Salicylic Acid Improves the Salt Tolerance Capacity of Saponaria officinalis by Modulating Its Photosynthetic Rate, Osmoprotectants, Antioxidant Levels, and Ion Homeostasis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Determination of Assays and Contents
2.3. Statistical Analysis
3. Results
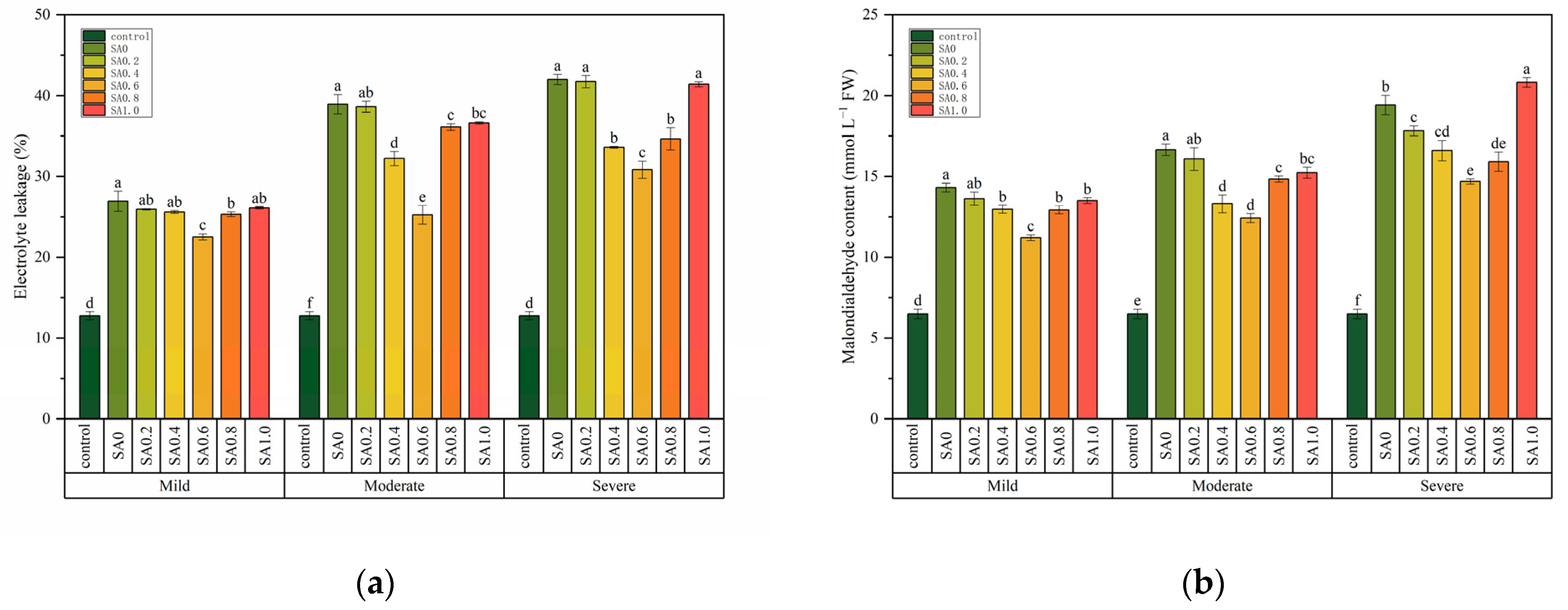
3.1. Salt Damage
3.2. Chlorophyll Content and Photosynthesis Rate
3.3. Osmoregulatory Material Content
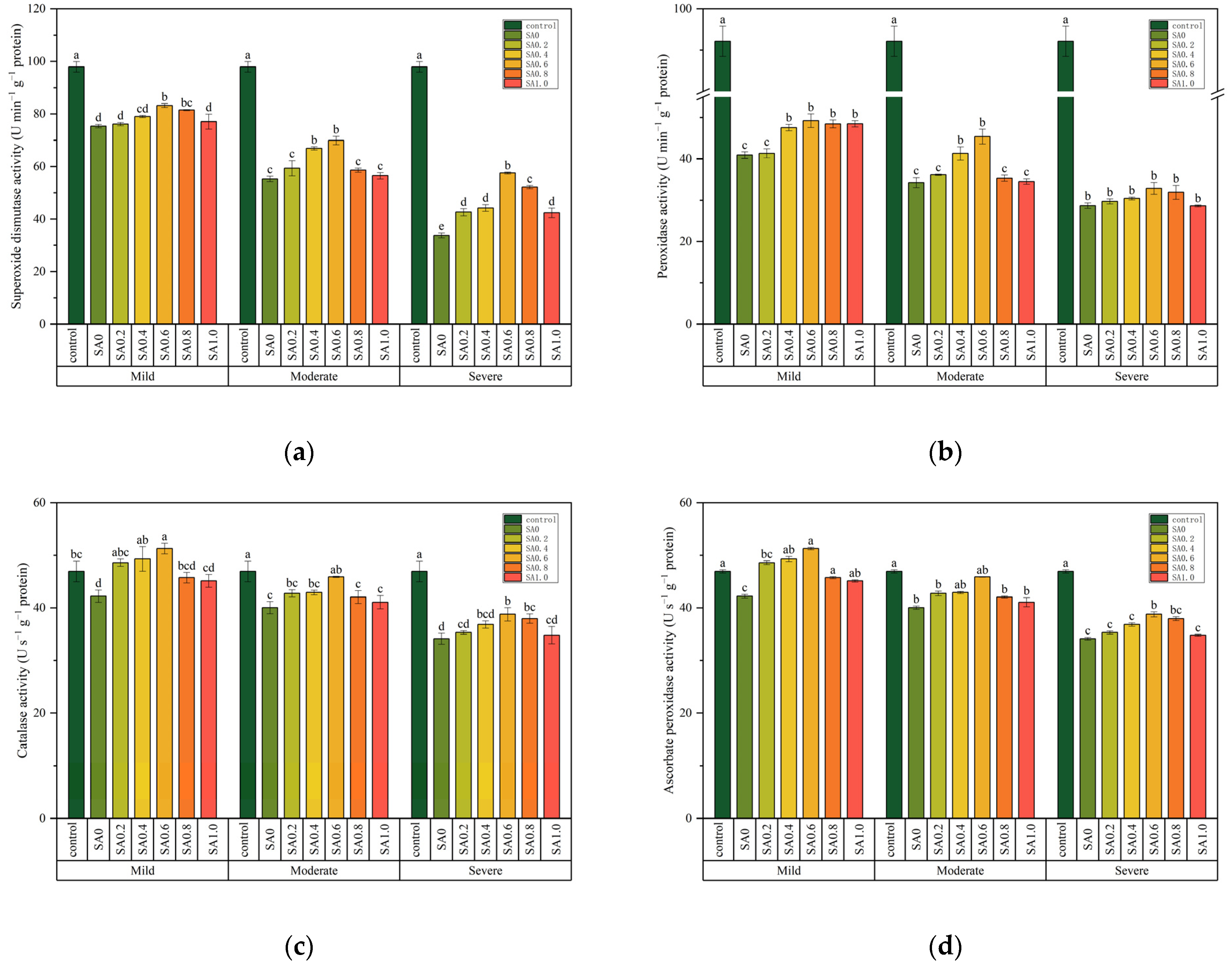
3.4. Antioxidase Activity
3.5. Ion Homeostasis
3.6. Factor Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rahman, M.M.; Mostofa, M.G.; Keya, S.S.; Siddiqui, M.N.; Ansary, M.M.U.; Das, A.K.; Rahman, M.A.; Tran, L.S.-P. Adaptive Mechanisms of Halophytes and Their Potential in Improving Salinity Tolerance in Plants. Int. J. Mol. Sci. 2021, 22, 10733. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jie, W.; Peng, X.; Hua, X.; Yan, X.; Zhou, Z.; Lin, J. Physiological Adaptive Strategies of Oil Seed Crop Ricinus communis Early Seedlings (Cotyledon vs. True Leaf) Under Salt and Alkali Stresses: From the Growth, Photosynthesis and Chlorophyll Fluorescence. Front. Plant Sci. 2019, 9, 1939. [Google Scholar] [CrossRef] [PubMed]
- Kulak, M.; Gul, F.; Sekeroglu, N. Changes in growth parameter and essential oil composition of sage (Salvia officinalis L.) leaves in response to various salt stresses. Ind. Crops Prod. 2020, 145, 112078. [Google Scholar] [CrossRef]
- Smulek, W.; Zdarta, A.; Pacholak, A.; Zgola-Grzeskowiak, A.; Marczak, L.; Jarzebski, M.; Kaczorek, E. Saponaria officinalis L. extract: Surface active properties and impact on environmental bacterial strains. Colloids Surf. B-Biointerfaces 2017, 150, 209–215. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Fujita, M.; Tran, L.-S.P. Nitric oxide mediates hydrogen peroxide- and salicylic acid-induced salt tolerance in rice (Oryza sativa L.) seedlings. Plant Growth Regul. 2015, 77, 265–277. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Fujita, M. Salicylic acid alleviates copper toxicity in rice (Oryza sativa L.) seedlings by up-regulating antioxidative and glyoxalase systems. Ecotoxicology 2013, 22, 959–973. [Google Scholar] [CrossRef]
- Horvath, E.; Szalai, G.; Janda, T. Induction of abiotic stress tolerance by salicylic acid signaling. J. Plant Growth Regul. 2007, 26, 290–300. [Google Scholar] [CrossRef]
- Hayat, Q.; Hayat, S.; Irfan, M.; Ahmad, A. Effect of exogenous salicylic acid under changing environment: A review. Environ. Exp. Bot. 2010, 68, 14–25. [Google Scholar] [CrossRef]
- Abdoli, S.; Ghassemi-Golezani, K.; Alizadeh-Salteh, S. Responses of ajowan (Trachyspermum ammi L.) to exogenous salicylic acid and iron oxide nanoparticles under salt stress. Environ. Sci. Pollut. Res. 2020, 27, 36939–36953. [Google Scholar] [CrossRef]
- Oueslati, S.; Karray-Bouraoui, N.; Attia, H.; Rabhi, M.; Ksouri, R.; Lachaal, M. Physiological and antioxidant responses of Mentha pulegium (Pennyroyal) to salt stress. Acta Physiol. Plant. 2010, 32, 289–296. [Google Scholar] [CrossRef]
- Zhong, Y.-p.; Qi, X.-j.; Chen, J.-y.; Li, Z.; Bai, D.-f.; Wei, C.-g.; Fang, J.-b. Growth and physiological responses of four kiwifruit genotypes to salt stress and resistance evaluation. J. Integr. Agric. 2019, 18, 83–95. [Google Scholar] [CrossRef] [Green Version]
- Bukhat, S.; Manzoor, H.; Athar, H.-u.-R.; Zafar, Z.U.; Azeem, F.; Rasul, S. Salicylic Acid Induced Photosynthetic Adaptability of Raphanus sativus to Salt Stress is Associated with Antioxidant Capacity. J. Plant Growth Regul. 2020, 39, 809–822. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Peng, X.; Yan, J.; Yan, X.; Zhou, Z.; Lin, J. Cotyledon removal decreases salt tolerance during seedling establishment of Ricinus communis, an oilseed energy crop species. Ind. Crops Prod. 2019, 142, 111857. [Google Scholar] [CrossRef]
- Bai, J.-h.; Liu, J.-h.; Zhang, N.; Yang, J.-h.; Sa, R.-l.; Wu, L. Effect of Alkali Stress on Soluble Sugar, Antioxidant Enzymes and Yield of Oat. J. Integr. Agric. 2013, 12, 1441–1449. [Google Scholar] [CrossRef]
- Tuna, A.L.; Kaya, C.; Dikilitas, M.; Yokas, I.; Burun, B.; Altunlu, H. Comparative effects of various salicylic acid derivatives on key growth parameters and some enzyme activities in salinity stressed maize (Zea mays L.) plants. Pak. J. Bot. 2007, 39, 787–798. [Google Scholar] [CrossRef] [Green Version]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Seckin, B.; Sekmen, A.H.; Turkan, I. An Enhancing Effect of Exogenous Mannitol on the Antioxidant Enzyme Activities in Roots of Wheat Under Salt Stress. J. Plant Growth Regul. 2009, 28, 12–20. [Google Scholar] [CrossRef]
- Nakano, Y.N.; Asada, K. Hydrogen Peroxide is Scavenged by Ascorbate-specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Souana, K.; Taibi, K.; Abderrahim, L.A.; Amirat, M.; Achir, M.; Boussaid, M.; Mulet, J.M. Salt-tolerance in Vicia faba L. is mitigated by the capacity of salicylic acid to improve photosynthesis and antioxidant response. Sci. Hortic. 2020, 273, 109641. [Google Scholar] [CrossRef]
- Gunes, A.; Inal, A.; Alpaslan, M.; Eraslan, F.; Bagci, E.G.; Cicek, N. Salicylic acid induced changes on some physiological parameters symptomatic for oxidative stress and mineral nutrition in maize (Zea mays L.) grown under salinity. J. Plant Physiol. 2007, 164, 728–736. [Google Scholar] [CrossRef]
- Selem, E.E.; Hamed, R.E.A.; Kamel, H.A.; Hegazy, H.S. Physiological and Biochemical Response of Gamma Irradiated Sesamum indicum L. Seed Grown in Heavy Metal Contaminated Soil. Biosci. Res. 2018, 15, 1063–1072. [Google Scholar]
- Elhindi, K.M.; Al-Amri, S.M.; Abdel-Salam, E.M.; Al-Suhaibani, N.A. Effectiveness of salicylic acid in mitigating salt-induced adverse effects on different physio-biochemical attributes in sweet basil (Ocimum basilicum L.). J. Plant Nutr. 2017, 40, 908–919. [Google Scholar] [CrossRef]
- Abdelhameed, R.E.; Abdel Latef, A.A.H.; Shehata, R.S. Physiological Responses of Salinized Fenugreek (Trigonella foenum-graecum L.) Plants to Foliar Application of Salicylic Acid. Plants 2021, 10, 657. [Google Scholar] [CrossRef]
- Nazar, R.; Umar, S.; Khan, N.A. Exogenous salicylic acid improves photosynthesis and growth through increase in ascorbate-glutathione metabolism and S assimilation in mustard under salt stress. Plant Signal. Behav. 2015, 10, e1003751. [Google Scholar] [CrossRef] [Green Version]
- Miao, Y.; Luo, X.; Gao, X.; Wang, W.; Li, B.; Hou, L. Exogenous salicylic acid alleviates salt stress by improving leaf photosynthesis and root system architecture in cucumber seedlings. Sci. Hortic. 2020, 272, 109577. [Google Scholar] [CrossRef]
- Kamran, M.; Xie, K.; Sun, J.; Wang, D.; Shi, C.; Lu, Y.; Gu, W.; Xu, P. Modulation of growth performance and coordinated induction of ascorbate-glutathione and methylglyoxal detoxification systems by salicylic acid mitigates salt toxicity in choysum (Brassica parachinensis L.). Ecotoxicol. Environ. Saf. 2020, 188, 109877. [Google Scholar] [CrossRef]
- Shi, Q.; Zhu, Z. Effects of exogenous salicylic acid on manganese toxicity, element contents and antioxidative system in cucumber. Environ. Exp. Bot. 2008, 63, 317–326. [Google Scholar] [CrossRef]
- Faghih, S.; Ghobadi, C.; Zarei, A. Response of Strawberry Plant cv. ‘Camarosa’ to Salicylic Acid and Methyl Jasmonate Application Under Salt Stress Condition. J. Plant Growth Regul. 2017, 36, 651–659. [Google Scholar] [CrossRef]
- Botella, M.A.; Martinez, V.; Pardines, J.; Cerda, A. Salinity induced potassium deficiency in maize plants. J. Plant Physiol. 1997, 150, 200–205. [Google Scholar] [CrossRef]
- Cachorro, P.; Ortiz, A.; Cerda, A. Implications of Calcium Nutrition on the Response of Phaseolus vulgaris L. to Salinity. Plant Soil 1994, 159, 205–212. [Google Scholar] [CrossRef]
- Ghassemi-Golezani, K.; Farhadi, N. The Efficacy of Salicylic Acid Levels on Photosynthetic Activity, Growth, and Essential Oil Content and Composition of Pennyroyal Plants Under Salt Stress. J. Plant Growth Regul. 2021. [Google Scholar] [CrossRef]
- Sultan, I.; Khan, I.; Chattha, M.U.; Hassan, M.U.; Barbanti, L.; Calone, R.; Ali, M.; Majid, S.; Ghani, M.A.; Batool, M.; et al. Improved salinity tolerance in early growth stage of maize through salicylic acid foliar application. Ital. J. Agron. 2021, 16, 1810. [Google Scholar] [CrossRef]
- Misra, N.; Saxena, P. Effect of salicylic acid on proline metabolism in lentil grown under salinity stress. Plant Sci. 2009, 177, 181–189. [Google Scholar] [CrossRef]
- Sha, H.; Liu, H.; Wang, J.; Jia, Y.; Wang, X.; Zou, D.; Zhao, H. Physiological mechanism of salicylic acid regulating salt tolerance of crops. J. Northeast. Agric. Univ. 2017, 48, 80–88. [Google Scholar] [CrossRef]
- Farhadi, N.; Ghassemi-Golezani, K. Physiological changes of Mentha pulegium in response to exogenous salicylic acid under salinity. Sci. Hortic. 2020, 267, 109325. [Google Scholar] [CrossRef]



| Source of Variation | Variable | ||
|---|---|---|---|
| Salt | SA | Salt × SA | |
| Salt damage index (%) | 120.522 *** | 4.366 ** | 0.578 NS |
| Electrolyte leakage (%) | 1593.293 *** | 227.847 *** | 32.059 *** |
| Malondialdehyde content (mmol g−1 FW) | 890.767 *** | 134.467 *** | 17.678 *** |
| Chlorophyll content (mg g−1 FW) | 1001.17 *** | 34.134 *** | 1.739 NS |
| Photosynthesis rate (μmol CO2 m−2 s−1) | 2543.288 *** | 30.735 *** | 2.572 * |
| Soluble sugar content (μmol g−1 FW) | 62.318 *** | 3.202 * | 0.993 NS |
| Soluble protein content (mg g−1 FW) | 302.128 *** | 23.867 *** | 6.927 *** |
| Free proline content (μg g−1 FW) | 2218.661 *** | 44.961 *** | 8.897 *** |
| Superoxide dismutase activity (U min−1 g−1 protein) | 2459.838 *** | 134.081 *** | 21.971 *** |
| Peroxidase activity (U min−1 g−1 protein) | 1789.024 *** | 40.996 *** | 11.225 *** |
| Catalase activity (U s−1 g−1 protein) | 284.676 *** | 32.282 *** | 3.409 ** |
| Ascorbate peroxidase activity (U s−1 g−1 protein) | 91.259 *** | 14.435 *** | 4.164 *** |
| Na+ content (mg g−1 DW) | 3929.347 *** | 102.827 *** | 5.435 *** |
| K+ content (mg g−1 DW) | 3178.515 *** | 119.37 *** | 5.187 *** |
| Mg2+ content (mg g−1 DW) | 996.112 *** | 42.766 *** | 3.997 ** |
| Ca2+ content (mg g−1 DW) | 930.038 *** | 27.836 *** | 4.026 ** |
| K+/Na+ ratio | 3298.456 *** | 78.773 *** | 8.833 *** |
| Treatment | Salt Damage Index (%) | ||
|---|---|---|---|
| Mild Salt Stress | Moderate Salt Stress | Severe Salt Stress | |
| Control | 20.00 ± 0.00 b | 20.00 ± 0.00 b | 20.00 ± 0.00 b |
| SA0 | 51.67 ± 10.41 a | 66.67 ± 10.41 a | 80.00 ± 5.00 a |
| SA0.2 | 41.67 ± 11.55 a | 65.00 ± 8.66 a | 78.33 ± 7.64 a |
| SA0.4 | 33.33 ± 7.64 ab | 63.33 ± 5.77 a | 71.67 ± 7.64 a |
| SA0.6 | 31.67 ± 7.64 ab | 58.33 ± 2.89 a | 68.33 ± 5.77 a |
| SA0.8 | 35.00 ± 5.00 ab | 63.33 ± 2.89 a | 76.67 ± 2.89 a |
| SA1.0 | 43.33 ± 2.89 a | 65.00 ± 5.00 a | 78.33 ± 7.64 a |
| Treatment | Soluble Sugar (μmol g−1 FW) | Soluble Protein (mg g−1 FW) | Free Proline (μg g−1 FW) | |
|---|---|---|---|---|
| Mild salt stress | Control | 124.64 ± 0.92 b | 4.80 ± 0.15 c | 116.88 ± 2.32 d |
| SA0 | 157.56 ± 0.41 a | 5.51 ± 0.23 b | 174.14 ± 1.33 c | |
| SA0.2 | 161.50 ± 6.62 a | 5.60 ± 0.08 b | 184.66 ± 0.96 b | |
| SA0.4 | 163.64 ± 4.73 a | 6.05 ± 0.05 a | 190.91 ± 0.56 a | |
| SA0.6 | 164.63 ± 7.86 a | 6.11 ± 0.25 a | 193.78 ± 1.22 a | |
| SA0.8 | 159.37 ± 2.88 a | 5.52 ± 0.03 b | 182.60 ± 2.11 b | |
| SA1.0 | 159.22 ± 3.79 a | 5.75 ± 0.08 ab | 174.83 ± 1.48 c | |
| Moderate salt stress | Control | 124.64 ± 0.92 b | 4.80 ± 0.15 b | 116.88 ± 2.32 c |
| SA0 | 157.56 ± 0.41 a | 6.22 ± 0.20 a | 210.26 ± 2.65 b | |
| SA0.2 | 161.50 ± 6.62 a | 6.38 ± 0.13 a | 211.37 ± 1.30 b | |
| SA0.4 | 163.64 ± 4.73 a | 6.45 ± 0.02 a | 216.34 ± 1.01 ab | |
| SA0.6 | 164.63 ± 7.86 a | 6.56 ± 0.10 a | 220.05 ± 5.16 a | |
| SA0.8 | 159.37 ± 2.88 a | 6.47 ± 0.09 a | 219.94 ± 0.72 a | |
| SA1.0 | 159.22 ± 3.79 a | 6.36 ± 0.11 a | 218.58 ± 0.52 a | |
| Severe salt stress | Control | 124.64 ± 0.92 c | 4.80 ± 0.15 d | 116.88 ± 2.32 d |
| SA0 | 164.54 ± 1.36 b | 6.49 ± 0.07 c | 231.57 ± 2.25 c | |
| SA0.2 | 169.70 ± 1.36 ab | 6.88 ± 0.15 b | 234.51 ± 1.17 bc | |
| SA0.4 | 171.56 ± 4.83 ab | 7.19 ± 0.05 ab | 244.11 ± 2.94 ab | |
| SA0.6 | 172.49 ± 3.00 ab | 7.20 ± 0.15 ab | 250.88 ± 3.40 a | |
| SA0.8 | 178.55 ± 6.73 a | 7.27 ± 0.20 a | 251.43 ± 7.25 a | |
| SA1.0 | 168.74 ± 4.58 ab | 6.46 ± 0.02 c | 239.40 ± 2.39 bc | |
| Treatment | Na+ Content (mg g−1 DW) | K+ Content (mg g−1 DW) | Mg+ Content (mg g−1 DW) | Ca+ Content (mg g−1 DW) | K+/Na+ Ratio | |
|---|---|---|---|---|---|---|
| Mild salt stress | Control | 5.461 ± 0.243 d | 23.101 ± 0.166 a | 9.919 ± 0.192 a | 12.651 ± 0.186 a | 4.237 ± 0.220 a |
| SA0 | 9.443 ± 0.169 a | 20.230 ± 0.111 f | 7.912 ± 0.205 e | 10.458 ± 0.125 c | 2.143 ± 0.027 f | |
| SA0.2 | 8.742 ± 0.109 b | 21.520 ± 0.169 d | 8.531 ± 0.301 d | 11.411 ± 0.257 b | 2.462 ± 0.040 de | |
| SA0.4 | 7.935 ± 0.050 c | 22.102 ± 0.075 c | 9.163 ± 0.019 bc | 11.678 ± 0.175 b | 2.786 ± 0.019 bc | |
| SA0.6 | 7.840 ± 0.216 c | 22.614 ± 0.088 b | 9.779 ± 0.292 a | 11.716 ± 0.409 b | 2.886 ± 0.083 b | |
| SA0.8 | 8.187 ± 0.091 c | 21.239 ± 0.140 de | 9.324 ± 0.274 ab | 11.685 ± 0.108 b | 2.594 ± 0.012 cd | |
| SA1.0 | 9.026 ± 0.263 ab | 20.973 ± 0.289 e | 8.582 ± 0.051 cd | 11.492 ± 0.145 b | 2.324 ± 0.055 ef | |
| Moderate salt stress | Control | 5.461 ± 0.243 e | 23.101 ± 0.166 a | 9.919 ± 0.192 a | 12.651 ± 0.186 a | 4.237 ± 0.220 a |
| SA0 | 12.286 ± 0.081 a | 17.896 ± 0.058 f | 5.904 ± 0.173 e | 9.473 ± 0.123 d | 1.457 ± 0.005 d | |
| SA0.2 | 11.545 ± 0.205 b | 18.202 ± 0.159 ef | 6.658 ± 0.095 cd | 9.593 ± 0.288 d | 1.577 ± 0.03 cd | |
| SA0.4 | 11.098 ± 0.273 bc | 19.074 ± 0.141 bc | 7.138 ± 0.34 bc | 10.170 ± 0.291 bc | 1.719 ± 0.038 bc | |
| SA0.6 | 9.992 ± 0.116 d | 19.298 ± 0.028 b | 7.217 ± 0.048 b | 10.500 ± 0.027 b | 1.932 ± 0.024 b | |
| SA0.8 | 10.978 ± 0.082 c | 18.802 ± 0.119 cd | 6.916 ± 0.072 bcd | 9.793 ± 0.182 cd | 1.713 ± 0.012 bc | |
| SA1.0 | 11.435 ± 0.140 bc | 18.519 ± 0.323 de | 6.428 ± 0.128 d | 9.549 ± 0.170 d | 1.619 ± 0.009 cd | |
| Severe salt stress | Control | 5.461 ± 0.243 c | 23.101 ± 0.166 a | 9.919 ± 0.192 a | 12.651 ± 0.186 a | 4.237 ± 0.220 a |
| SA0 | 15.055 ± 0.331 a | 14.918 ± 0.105 e | 5.044 ± 0.337 c | 8.033 ± 0.122 c | 0.991 ± 0.025 c | |
| SA0.2 | 14.672 ± 0.281 a | 15.683 ± 0.206 d | 5.531 ± 0.421 bc | 8.322 ± 0.092 bc | 1.069 ± 0.012 bc | |
| SA0.4 | 13.897 ± 0.123 b | 16.343 ± 0.182 c | 5.362 ± 0.084 bc | 8.543 ± 0.141 b | 1.176 ± 0.008 bc | |
| SA0.6 | 13.545 ± 0.177 b | 17.081 ± 0.238 b | 5.974 ± 0.121 b | 8.662 ± 0.126 b | 1.261 ± 0.015 b | |
| SA0.8 | 14.692 ± 0.086 a | 16.131 ± 0.151 cd | 5.452 ± 0.058 bc | 8.532 ± 0.234 b | 1.098 ± 0.015 bc | |
| SA1.0 | 14.953 ± 0.224 a | 15.743 ± 0.385 cd | 5.311 ± 0.230 bc | 8.071 ± 0.182 c | 1.053 ± 0.040 bc | |
| Treatment | Comprehensive Score | ||
|---|---|---|---|
| Mild Salt Stress | Moderate Salt Stress | Severe Salt Stress | |
| Control | −0.809 ± 0.085 bc | −0.809 ± 0.085 d | −0.809 ± 0.085 e |
| SA0 | −0.085 ± 0.155 a | 0.803 ± 0.113 a | 1.681 ± 0.064 a |
| SA0.2 | −0.731 ± 0.063 b | 0.264 ± 0.215 b | 1.16 ± 0.1 bc |
| SA0.4 | −1.219 ± 0.301 c | −0.238 ± 0.095 c | 0.759 ± 0.162 c |
| SA0.6 | −1.733 ± 0.221 d | −0.708 ± 0.16 d | 0.032 ± 0.231 d |
| SA0.8 | −0.941 ± 0.072 bc | 0.242 ± 0.092 b | 0.336 ± 0.093 d |
| SA1.0 | −0.665 ± 0.065 b | 0.426 ± 0.102 b | 1.427 ± 0.195 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Chen, H.; Zhang, T.; Deng, Y.; Yan, J.; Wang, L. Salicylic Acid Improves the Salt Tolerance Capacity of Saponaria officinalis by Modulating Its Photosynthetic Rate, Osmoprotectants, Antioxidant Levels, and Ion Homeostasis. Agronomy 2022, 12, 1443. https://doi.org/10.3390/agronomy12061443
Xu L, Chen H, Zhang T, Deng Y, Yan J, Wang L. Salicylic Acid Improves the Salt Tolerance Capacity of Saponaria officinalis by Modulating Its Photosynthetic Rate, Osmoprotectants, Antioxidant Levels, and Ion Homeostasis. Agronomy. 2022; 12(6):1443. https://doi.org/10.3390/agronomy12061443
Chicago/Turabian StyleXu, Lingxin, Hong Chen, Tingting Zhang, Yanan Deng, Junxin Yan, and Lei Wang. 2022. "Salicylic Acid Improves the Salt Tolerance Capacity of Saponaria officinalis by Modulating Its Photosynthetic Rate, Osmoprotectants, Antioxidant Levels, and Ion Homeostasis" Agronomy 12, no. 6: 1443. https://doi.org/10.3390/agronomy12061443
APA StyleXu, L., Chen, H., Zhang, T., Deng, Y., Yan, J., & Wang, L. (2022). Salicylic Acid Improves the Salt Tolerance Capacity of Saponaria officinalis by Modulating Its Photosynthetic Rate, Osmoprotectants, Antioxidant Levels, and Ion Homeostasis. Agronomy, 12(6), 1443. https://doi.org/10.3390/agronomy12061443







