Nitrogen Reduction with Bio-Organic Fertilizer Altered Soil Microorganisms, Improved Yield and Quality of Non-Heading Chinese Cabbage (Brassica campestris ssp. chinensis Makino)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experiment Design
2.3. Determination Index and Method
2.3.1. Determination of Soil Characteristics
2.3.2. DNA Extraction and PCR Amplification
2.3.3. Growth, Yield, Quality, and Photosynthetic Parameter Indexes
2.4. Statistical Analysis
3. Results
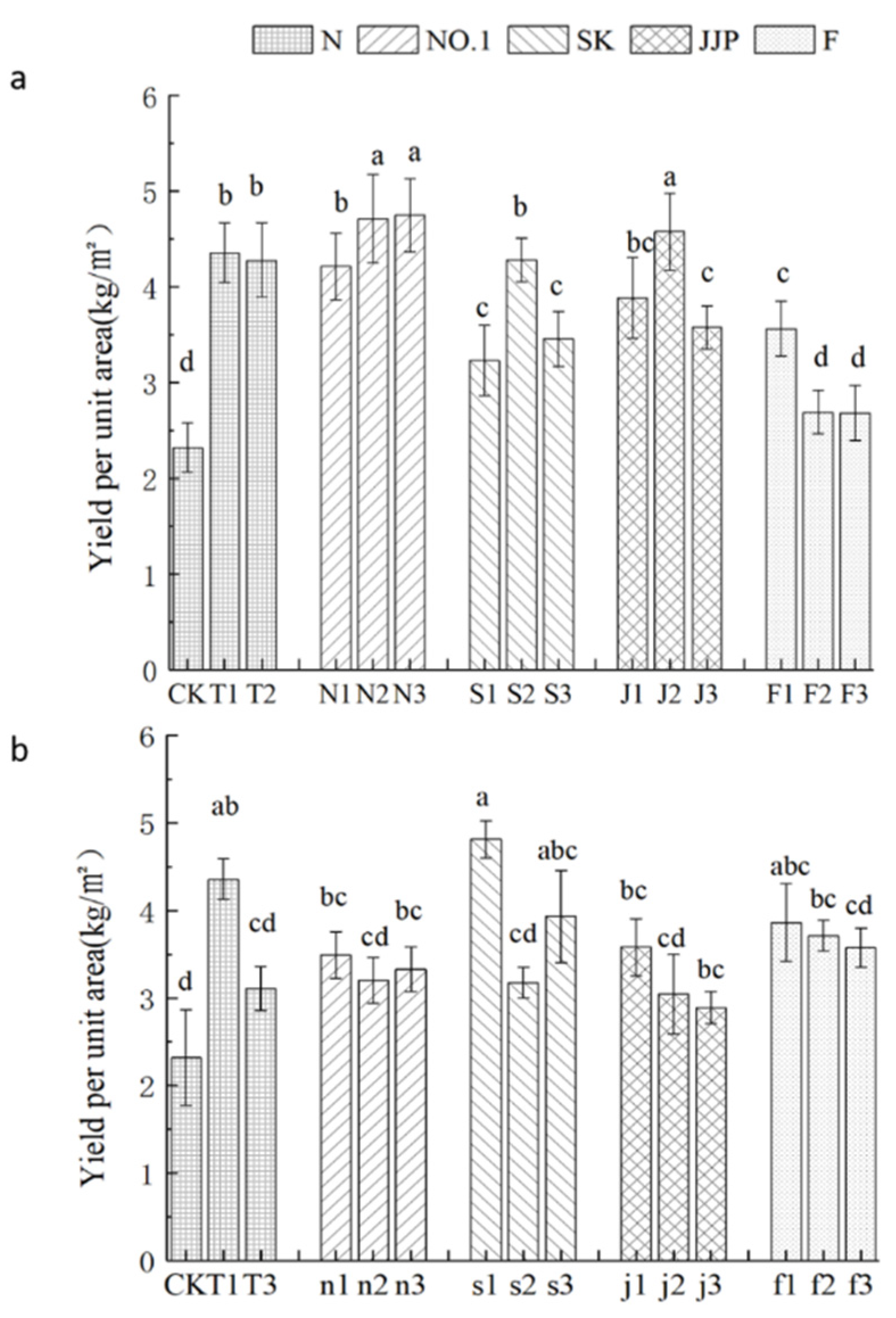
3.1. Soil Chemical Properties, Yield, and Quality of the First Experiment
3.2. Soil Chemical Properties, Soil Element Content, and Soil Enzyme Activities of the Second Experiment
3.3. Soil Microbiomes
3.4. Vitality of the Root, Photosynthetic Characteristics, Quality, Growth, and Yield of the Second Experiment
4. Discussion
4.1. Effects of Nitrogen Reduction with Bio-Organic Fertilizer on Soil Chemical Properties
4.2. Effects of Nitrogen Reduction with Bio-Organic Fertilizer on the Soil Microbial Community
4.3. Effects of Nitrogen Reduction with Bio-Organic Fertilizer on the Growth, Yield, and Quality of Non-Heading Chinese Cabbage
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gai, X.; Liu, H.; Zhai, L.; Tan, G.; Liu, J.; Ren, T.; Wang, H. Vegetable yields and soil biochemical properties as influenced by fertilization in southern China. Appl. Soil Ecol. 2016, 107, 170–181. [Google Scholar] [CrossRef]
- Xin, L.; Li, X.; Tan, M. Temporal and regional variations of China’s fertilizer consumption by crops during 1998–2008. J. Geogr. Sci. 2016, 22, 643–652. [Google Scholar] [CrossRef]
- Ye, L.; Zhao, X.; Bao, E.; Li, J.; Zou, Z.; Cao, K. Bio-organic fertilizer with reduced rates of chemical fertilization improves soil fertility and enhances tomato yield and quality. Sci. Rep. 2020, 10, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Z.; He, J.; Quan, Z.; Wu, C.; Geisen, S. Fertilization changes soil microbiome functioning, especially phagotrophic protists. Soil Biol. Biochem. 2020, 148, 107863. [Google Scholar] [CrossRef]
- Kang, A.; Zhang, N.; Xun, W.; Dong, X.; Xiao, M.; Liu, Z.; Xu, Z.; Feng, H.; Zou, J.; Shen, Q. Nitrogen fertilization modulates beneficial rhizosphere interactions through signaling effect of nitric oxide. Plant Physiol. 2021, 188, 1129–1140. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Tang, J.; Li, C.; Zhang, H.; Yuan, S. Reducing potential of chemical fertilizers and scientific fertilization countermeasure in vegetable production in China. J. Plant Nutr. 2017, 23, 1480–1493. [Google Scholar] [CrossRef]
- Li, R.; Tao, R.; Ling, N.; Chu, G. Chemical, organic and bio-fertilizer management practices effect on soil physicochemical property and antagonistic bacteria abundance of a cotton field: Implications for soil biological quality. Soil Tillage Res. 2017, 167, 30–38. [Google Scholar] [CrossRef]
- Xue, F.; Yan, T.; Yang, L.; Qiao, J. Influences of organic fertilizer application on soil biological properties. Chin. J. Eco-Agric. 2010, 18, 1372–1377. [Google Scholar] [CrossRef]
- Bandyopadhyay, K.K.; Misra, A.K.; Ghosh, P.K.; Hati, K.M. Effect of integrated use of farmyard manure and chemical fertilizers on soil physical properties and productivity of soybean. Soil Tillage Res. 2010, 110, 115–125. [Google Scholar] [CrossRef]
- Fierer, N. Embracing the unknown: Disentangling the complexities of the soil microbiome. Nat. Rev. Microbiol. 2017, 15, 579–589. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, Z.; Ling, N.; Yuan, Y.; Zheng, X.; Shen, B.; Shen, Q. Bacillus subtilis SQR9 can control Fusarium wilt in cucumber by colonizing plant roots. Biol. Fertil. Soils 2011, 47, 495–506. [Google Scholar] [CrossRef]
- Sun, X.; Xu, Z.; Xie, J.; Hesselberg, T.; Tan, T.; Zheng, D.; Strube, L.; Dragoš, A.; Shen, Q.; Zhang, R.; et al. Bacillus velezensis stimulates resident rhizosphere pseudomonas stutzeri for plant health through metabolic interactions. ISME J. 2011, 16, 774–787. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, X.; Raza, W.; Li, J.; Liu, Y.; Qiu, M.; Zhang, F.; Shen, Q. Trichoderma harzianum SQR-T037 rapidly degrades allelochemicals in rhizospheres of continuously cropped cucumbers. Appl. Microbiol. Biotechnol. 2011, 89, 1653–1663. [Google Scholar] [CrossRef] [PubMed]
- Cai, F.; Pang, G.; Li, R.; Li, R.; Gu, X.; Shen, Q.; Chen, W. Bioorganic fertilizer maintains a more stable soil microbiome than chemical fertilizer for monocropping. Biol. Fertil. Soils 2017, 53, 861–872. [Google Scholar] [CrossRef]
- Gao, M.; Yang, J.; Liu, C.; Gu, B.; Han, M.; Li, J.; Li, N.; Liu, N.; An, N.; Dai, J.; et al. Effects of long-term biochar and biochar-based fertilizer application on brown earth soil bacterial communities. Agric. Ecosyst. Environ. 2020, 309, 107285. [Google Scholar] [CrossRef]
- Li, J.; Li, S.; Li, Q. Effects of different amounts of fulvic acid on tomato yield and quality. J. Agric. 2022, 12, 54–59. [Google Scholar]
- Hou, X.; Song, X. Research and utilization of Brassica campestris ssp. chinensis Makino (non-heading Chinese cabbage) germplasm resources. J. Nanjing Agric. Univ. 2012, 35, 35–42. [Google Scholar]
- Ding, H.; Fan, J.; Jia, C.; Qin, C.; Yang, Y.; Zhang, H.; Zhang, F.; Wen, C.; Yu, S.; Xu, Y. Current situation and trend of vegetable seed industry development in China. China Veg. 2020, 9, 1–8. [Google Scholar]
- Raigón, M.D.; García, M.; Maquieira, A.; Puchades, R. Determination of available nitrogen (nitic and ammoniacal) in soils by flow-injection analysis. Analysis 1992, 20, 483–487. [Google Scholar] [CrossRef]
- Wang, Q.; Ren, Y.; Meng, L.; Hong, L.; Hui-Min, F.; Wang, H. Simultaneous determination of total nitrogen and organic carbon in soil with an elemental analyzer. Chin. J. Anal. Lab. 2013, 32, 41–45. [Google Scholar]
- Bao, S.D. Analysis Method of Soil and Agricultural Chemistry; China Agricultural Press: Beijing, China, 2000; pp. 25–108. [Google Scholar]
- Falciani, R.; Novaro, E.; Marchesini, M.; Gucciardi, M. Multi-element analysis of soil and sediment by icp-ms after a microwave assisted digestion method. J. Anal. At. Spectrom. 2000, 15, 561–565. [Google Scholar] [CrossRef]
- Sun, X.; Zhu, L.; Wang, J.; Wang, J.; Su, B.; Liu, T.; Zhang, C.; Gao, C.; Shao, Y. Toxic effects of ionic liquid 1-octyl-3-methylimidazolium tetrafluoroborate on soil enzyme activity and soil microbial community diversity. Ecotoxicol. Environ. Saf. 2017, 135, 201208. [Google Scholar] [CrossRef]
- Guan, S.Y. Methodology of soil enzyme measurement. In Methods of Soil Enzymology; Guan, Y., Ed.; China Agricultural Press: Beijing, China, 1986; pp. 274–314. [Google Scholar]
- Taylor, J.P.; Wilson, B.; Mills, M.S.; Burns, R.G. Comparison of microbial numbers and enzymatic activities in surface soils and subsoils using various techniques. Soil Biol. Biochem. 2002, 34, 387–401. [Google Scholar] [CrossRef]
- Bates, S.; Berg-Lyons, D.; Caporaso, J.; Walters, W.; Knight, R.; Fierer, N. Examining the global distribution of dominant archaeal populations in soil. ISME J. 2011, 5, 908–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rousk, J.; Baath, E.; Brookes, P.; Lauber, C.; Lozupone, C.; Caporaso, J.; Knight, R.; Fierer, N. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef] [PubMed]
- Arono, D.I. Copper enzymes in isolated chloroplasts, polyphenol oxidase in Brta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Clemensson, A.; Persson, H. Fine-root vitality in a nor way spruce stand subjected to various nutrient supplies. Plant Soil 1995, 168, 167–172. [Google Scholar] [CrossRef]
- Cataldo, D.A.; Maroon, M.; Schrader, L.E.; Youngs, A.L. Rapid colorimetric determination of nitrate in plant-tissue by nitration of salicylic-acid. Commun. Soil Sci. Plant Anal. 1975, 6, 7–80. [Google Scholar] [CrossRef]
- Zhang, J.; Kirkham, M.B. Antioxidant responses to drought in sunflower and sorghum seedlings. New Phytol. 1996, 132, 361–373. [Google Scholar] [CrossRef]
- Buysse, J.A.N.; Merckx, R. An improved colorimetric method to quantify sugar content of plant tissue. J. Exp. Bot. 1993, 44, 1627–1629. [Google Scholar] [CrossRef]
- Eltun, R.; Korsth, A.; Nordheim, O. A comparison of environmental, soil fertility, yield, and economical effects in six cropping systems based on an 8-year experiment in Norway. Agric. Ecosyst. Environ. 2002, 90, 155–168. [Google Scholar] [CrossRef]
- Bozkurt, S.; Agca, N.; Odemis, B. Influence of different nitrogen sources and leaching practices on soil chemical properties under tomato vegetation in a greenhouse. J. Agron. 2008, 7, 210–219. [Google Scholar] [CrossRef] [Green Version]
- Youssef, I.; Ali, M.; Noufal, E.; Ismail, S.; Ali, M. Effect of different sources and levels of nitrogen fertilizers with and without organic and bio-fertilizers on growth and yield components of fennel plants (foeniculum vulgare mill.). Asian J. Soil Sci. Plant Nutr. 2020, 6, 6–14. [Google Scholar] [CrossRef]
- Whalen, J.; Chang, C.; Clayton, G.; Carefoot, J. Cattle manure amendments can increase the pH of acid soils. Soil Sci. Soc. Am. J. 2000, 64, 962–966. [Google Scholar] [CrossRef] [Green Version]
- Gu, Y.; Wang, Y.; Lu, S.; Xiang, Q.; Yu, X.; Zhao, K.; Zou, L.; Chen, Q.; Tu, S.; Zhang, X. Long-term fertilization structures bacterial and archaeal communities along soil depth gradient in a paddy soil. Front. Microbiol. 2017, 8, 1516. [Google Scholar] [CrossRef] [Green Version]
- López, A.; Fenoll, J.; Hellín, P.; Flores, P. Physical characteristics and mineral composition of two pepper cultivars under organic, conventional and soilless cultivation. Sci. Hortic. 2013, 150, 259–266. [Google Scholar] [CrossRef]
- Yang, P.; Jian, L.; Sohail, H.; Yu, J.; Li, J. Partial substitution of mineral fertilizer with biofertilizer enhances cauliflower nutritional quality, yield, and soil characteristics. Crop Sci. 2020, 60, 934–944. [Google Scholar] [CrossRef]
- Li, B.; Zhou, D.; Cang, L.; Zhang, H.; Fan, X.; Qin, W. Soil micronutrient availability to crops as affected by long-term inorganic and organic fertilizer applications. Soil Tillage Res. 2007, 96, 66–173. [Google Scholar] [CrossRef]
- Gou, J.Y.; Suo, S.Z.; Shao, K.Z.; Zhao, Q.; Rensing, C. Biofertilizers with beneficial rhizobacteria improved plant growth and yield in chili (Capsicum annuum L.). World J. Microbiol. 2020, 36, 86. [Google Scholar] [CrossRef]
- Wang, N.; Nan, H.; Feng, K. Effects of reduced chemical fertilizer with organic fertilizer application on soil microbial biomass, enzyme activity and cotton yield. J. Appl. Ecol. 2020, 31, 173–181. [Google Scholar]
- Liao, J.; Ye, J.; Liang, Y.; Khalid, M.; Huang, D. Pakchoi antioxidant improvement and differential rhizobacterial community composition under organic fertilization. Sustainability 2019, 11, 2424. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.; Han, M.; Hu, Y.; Li, Z.; Ma, Z. Effects of continuous cropping of sweet potato on the fungal community structure in rhizospheric soil. Front. Microbiol. 2019, 10, 2269. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zheng, A.; Bromfield, E.; Zhu, J.; Li, S.; Wang, S.; Deng, Q.; Li, P. 16S rRNA gene sequence analysis of halophilic and halotolerant bacteria isolated from a hypersaline pond in Sichuan, China. Ann. Microbiol. 2011, 61, 375–381. [Google Scholar] [CrossRef]
- Kami, K.; Ghane, M.; Bababeekhou, L. Hydrolase-producing moderately halophilic bacteria from eshtehard desert (iran). Microbiology 2020, 89, 769–777. [Google Scholar] [CrossRef]
- Zhang, H. Gemmatimonas aurantiaca gen. nov., sp. nov., a Gram-negative, aerobic, polyphosphate-accumulating micro-organism, the first cultured representative of the new bacterial phylum Gemmatimonadetes phyl. nov. Int. J. Syst. Evol. Microbiol. 2003, 53, 1155–1163. [Google Scholar] [CrossRef]
- Nemergut, D.R.; Townsend, A.R.; Sattin, S.R.; Freeman, K.R.; Fierer, N.; Neff, J.C.; Bowman, W.D.; Schadt, C.W.; Weintraub, M.N.; Schmidt, S.K. The effects of chronic nitrogen fertilization on alpine tundra soil microbial communities: Implications forcarbon and nitrogen cycling. Environ. Microbiol. 2010, 10, 3093–3105. [Google Scholar] [CrossRef]
- Feng, C.; Yue, S.; Jian, A.; Chen, L.; Guo, Y.; Zheng, R.; Su, J. The effect of continuous cropping of selenium melon on soil fungal community structure. Chin. J. Eco-Agric. 2019, 10, 2269. [Google Scholar]
- Luo, X.; Wang, Y.; Li, Y.; An, j.; Wang, G.; Li, M.; Zheng, S. Microbial oxidation of organic and elemental selenium to selenite. Sci. Total Environ. 2022, 833, 155203. [Google Scholar] [CrossRef]
- Lajudie, P. Polyphasic Taxonomy of Rhizobia: Emendation of the genus sinorhizobium and description of Sinorhizobium meliloti comb. nov. Sinorhizobium saheli sp. nov. and Sinorhizobium teranga sp. nov. Int. J. Syst. Bacteriol 1994, 44, 715–733. [Google Scholar] [CrossRef]
- Osorio, N.W.; Habte, M. Soil phosphate desorption induced by a phosphate-solubilizing fungus. Commun. Soil Sci. Plant Anal. 2014, 45, 451–460. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, X.; Gang, L.; Qin, P. Interactions between arbuscular mycorrhizal fungi and phosphate-solubilizing fungus (Mortierella sp.) and their effects on Kostelelzkya virginica growth and enzyme activities of rhizosphere and bulk soils at different salinities. Biol. Fertil. Soils 2011, 47, 543–554. [Google Scholar] [CrossRef] [Green Version]
- Malik, K.; Sandhu, G. Decomposition of organic matter by fungi in saline soils. Mycopathol. Mycol. Appl. 1973, 50, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Hu, G. Discussion on the origin of sweet potato root rot in China. J. Henan Univ. Sci. Technol. (Agric. Ed.) 1984, 1, 10–13. [Google Scholar]
- Shen, Z.; Wang, D.; Ruan, Y.; Xue, C.; Zhang, J.; Li, R.; Shen, Q. Deep 16S rRNA pyrosequencing reveals a bacterial community associated with banana Fusarium wilt disease suppression induced by bioorganic fertilizer application. PLoS ONE 2014, 9, e98420. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, R.; Xue, C.; Xun, W.; Sun, L.; Xu, Y.; Shen, Q. Pyrosequencing reveals contrasting soil bacterial diversity and com-munity structure of two main winter wheat cropping systems in China. Microb. Ecol. 2014, 67, 443–453. [Google Scholar] [CrossRef]
- Lin, Y.; Ye, G.; Kuzyakov, Y.; Liu, D.; Fan, J.; Ding, W. Long-term manure application increases soil organic matter and aggregation, and alters microbial community structure and keystone taxa. Soil Biol. Biochem. 2019, 134, 187–196. [Google Scholar] [CrossRef]
- Hu, J.; Lin, X.; Wang, J.; Dai, J.; Chen, R.; Zhang, J.; Ming, H. Microbial functional diversity, metabolic quotient, and invertase activity of a sandy loam soil as affected by long-term application of organic amendment and mineral fertilizer. J. Soils Sediments 2011, 11, 271–280. [Google Scholar] [CrossRef]
- Mauromicale, G.; Ierna, A.; Marchese, M. Chlorophyll fluorescence and chlorophyll content in field-grown potato as affected by nitrogen supply, genotype, and plant age. Photosynthetica 2006, 44, 76. [Google Scholar] [CrossRef]
- Fan, M.; Shen, J.; Yuan, L.; Jiang, R.; Zhang, F. Improving crop productivity and resource use efficiency to ensure food security and environmental quality in China. J. Exp. Bot. 2012, 63, 13–24. [Google Scholar] [CrossRef]
- Acon, C.; Palencia, E.; Hinton, D. Abiotic and biotic plant stress-tolerant and beneficial secondary metabolites produced by endophytic Bacillus species. In Plant Microbes Symbiosis: Applied Facets; Springer: New Delhi, India, 2015; pp. 163–177. [Google Scholar] [CrossRef]
- Gomes, F.; Moraes, J.; Santos, C.; Goussain, M. Resistance induction in wheat plants by silicon and aphids. Sci. Agric. 2005, 62, 547–551. [Google Scholar] [CrossRef]
- Van, R.; Van, E.; Jetten, M.; Hefting, M.; Kartal, B. De-nitrification at pH 4 by a soil-derived Rhodanobacter-dominated community. Environ. Microbiol. 2010, 12, 3264–3271. [Google Scholar] [CrossRef]
- Wang, Y. Effects of Nitrogen Fertilizer Reduction on Yield and Quality of Non-Heading Chinese Cabbage; Nanjing Agricultural University: Nanjing, China, 2017. [Google Scholar]
- Qi, Y.; Jiang, F.; Zhou, R.; Wu, Y.; Hou, X.; Li, J.; Lin, W.; Wu, Z. Effects of reduced nitrogen with bio-organic fertilizer on soil properties, yield and quality of non-heading Chinese cabbage. Agronomy 2021, 11, 2196. [Google Scholar] [CrossRef]
- Feng, N.; Liang, Q.; Feng, Y.; Xiang, L.; Wong, M. Improving yield and quality of vegetable grown in paes-contaminated soils by using novel bioorganic fertilizer. Sci. Total Environ. 2020, 739, 139883. [Google Scholar] [CrossRef] [PubMed]
- Briseis, A.; Xiao, O.; Yu, T.; Bu, T.; Gong, Y.; Hong, L.; Nathaniel, R.; Wong, H.; Mary, H. Thyroid cancer risk and dietary nitrate and nitrite intake in the Shanghai women’s health study. Int. J. Cancer 2013, 132, 897–904. [Google Scholar] [CrossRef]
- Simion, V.; Cmpeanu, G.; Vasile, G.; Artimon, M.; Negoi, M. Nitrate and nitrite accumulation in tomatoes and derived products. Rom. Biotechnol. Lett. 2008, 13, 3785–3790. [Google Scholar] [CrossRef]
- Siciliano, J.; Krulick, S.; Heisler, E.G.; Schwartz, J.H.; White, J.W. Nitrate and nitrite content of some fresh and processed market vegetables. J. Agric. Food Chem. 1975, 23, 461–464. [Google Scholar] [CrossRef]


| Treatment | Mineral Fertilizer | Bio-Organic Fertilizer | ||||
|---|---|---|---|---|---|---|
| N kg·ha−1 | P2O5 kg·ha−1 | K2O kg·ha−1 | No.1 kg·ha−1 | Seek kg·ha−1 | Jiajiapei kg·ha−1 | |
| No fertilizer (CK) | - | - | - | - | - | - |
| Conventional fertilizer (T1) | 193.2 | 135 | 135 | - | - | - |
| −N20% (T2) | 154.5 | 135 | 135 | - | - | - |
| −N20% + No.1 (N2) | 109.5 | 60 | 125 | 1500 | - | - |
| −N20% + Seek (S2) | 109.5 | 60 | 125 | - | 1500 | - |
| −N20% + Jiajiapei (J2) | 140.1 | 120.6 | 127.8 | - | - | 720 |
| Treatment | pH | EC ms·m−1 | Organic Matter g·kg−1 | Available Phosphorus g·kg−1 | Total Nitrogen g·kg−1 | Ammonium Nitrogen g·kg−1 | Nitrate Nitrogen g·kg−1 | Organic Carbon g·kg−1 |
|---|---|---|---|---|---|---|---|---|
| CK | 5.15 ± 0.04 bc | 75.2 ± 7.15 e | 13.47 ± 0.37 b | 18.45 ± 1.14 b | 1.80 ± 0.02 b | 5.70 ± 1.63 ab | 8.78 ± 0.19 d | 8.06 ± 0.3 b |
| TI | 5.14 ± 0.07 c | 148.60 ± 6.03 a | 16.44 ± 1.44 ab | 31.45 ± 5.10 b | 1.81 ± 0.04 b | 4.50 ± 0.18 b | 18.95 ± 0.52 a | 9.54 ± 0.84 ab |
| T2 | 5.18 ± 0.03 bc | 111.27 ± 5.46 ab | 15.57 ± 0.39 ab | 30.58 ± 2.5 b | 1.85 ± 0.03 b | 8.34 ± 1.04 ab | 15.80 ± 0.47 b | 9.03 ± 0.23 ab |
| N2 | 5.38 ± 0.04 a | 92.83 ± 8.94 de | 18.07 ± 0.31 a | 64.93 ± 3.07 a | 2.07 ± 0.02 a | 12.57 ± 2.69 a | 11.29 ± 0.57 c | 10.59 ± 0.13 a |
| S2 | 5.32 ± 0.04 ab | 95.23 ± 10.65 ed | 17.08 ± 1 ab | 22.80 ± 2.87 b | 1.80 ± 0.01 b | 5.68 ± 0.95 ab | 11.18 ± 0.24 c | 9.91 ± 0.58 a |
| J2 | 5.07 ± 0.03 c | 134.67 ± 2.34 ab | 14.88 ± 0.45 ab | 21.82 ± 1.76 b | 1.83 ± 0.04 b | 7.18 ± 0.64 ab | 9.40 ± 0.72 d | 9.79 ± 0.55 a |
| Treatments | Soluble Sugar (mg·g−1) | Cellulose % | Soluble Protein (mg·g−1) | Vitamin C (mg·100 g−1) | Nitrate (mg·kg−1) |
|---|---|---|---|---|---|
| CK | 1.07 ± 0.03 a | 27.31 ± 1.36 a | 10.51 ± 0.34 d | 68.13 ± 2.45 cd | 551.83 ± 24.66 bc |
| T1 | 0.53 ± 0.06 bc | 18.87 ± 0.47 bc | 11.15 ± 0.38 c | 69.74 ± 1.38 ab | 564.04 ± 25.27 b |
| T2 | 0.40 ± 0.05 c | 16.94 ± 0.40 c | 11.62 ± 0.12 ab | 69.61 ± 3.55 ab | 507.03 ± 42.90 bc |
| N2 | 0.63 ± 0.02 b | 18.42 ± 1.23 c | 11.20 ± 0.48 ab | 73.23 ± 2.36 a | 446.15 ± 29.04 c |
| S2 | 0.52 ± 0.01 bc | 19.76 ± 0.42 b | 10.56 ± 0.27 c | 72.62 ± 2.77 ab | 475.03 ± 21.91 bc |
| J2 | 0.56 ± 0.84 bc | 18.13 ± 1.72 c | 11.87 ± 0.29 a | 68.90 ± 2.77 bcd | 1309.40 ± 52.77 a |
| Yield | VC | Nitrate | Soluble Sugar | Total Chloro- phyll | Root Activity | pH | OM | TN | TP | TK | TCa | TZn | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yield | 1 | |||||||||||||
| VC | −0.098 | 1 | ||||||||||||
| Nitrate | 0.062 | 0.392 | 1 | 1 | ||||||||||
| Soluble sugar | −0.688 ** | 0.097 | −0.078 | 1 | 0.5 | |||||||||
| Total chlorophyll | 0.804 ** | −0.339 | 0.09 | −0.602 ** | 1 | 0 | ||||||||
| Root activity | 0.487 * | 0.196 | −0.245 | −0.387 | 0.289 | 1 | −0.5 | |||||||
| pH | 0.588 * | −0.36 | −0.624 ** | −0.291 | 0.407 | 0.434 | 1 | −1 | ||||||
| OM | 0.565 * | 0.274 | 0.071 | −0.014 | 0.338 | 0.311 | 0.328 | 1 | ||||||
| TN | 0.066 | −0.073 | 0.156 | 0.023 | 0.048 | 0.201 | −0.006 | −0.293 | 1 | |||||
| TP | 0.007 | −0.422 | −0.336 | 0.171 | 0.224 | −0.079 | 0.363 | −0.115 | −0.223 | 1 | ||||
| TK | −0.311 | −0.096 | −0.408 | −0.046 | −0.399 | −0.045 | −0.014 | −0.249 | −0.366 | 0.051 | 1 | |||
| TCa | −0.203 | −0.422 | −0.346 | 0.264 | −0.026 | −0.282 | 0.217 | −0.326 | −0.375 | 0.856 ** | 0.323 | 1 | ||
| TZn | −0.578 * | −0.56 * | −0.553 * | 0.532 * | −0.38 | −0.301 | 0.127 | −0.273 | −0.167 | 0.557 * | 0.434 | 0.692 ** | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, Y.; Wu, Z.; Zhou, R.; Hou, X.; Yu, L.; Cao, Y.; Jiang, F. Nitrogen Reduction with Bio-Organic Fertilizer Altered Soil Microorganisms, Improved Yield and Quality of Non-Heading Chinese Cabbage (Brassica campestris ssp. chinensis Makino). Agronomy 2022, 12, 1437. https://doi.org/10.3390/agronomy12061437
Qi Y, Wu Z, Zhou R, Hou X, Yu L, Cao Y, Jiang F. Nitrogen Reduction with Bio-Organic Fertilizer Altered Soil Microorganisms, Improved Yield and Quality of Non-Heading Chinese Cabbage (Brassica campestris ssp. chinensis Makino). Agronomy. 2022; 12(6):1437. https://doi.org/10.3390/agronomy12061437
Chicago/Turabian StyleQi, Yingbin, Zhen Wu, Rong Zhou, Xilin Hou, Lu Yu, Yuxin Cao, and Fangling Jiang. 2022. "Nitrogen Reduction with Bio-Organic Fertilizer Altered Soil Microorganisms, Improved Yield and Quality of Non-Heading Chinese Cabbage (Brassica campestris ssp. chinensis Makino)" Agronomy 12, no. 6: 1437. https://doi.org/10.3390/agronomy12061437
APA StyleQi, Y., Wu, Z., Zhou, R., Hou, X., Yu, L., Cao, Y., & Jiang, F. (2022). Nitrogen Reduction with Bio-Organic Fertilizer Altered Soil Microorganisms, Improved Yield and Quality of Non-Heading Chinese Cabbage (Brassica campestris ssp. chinensis Makino). Agronomy, 12(6), 1437. https://doi.org/10.3390/agronomy12061437









