin, M.; Farrant, J.M.; Driouich, A. Adaptations of higher plant cell walls to water loss: Drought vs. desiccation. Physiol. Plant. 2008, 134, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Kaur, M.; Kanwar, S.; Kaur, G.; Sharma, S.; Ganguli, S.; Kumari, V.; Mazumder, K.; Pandey, P.; Rouached, H.; et al. Wheat inositol pyrophosphate kinase TaVIH2-3B modulates cell-wall composition and drought tolerance in Arabidopsis. BMC Biol. 2021, 19, 261. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, T.; Shan, Z.; Zhou, S.; Yang, Q.; Gai, J.; Li, Y. GmDNAJC7 from Soybean Is Involved in Plant Tolerance to Alkaline-Salt, Salt, and Drought Stresses. Agronomy 2022, 12, 1419. https://doi.org/10.3390/agronomy12061419
Jin T, Shan Z, Zhou S, Yang Q, Gai J, Li Y. GmDNAJC7 from Soybean Is Involved in Plant Tolerance to Alkaline-Salt, Salt, and Drought Stresses. Agronomy. 2022; 12(6):1419. https://doi.org/10.3390/agronomy12061419
Chicago/Turabian StyleJin, Ting, Zhong Shan, Shuang Zhou, Qianqian Yang, Junyi Gai, and Yan Li. 2022. "GmDNAJC7 from Soybean Is Involved in Plant Tolerance to Alkaline-Salt, Salt, and Drought Stresses" Agronomy 12, no. 6: 1419. https://doi.org/10.3390/agronomy12061419





