Identification of Low-Light-Resistant Germplasm and Related Loci of Soybean
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Field Experiments
2.2. Phenotypic Trait Measurement
2.3. DNA Extraction
2.4. Primer Information and PCR Amplification Reaction
2.5. Assessment of SSR and SNP Polymorphisms
2.6. Population Structural Analysis
2.7. Association Mapping
2.8. Genetic Map Construction
3. Results
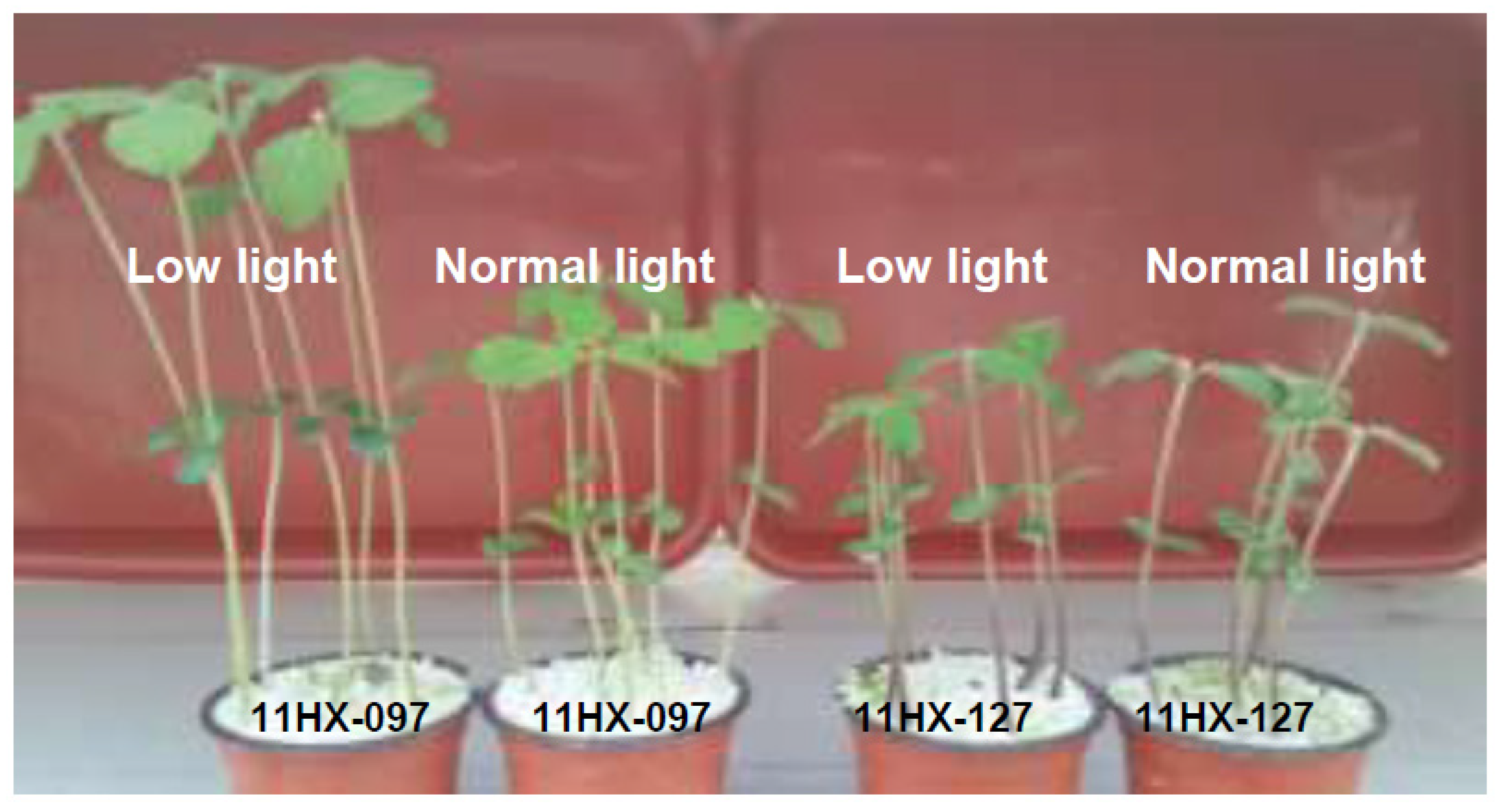
3.1. Stem Height and Thickness Variation of Soybean Accessions under Low-Light
3.2. Accessions with Outstanding Performance in Low-Light Conditions
3.3. Population Structure Analysis of Accession Material
3.4. Identification of SNP and SSR Loci Related to Low-Light Resistance
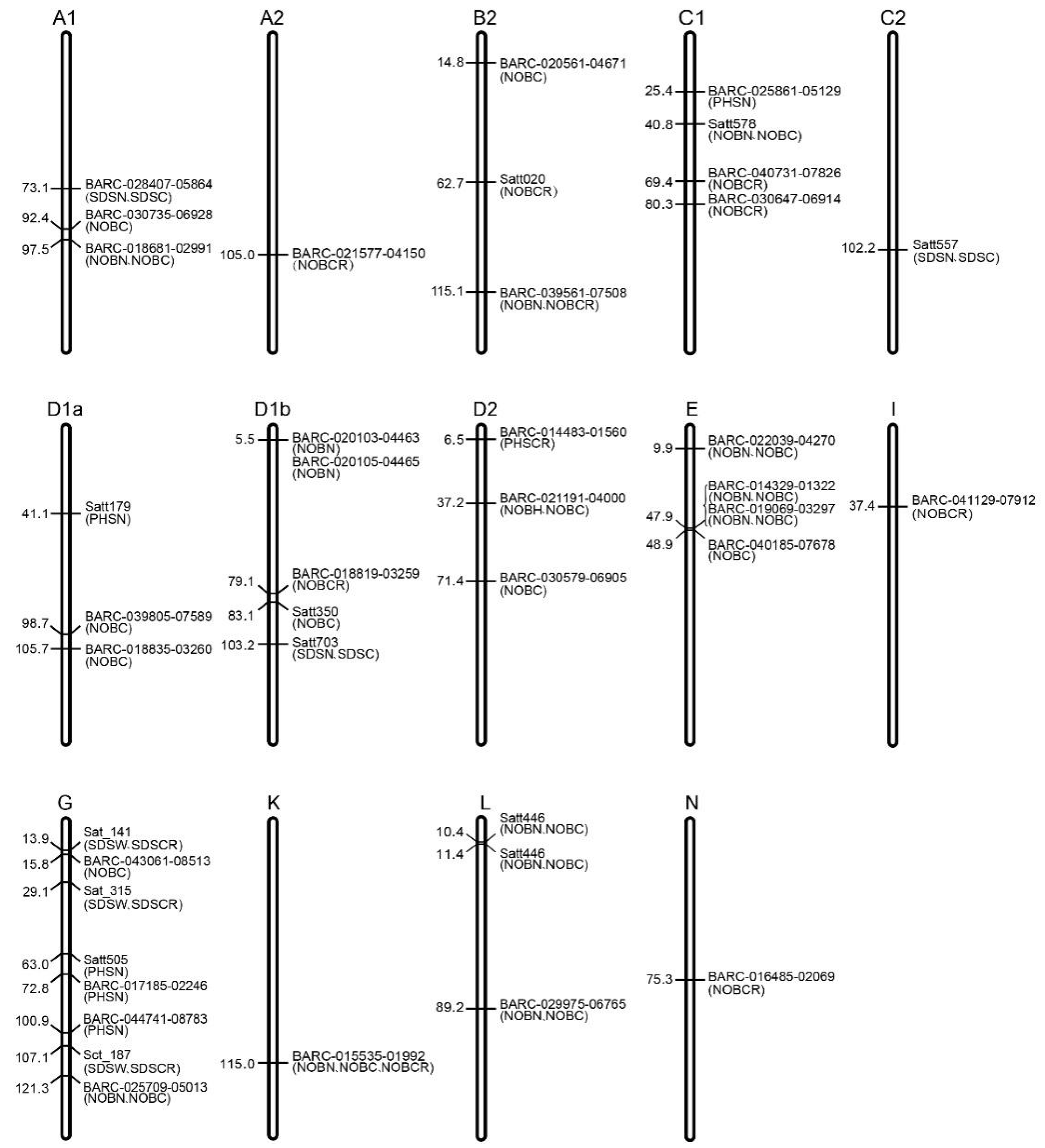
3.5. Distribution of the Markers Related to Low-Light Resistance in Soybean
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fan, Y.F.; Chen, J.X.; Wang, Z.L.; Tan, T.T.; Li, S.L.; Li, J.F.; Wang, B.B.; Zhang, J.W.; Cheng, Y.J.; Wu, X.L.; et al. Soybean (Glycine max L. Merr.) seedlings response to shading: Leaf structure, photosynthesis and proteomic analysis. BMC Plant Biol. 2019, 19, 34. [Google Scholar] [CrossRef] [Green Version]
- Coltman, R.R.; Wolff, X.Y. Productivity under shade in Hawaii of five crops grown as vegetables in tropics. J. Am. Soci. Hort. Sci. 1990, 115, 175–181. [Google Scholar]
- Su, B.Y.; Chen, S.B.; Li, Y.G.; Yang, W.Y. Intercropping enhances the farmland ecosystem services. Acta Ecol. Sin. 2013, 33, 4505–4514. [Google Scholar]
- Yao, X.D.; Li, C.H.; Li, S.Y.; Zhu, Q.; Zhang, H.J.; Wang, H.Y.; Yu, C.M.; Martin, S.K.S.; Xie, F. Effect of shade on leaf photosynthetic capacity, light-intercepting, electron transfer and energy distribution of soybeans. Plant Growth Regul. 2017, 83, 409–416. [Google Scholar] [CrossRef]
- Liu, W.G.; Zou, J.L.; Zhang, J.; Yang, F.; Wan, Y.; Yang, W.Y. Evaluation of soybean (Glycine max) stem vining in maize-soybean relay strip intercropping system. Plant Prod. Sci. 2015, 18, 69–75. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Yong, T.; Ren, W.; Fan, G.; Mu, J.; Lu, X. Develop relay-planting soybean, revitalize soybean industry. Soybean Sci. 2008, 27, 1. [Google Scholar]
- Umezaki, T.; Yoshida, T. Effect of shading on the internode elongation of late maturing soybean. J. Fac. Agric. Kyushu Univ. 1992, 36, 267–272. [Google Scholar] [CrossRef]
- Behairy, T.G. Effect of intercropping patterns on soybean growth and photosynthetic appa-ratus. J. Physiol. Sci. 1994, 18, 168–178. [Google Scholar]
- Sharma, K.; Walia, N. Growth and yield of soybean Glycine max (L.) Merrill as influenced by light intensity and cytokinin. Environ. Ecol. 1996, 14, 307–310. [Google Scholar]
- Tateno, M.; Taneda, H. Photosynthetically versatile thin shade leaves: A paradox of irradiance-response curves. Photosynthetica 2007, 45, 299–302. [Google Scholar] [CrossRef]
- Liu, W.G.; Deng, Y.C.; Hussain, S.; Zou, J.L.; Yuan, J.; Luo, L.; Yang, C.Y.; Yuan, X.Q.; Yang, W.Y. Relationship between cellulose accumulation and lodging resistance in the stem of relay intercropped soybean [Glycine max (L.) Merr.]. Field Crop Res. 2016, 196, 261–267. [Google Scholar] [CrossRef]
- Boardman, N.K. Photosynthesis of sun and shading plant. Annu. Rev. Physiol. 1977, 28, 355–371. [Google Scholar] [CrossRef]
- Liu, W.G.; Jiang, T.; Zhou, X.R.; Yang, W.Y. Characteristics of expansins in soybean (Glycine max) internodes and responses to shade stress. Asian J. Crop Sci. 2011, 3, 26–34. [Google Scholar] [CrossRef] [Green Version]
- Alam, B.; Singh, R.; Newaj, R. Comparative adaptive traits in green gram (Vigna radiata L.) and soybean (Glycine max L.) as influenced by varying regimes of shade. Range Manag. Agrofor. 2012, 33, 142–146. [Google Scholar]
- Wu, Y.S.; Gong, W.Z.; Yang, W.Y. Shade inhibits leaf size by controlling cell proliferation and enlargement in soybean. Sci. Rep. 2017, 7, 9259. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Smith, D.L.; Liu, W.G.; Chen, X.F.; Yang, W.Y. Effects of shade and drought stress on soybean hormones and yield of main-stem and branch. Afr. J. Biotechnol. 2011, 10, 14392–14398. [Google Scholar]
- Jiang, H.K.; Shui, Z.W.; Xu, L.; Yang, Y.H.; Li, Y.; Yuan, X.Q.; Shang, J.; Asghar, M.A.; Wu, X.L.; Yu, L.; et al. Gibberellins modulate shade-induced soybean hypocotyl elongation downstream of the mutual promotion of auxin and brassinosteroids. Plant Physiol. Biochem. 2020, 150, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Myles, S.; Peiffer, J.; Brown, P.J.; Ersoz, E.S.; Zhang, Z.W.; Costich, D.E.; Buckler, E.S. Association Mapping: Critical Considerations Shift from Genotyping to Experimental Design. Plant Cell 2009, 21, 2194–2202. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Cheng, W.Q.; Xiong, C.Y.; Jiang, X.B.; Wu, K.Y.; Gong, B.C. Genetic Diversity and Association Analysis among Germplasms of Diospyros kaki in Zhejiang Province Based on SSR Markers. Forests 2021, 12, 422. [Google Scholar] [CrossRef]
- Cardon, L.R.; Bell, J.I. Association study designs for complex diseases. Nat. Rev. Genet. 2001, 2, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Smulders, M.J.M.; Chang, R.Z.; Qiu, L.J. Genetic diversity and association mapping in a collection of selected Chinese soybean accessions based on SSR marker analysis. Conserv. Genet. 2011, 12, 1145–1157. [Google Scholar] [CrossRef]
- Korir, P.C.; Zhang, J.; Wu, K.J.; Zhao, T.J.; Gai, J.Y. Association mapping combined with linkage analysis for aluminum tolerance among soybean cultivars released in Yellow and Changjiang River Valleys in China. Theor. Appl. Genet. 2013, 126, 1659–1675. [Google Scholar] [CrossRef] [PubMed]
- Breseghello, F.; Sorrells, M.E. Association mapping of kernel size and milling quality in wheat (Triticum aestivum L.) cultivars. Genetics 2006, 172, 1165–1177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agrama, H.; Eizenga, G.; Yan, W. Association mapping of yield and its components in rice cultivars. Mol. Breed. 2007, 19, 341–356. [Google Scholar] [CrossRef]
- Casa, A.M.; Pressoir, G.; Brown, P.J.; Mitchell, S.E.; Rooney, W.L.; Tuinstra, M.R.; Franks, C.D.; Kresovich, S. Community resources and strategies for association mapping in sorghum. Crop. Sci. 2008, 48, 30–40. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.H.; Li, W.; Zhang, C.; Yang, L.A.; Chang, R.Z.; Gaut, B.S.; Qiu, L.J. Genetic diversity in domesticated soybean (Glycinemax) and its wild progenitor (Glycine soja) for simple sequence repeat and single-nucleotide polymorphism loci. New Phytol. 2010, 188, 242–253. [Google Scholar] [CrossRef]
- Murray, M.G.; Thompson, W.F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980, 8, 4321–4326. [Google Scholar] [CrossRef] [Green Version]
- Song, Q.J.; Marek, L.F.; Shoemaker, R.C.; Lark, K.G.; Concibido, V.C.; Delannay, X.; Specht, J.E.; Cregan, P.B. A new integrated genetic linkage map of the soybean. Theor. Appl. Genet. 2004, 109, 122–128. [Google Scholar] [CrossRef]
- Li, Y.H.; Zhao, S.C.; Ma, J.X.; Li, D.; Yan, L.; Li, J.; Qi, X.T.; Guo, X.S.; Zhang, L.; He, W.M.; et al. Molecular footprints of domestication and improvement in soybean revealed by whole genome re-sequencing. BMC Genom. 2013, 14, 579. [Google Scholar] [CrossRef] [Green Version]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef]
- Bradbury, P.J.; Zhang, Z.; Kroon, D.E.; Casstevens, T.M.; Ramdoss, Y.; Buckler, E.S. TASSEL: Software for association mapping of complex traits in diverse samples. Bioinformatics 2007, 23, 2633–2635. [Google Scholar] [CrossRef] [PubMed]
- Voorrips, R.E. MapChart: Software for the graphical presentation of linkage maps and QTLs. J. Hered. 2002, 93, 77–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slattery, R.A.; VanLoocke, A.; Bernacchi, C.J.; Zhu, X.G.; Ort, D.R. Photosynthesis, light use efficiency, and yield of reduced-chlorophyll soybean mutants in field conditions. Front. Plant Sci. 2017, 8, 549. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Huang, S.; Gao, R.C.; Liu, W.G.; Yong, T.W.; Wang, X.C.; Wu, X.L.; Yang, W. Growth of soybean seeeding in relay strip intercropping systems in telation to light quantity and red: Far-red ratio. Field Crop. Res. 2014, 155, 245–253. [Google Scholar] [CrossRef]
- Xie, Y.H.; Li, X.H.; Wang, Y.J.; Li, L.; Zhou, H. Influences of different intercropping row-ratios between maize and soybean on agronomic characters and yield of precocious spring soybean. Hunan Agric. Sci. 2011, 5, 26–28. [Google Scholar]
- Liu, W.G.; Ren, M.L.; Liu, T.; Du, Y.L.; Zhou, T.; Liu, X.M.; Liu, J.; Hussain, S.; Yang, W.Y. Effect of shade stress on lignin biosynthesis in soybean stems. J. Integr. Agric. 2018, 11, 1594–1604. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Gong, W.Z.; Yang, W.Y.; Ma, Z. Correlation between agronomic characters and yield in relay-planting soybeans. Soybean Sci. 2007, 5, 680–686. [Google Scholar]
- Wang, Y.; Yang, W.Y.; Zhang, X.; Yong, T.W.; Liu, W.G.; Su, B.Y. Effects of shading at different growth stages on different traits and yield of soybean. Acta Agron. Sin. 2013, 39, 1871–1879. [Google Scholar] [CrossRef]
- He, L.Y.; Yin, C.J.; Huang, S.C.; He, Q.Y.; Shu, Y.J. Correlation, clustering and principal component analysis of primary agronomic traits of vegetable soybean. Acta Agric. Zhejiangensis 2018, 30, 50–57. [Google Scholar]
- Fan, Y.F.; Chen, J.X.; Cheng, Y.J.; Raza, M.A.; Wu, X.L.; Wan, Z.L.; Liu, Q.L.; Wang, R.; Wan, X.C.; Yong, T.W.; et al. Effect of shading and light recovery on the growth, leaf structure, and photosynthetic performance of soybean in a maize-soybean relay-strip intercropping system. Field Crop. Res. 2014, 155, 245–253. [Google Scholar] [CrossRef]
- Li, J.J.; Zhao, J.H.; Li, Y.H.; Gao, Y.L.; Hua, S.N.; Nadeem, M.; Sun, G.L.; Zhang, W.M.; Hou, J.F.; Wang, X.B.; et al. Identification of a novel seed size associated locus SW9-1 in soybean. Crop J. 2019, 7, 548–559. [Google Scholar] [CrossRef]
- Zhou, Q.H.; Han, D.P.; Mason, A.S.; Zhou, C.; Zheng, W.; Li, Y.Z.; Wu, C.J.; Fu, D.H.; Huang, Y.J. Earliness traits in rapeseed (Brassica napus): SNP loci and candidate genes identified by genome-wide association analysis. DNA Res. 2018, 25, 229–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michno, J.M.; Virdi, K.; Stec, A.O.; Liu, J.Q.; Wang, X.B.; Xiong, Y.; Stupar, R.M. Integration, abundance, and transmission of mutations and transgenes in a series of CRISPR/Cas9 soybean lines. BMC Biotechnol. 2020, 20, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Sub-Traits | Max. 1 | Min. 2 | Mean | SD | CV 3 | |
|---|---|---|---|---|---|---|
| Plant height | PHN (cm) | 143.67 | 39.00 | 82.03 | 22.10 | 0.27 |
| PHL (cm) | 215.67 | 41.00 | 102.09 | 30.05 | 0.29 | |
| PHC (cm) | 91.67 | 0.17 | 22.75 | 17.92 | 0.79 | |
| PHCR | 0.99 | 0.00 | 0.28 | 0.21 | 0.76 | |
| Stem diameter | SDN (mm) | 12.43 | 4.15 | 7.62 | 1.47 | 0.19 |
| SDL (mm) | 9.18 | 3.94 | 6.29 | 1.18 | 0.19 | |
| SDC (mm) | 6.07 | 0.00 | 1.52 | 1.18 | 0.78 | |
| SDCR | 0.49 | 0.00 | 0.18 | 0.11 | 0.62 | |
| Number of bean pods (individual) | NOBN | 242.67 | 7.33 | 49.52 | 27.51 | 0.56 |
| NOBL | 116.33 | 3.67 | 28.43 | 17.83 | 0.63 | |
| NOBC | 186.67 | 0.67 | 24.87 | 21.59 | 0.87 | |
| NOBCR | 2.75 | 0.01 | 0.51 | 0.34 | 0.67 | |
| Cotyledon height (seedling stage) | CNHSN (cm) | 8.85 | 0.57 | 3.62 | 1.22 | 0.34 |
| CNHSL (cm) | 15.50 | 4.23 | 8.40 | 2.01 | 0.24 | |
| CNHSC (cm) | 12.50 | 0.13 | 4.81 | 2.26 | 0.47 | |
| CNHSCR | 9.94 | 0.03 | 1.62 | 1.29 | 0.80 | |
| Plant height (seedling stage) | PHSN (cm) | 25.00 | 1.14 | 7.03 | 2.38 | 0.34 |
| PHSL (cm) | 32.50 | 8.03 | 19.46 | 3.67 | 0.19 | |
| PHSC (cm) | 26.17 | 1.62 | 12.38 | 4.09 | 0.33 | |
| PHSCR | 16.71 | 0.17 | 2.11 | 1.59 | 0.75 | |
| Stem diameter (seedling stage) | SDSN (mm) | 26.60 | 1.31 | 2.66 | 1.90 | 0.71 |
| SDSL (mm) | 13.66 | 1.40 | 2.43 | 1.10 | 0.45 | |
| SDSC (mm) | 26.60 | 0.01 | 0.63 | 2.31 | 3.66 | |
| SDSCR | 4.12 | 0.00 | 0.18 | 0.39 | 2.17 |
| Trait | 11HX-020 | 11HX-025 | 11HX-029 | 11HX-124 | 11HX-127 | 11HX-166 | 11HX-183 | 11HX-216 |
|---|---|---|---|---|---|---|---|---|
| PHCR | 0.01 | 0.42 | 0.46 | 0.05 | 0.16 | 0.12 | 0.04 | 0.49 |
| SDCR | 0.25 | 0.01 | 0.02 | 0.05 | 0.02 | 0.01 | 0.28 | 0.05 |
| NOBCR | 0.08 | 0.05 | 0.10 | 0.14 | 0.09 | -- | 0.04 | 0.04 |
| SDSCR | -- | 0.33 | 0.12 | -- | 0.07 | 0.01 | 0.10 | 0.03 |
| Marker Loci | PHSN | PHSCR | SDSN | SDSL | SDSC | SDSCR |
|---|---|---|---|---|---|---|
| BARC-017185-02246 | 0.17 | |||||
| BARC-016069-02054 | 0.18 | |||||
| BARC-044741-08783 | 0.35 | |||||
| BARC-025861-05129 | 0.36 | |||||
| Satt505 | 0.16 | |||||
| Satt179 | 0.18 | |||||
| BARC-019061-03295 | 0.10 | |||||
| BARC-014483-01560 | 0.12 | |||||
| BARC-038949-07404 | 0.13 | |||||
| BARC-021793-04213 | 0.13 | |||||
| Satt144 | 0.29 | |||||
| BARC-041903-08129 | 0.10 | |||||
| BARC-028407-05864 | 0.19 | 0.20 | ||||
| Satt557 | 0.33 | 0.19 | ||||
| Satt282 | 0.33 | 0.11 | ||||
| Satt703 | 0.34 | 0.18 | ||||
| Sat_315 | 0.15 | 0.12 | ||||
| Sct_187 | 0.09 | 0.08 | ||||
| Sat_141 | 0.28 | 0.31 |
| Marker Loci | NOBN | NOBC | NOBCR |
|---|---|---|---|
| BARC-014329-01322 | 0.13 | 0.16 | |
| BARC-015535-01992 | 0.27 | 0.34 | 0.26 |
| BARC-016413-02582 | 0.10 | ||
| BARC-018681-02991 | 0.13 | 0.14 | |
| BARC-019069-03297 | 0.14 | 0.16 | |
| BARC-020103-04463 | 0.11 | ||
| BARC-020105-04465 | 0.11 | ||
| BARC-020139-04480 | 0.14 | 0.16 | 0.27 |
| BARC-021191-04000 | 0.10 | 0.15 | |
| BARC-022039-04270 | 0.11 | 0.12 | |
| BARC-025709-05013 | 0.29 | 0.31 | |
| BARC-029975-06765 | 0.13 | 0.15 | |
| BARC-039561-07508 | 0.27 | 0.33 | |
| rhg1-597-606 | 0.12 | 0.15 | |
| Satt446 | 0.16 | 0.20 | |
| Satt578 | 0.23 | 0.24 | |
| BARC-018835-03260 | 0.11 | ||
| BARC-020561-04671 | 0.13 | ||
| BARC-025791-05069 | 0.11 | ||
| BARC-030735-06928 | 0.11 | ||
| BARC-039805-07589 | 0.13 | ||
| BARC-040185-07678 | 0.14 | ||
| BARC-043061-08513 | 0.15 | ||
| Satt350 | 0.19 | ||
| Satt365 | 0.26 | ||
| BARC-016485-02069 | 0.12 | ||
| BARC-018013-02496 | 0.26 | ||
| BARC-018819-03259 | 0.16 | ||
| BARC-021577-04150 | 0.12 | ||
| BARC-030579-06905 | 0.15 | ||
| BARC-030647-06914 | 0.26 | ||
| BARC-040731-07826 | 0.23 | ||
| BARC-041129-07912 | 0.13 | ||
| Satt020 | 0.20 |
| Traits | Marker Loci |
|---|---|
| Plant height-related traits (PHSN, PHSCR) | BARC-017185-02246, BAR5 C-016069-02054, BARC-044741-08783, BARC-025861-05129, Satt505, Satt179 |
| Stem diameter-related traits (SDSN, SDSL, SDSC, SDSCR) | BARC-041903-08129. BARC-028407-05864, Satt557, Satt282, Satt703, Sat_315, Sct_187, Sat_141 |
| Number of bean pod-related traits (NOBN, NOBC, NOBCR) | BARC-014329-01322, BARC-015535-01992, BARC-016413-02582, BARC-018681-02991, BARC-019069-03297, BARC-020103-04463, BARC-020105-04465, BARC-020139-04480, BARC-021191-04000, BARC-022039-04270, BARC-025709-05013, BARC-029975-06765, BARC-039561-07508, rhg1-597-606, Satt446, Satt578, BARC-018835-03260, BARC-020561-04671, BARC-025791-05069, BARC-030735-06928, BARC-039805-07589, BARC-040185-07678, BARC-043061-08513, Satt350, Satt365, BARC-016485-02069, BARC-018013-02496, BARC-018819-03259, BARC-021577-04150, BARC-030579-06905, BARC-030647-06914, BARC-040731-07826, BARC-041129-07912, Satt020 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, J.; Wang, S.; Shan, G.; Yuan, L.; Wang, C.; Zhu, S.; Wang, X.; Qiu, L. Identification of Low-Light-Resistant Germplasm and Related Loci of Soybean. Agronomy 2022, 12, 1483. https://doi.org/10.3390/agronomy12071483
Hou J, Wang S, Shan G, Yuan L, Wang C, Zhu S, Wang X, Qiu L. Identification of Low-Light-Resistant Germplasm and Related Loci of Soybean. Agronomy. 2022; 12(7):1483. https://doi.org/10.3390/agronomy12071483
Chicago/Turabian StyleHou, Jinfeng, Shuangshuang Wang, Guolei Shan, Lingyun Yuan, Chenggang Wang, Shidong Zhu, Xiaobo Wang, and Lijuan Qiu. 2022. "Identification of Low-Light-Resistant Germplasm and Related Loci of Soybean" Agronomy 12, no. 7: 1483. https://doi.org/10.3390/agronomy12071483
APA StyleHou, J., Wang, S., Shan, G., Yuan, L., Wang, C., Zhu, S., Wang, X., & Qiu, L. (2022). Identification of Low-Light-Resistant Germplasm and Related Loci of Soybean. Agronomy, 12(7), 1483. https://doi.org/10.3390/agronomy12071483







