Competition and Niche Differentiation of Water and Nutrients between Broussonetia papyrifera and Platycladus orientalis under Prolonged Drought Stress
Abstract
:1. Introduction
- (1)
- Does the niche differentiation of nitrogen and phosphorus occur between B. papyrifera and P. orientalis under drought stress?
- (2)
- Does interspecific competition affect the status of the water, nitrogen, and phosphorus nutrition of B. papyrifera and P. orientalis under drought stress, and who is the winner?
2. Materials and Methods
2.1. Plant Material and Drought Treatment
2.2. Measurement of Leaf Gas Exchange
2.3. Nitrogen and Phosphorus Contents
2.4. Carbon (δ13C) and Nitrogen (δ15N) Isotope Compositions
2.5. Root PME and PDE Activities
2.6. Data Analysis
3. Results
3.1. Soil Water Content
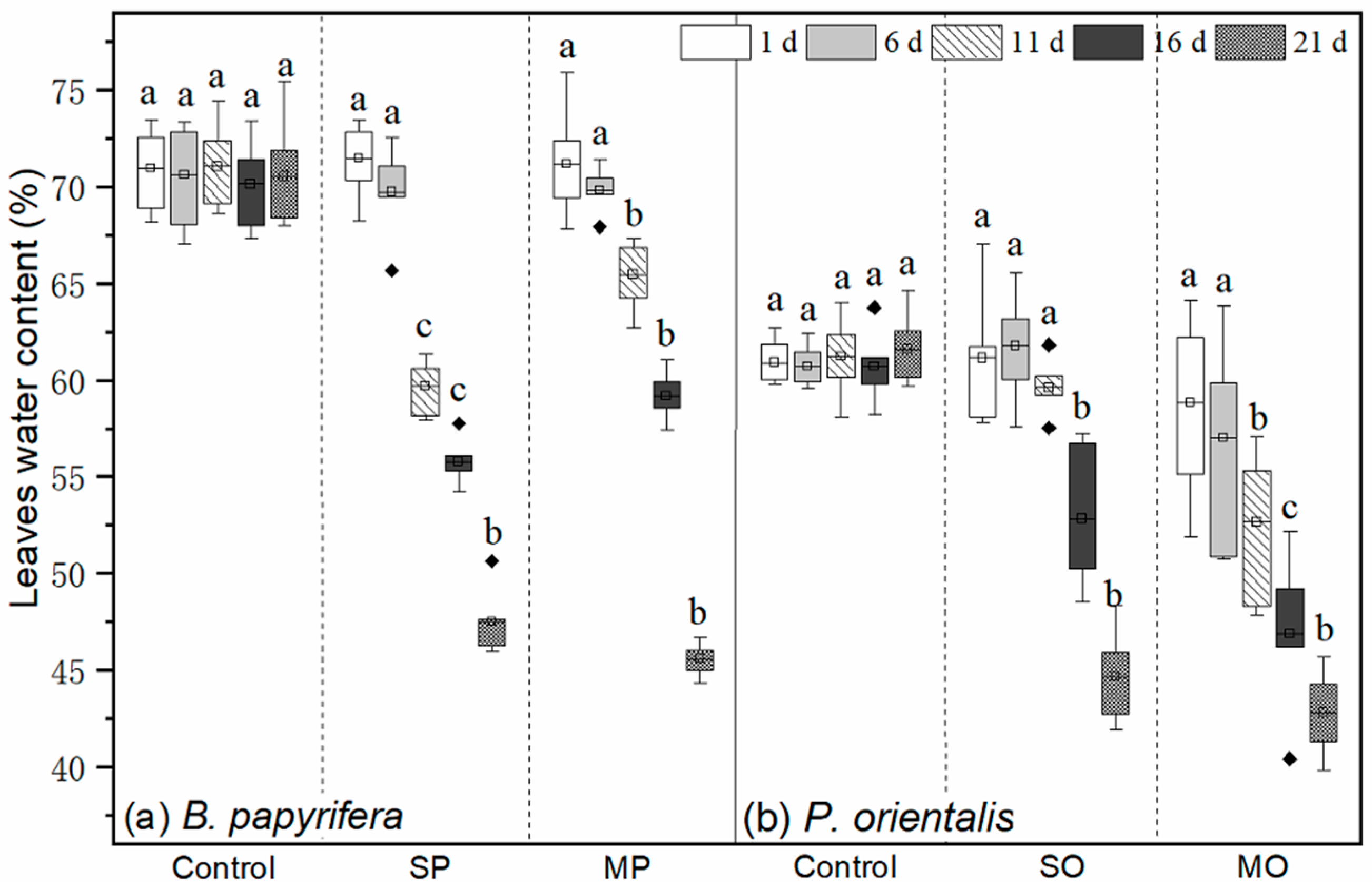
3.2. Leaf Water Contents, δ13C Value, and gs
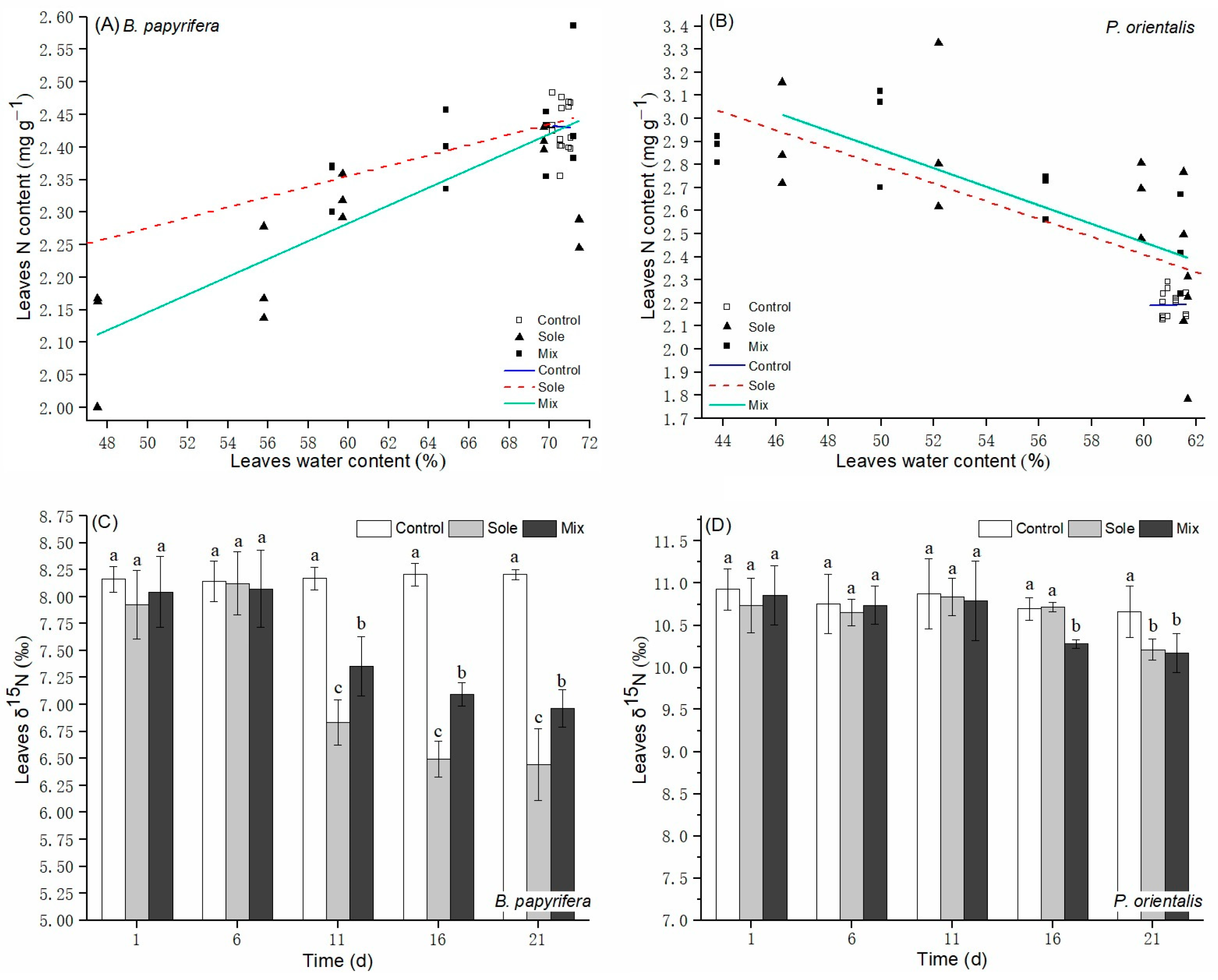
3.3. N Contents and δ15N Values
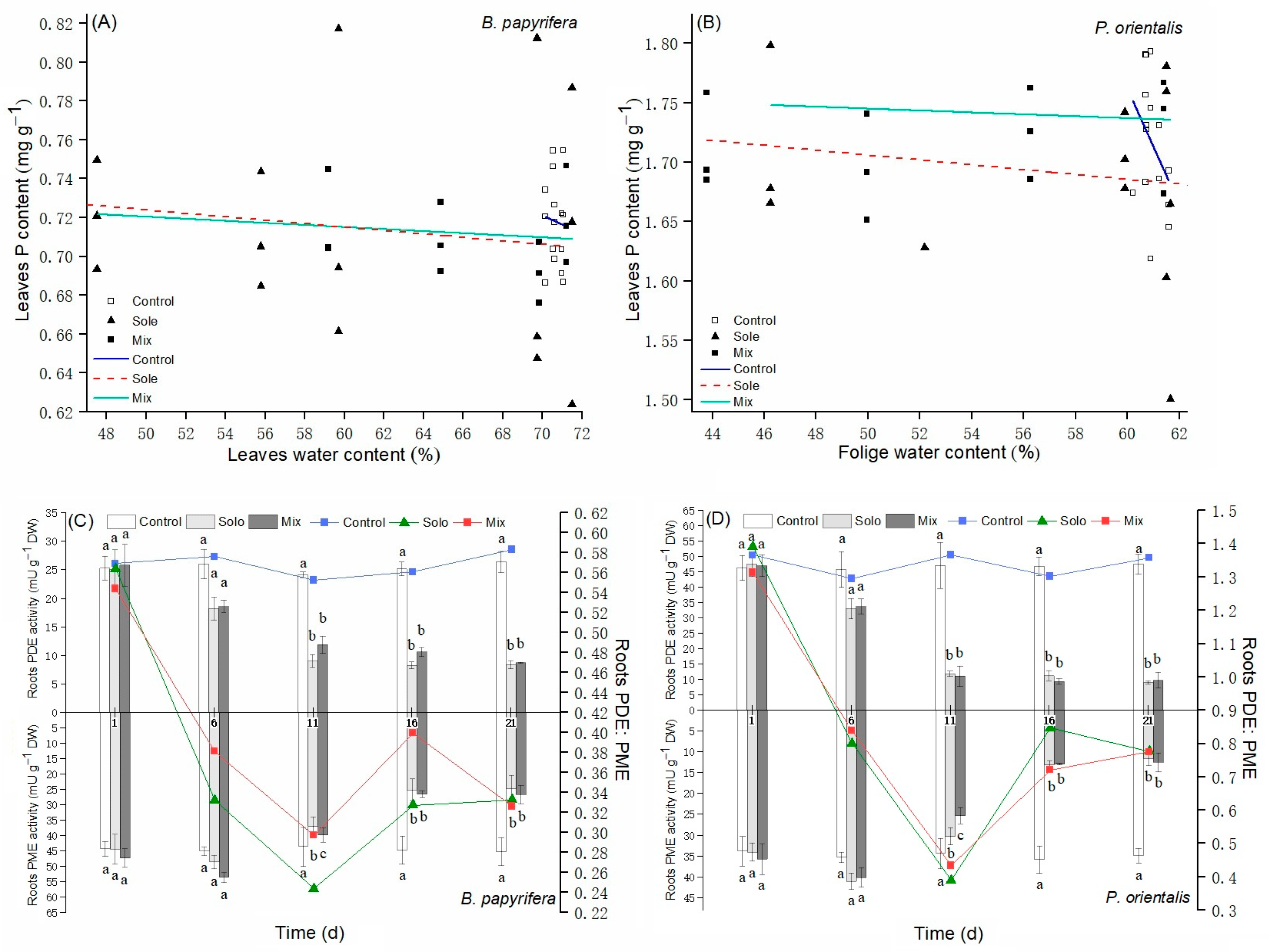
3.4. P Contents and PDE: PME Ratio
3.5. Correlation and Difference Analysis
4. Discussion
4.1. Plants’ Water Status and Water Source Competition
4.2. Plants N Status and N Sources Change
4.3. Plants P Status and P Sources Change
4.4. Interspecific Competition Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cramer, W.; Guiot, J.; Fader, M.; Garrabou, J.; Gattuso, J.P.; Iglesias, A.; Lange, M.A.; Lionello, P.; Llasat, M.C.; Paz, S. Climate change and interconnected risks to sustainable development in the Mediterranean. Nat. Clim. Chang. 2018, 8, 972–980. [Google Scholar] [CrossRef] [Green Version]
- Kunstler, G.; Falster, D.; Coomes, D.A.; Hui, F.; Kooyman, R.M.; Laughlin, D.C.; Poorter, L.V.; Vanderwel, M.; Vieilledent, G.; Wright, S.J.; et al. Plant functional traits have globally consistent effects on competition. Nature 2016, 529, 204–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calama, R.; Conde, M.; Dios-García, J.; Madrigal, G.; Vázquez-Piquéc, J. Linking climate, annual growth and competition in a Mediterranean forest: Pinus pinea in the Spanish Northern Plateau. Agric. For. Meteorol. 2019, 264, 309–321. [Google Scholar] [CrossRef]
- Lorts, C.M.; Lasky, J.R. Competition × drought interactions change phenotypic plasticity and the direction of selection on Arabidopsis traits. New Phytol. 2020, 227, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Grossiord, C. Having the right neighbors: How tree species diversity modulates drought impacts on forests. New Phytol. 2019, 228, 42–49. [Google Scholar] [CrossRef] [Green Version]
- Jourdan, M.; Lebourgeois, F.; Morin, X. The effect of tree diversity on the resistance and recovery of forest stands in the French Alps may depend on species differences in hydraulic features. For. Ecol. Manag. 2019, 450, 117486. [Google Scholar] [CrossRef]
- Schwarz, J.A.; Bauhus, J. Benefits of mixtures on growth performance of silver fir (Abies alba) and european beech (Fagus sylvatica) increase with tree size without reducing drought tolerance. Front. For. Glob. Chang. 2019, 2, 79. [Google Scholar] [CrossRef]
- Forrester, D.I.; Bauhus, J. A review of processes behind diversity-productivity relationships in forests. Curr. For. Rep. 2016, 2, 45–61. [Google Scholar] [CrossRef] [Green Version]
- He, M.; Dijkstra, F.A. Drought effect on plant nitrogen and phosphorus: A meta-analysis. New Phytol. 2014, 204, 924–931. [Google Scholar] [CrossRef]
- Sardans, J.; Urbina, I.; Grau, O.; Asensio, D.; Ogaya, R.; Peñuelas, J. Long-term drought decreases ecosystem C and nutrient storage in a Mediterranean holm oak forest. Environ. Exp. Bot. 2020, 114, 104135. [Google Scholar] [CrossRef]
- Suriyagoda, L.D.B.; Ryan, M.H.; Renton, M.; Lambers, H. Plant responses to limited moisture and phosphorus availability: A meta-analysis. Adv. Agron. 2014, 124, 143–200. [Google Scholar]
- Cregger, M.A.; McDowell, N.G.; Pangle, R.E.; Pockman, W.T.; Classen, A.T. The impact of precipitation change on nitrogen cycling in a semi-arid ecosystem. Funct. Ecol. 2014, 28, 1534–1544. [Google Scholar] [CrossRef]
- Agren, G.I. Stoichiometry and nutrition of plant growth in natural communities. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 153–170. [Google Scholar] [CrossRef]
- Le Bauer, D.S.; Treseder, K.K. Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 2008, 89, 371–379. [Google Scholar] [CrossRef] [Green Version]
- Vitousek, P.M.; Porder, S.; Houlton, B.Z.; Chadwick, O.A. Terrestrial phosphorus limitation: Mechanisms, implications, and nitrogen-phosphorus interactions. Ecol. Appl. 2010, 20, 5–15. [Google Scholar] [CrossRef] [Green Version]
- Homyak, P.M.; Slessarev, E.W.; Hagerty, S.; Greene, A.C.; Marchus, K.; Dowdy, K.; Iverson, S.; Schimel, J.P. Amino acids dominate diffusive nitrogen fluxes across soil depths in acidic tussock tundra. New Phytol. 2021, 231, 2162–2173. [Google Scholar] [CrossRef]
- Leake, J.R.; Miles, W. Phosphodiesters as mycorrhizal P sources: I. Phosphodiesterase production and the utilization of DNA as a phosphorus source by the ericoid mycorrhizal fungus Hymenoscyphus ericae. New Phytol. 1996, 132, 435–443. [Google Scholar] [CrossRef]
- Makarov, M.I.; Haumaier, L.; Zech, W. The nature and origins of diester phosphates in soils: A 31P-NMR study. Biol. Fertil. Soils 2002, 35, 136–146. [Google Scholar]
- Turner, B.L.; Newman, S. Phosphorus cycling in wetland soils: The importance of phosphate diesters. J. Environ. Qual. 2005, 34, 1921–1929. [Google Scholar] [CrossRef] [Green Version]
- Turner, B.L. Resource partitioning for soil phosphorus: A hypothesis. J. Ecol. 2008, 96, 698–702. [Google Scholar] [CrossRef]
- Rejmάnkovά, E.; Dagmara Sirovά, D.; Carlson, E. Patterns of activities of root phosphomonoesterase and phosphodiesterase in wetland plants as a function of macrophyte species and ambient phosphorus regime. New Phytol. 2011, 190, 968–976. [Google Scholar] [CrossRef]
- Huston, M.A. Management strategies for plant invasions: Manipulating productivity, disturbance, and competition. Divers. Distrib. 2004, 10, 167–178. [Google Scholar] [CrossRef]
- Lei, H.P.; Wang, Y.G.; Liang, F.Y.; Su, W.W.; Feng, Y.F.; Guo, X.L.; Wang, N. Composition and variability of essential oils of Platycladus orientalis growing in China. Biochem. Syst. Ecol. 2010, 38, 1000–1006. [Google Scholar] [CrossRef]
- Li, J.Y. Studies on drought tolerance of some main used in afforestation in Taihang mountain tree species region (III)—Water parameters. J. Beijing For. Univ. 1990, 13, 230–239. [Google Scholar]
- Tabatabai, M.A.; Bremmer, J.A. Use of p-nitrophenylphosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabai, M.A. Phosphatases in soils. Soil Biol. Biochem. 1977, 9, 167–172. [Google Scholar] [CrossRef]
- Farrior, C.E.; Dybzinski, R.; Levin, S.A.; Pacala, S.W. Competition for water and light in closed-canopy forests: A tractable model of carbon allocation with implications for carbon sinks. Am. Nat. 2013, 181, 314–330. [Google Scholar] [CrossRef] [Green Version]
- Jucker, T.; Grossiord, C.; Bonal, D.; Bouriaud, O.; Gessler, A.; Coomes, D.A. Detecting the fingerprint of drought across Europe’s forest: Do carbon isotope ratios and stem growth rates tell similar stories? For. Ecosyst. 2017, 4, 24. [Google Scholar] [CrossRef] [Green Version]
- DeSoto, L.; Cailleret, M.; Sterck, F.; Jansen, S.; Kramer, K.; Robert, E.M.R.; Aakala, T.; Amoroso, M.M.; Bigler, C.; Camarero, J.J.; et al. Low growth resilience to drought is related to future mortality risk in trees. Nat. Commun. 2020, 11, 545. [Google Scholar] [CrossRef]
- Adams, H.D.; Zeppel, M.J.B.; Anderegg, W.R.L.; Hartmann, H.; Landhäusser, S.M.; Tissue, D.T.; Huxman, T.E.; Hudson, P.J.; Franz, T.E.; Allen, C.D.; et al. A multi-species synthesis of physiological mechanisms in drought-induced tree mortality. Nat. Ecol. Evol. 2017, 1, 1285–1291. [Google Scholar] [CrossRef]
- Zenes, N.; Kerr, K.L.; Trugman, A.T.; Anderegg, W.R.L. Competition and drought alter optimal stomatal strategy in tree seedlings. Front. Plant Sci. 2020, 11, 478. [Google Scholar] [CrossRef] [PubMed]
- Brodribb, T.J.; Field, T.S. Leaf hydraulic evolution led a surge in leaf photosynthetic capacity during early angiosperm diversification. Ecol. Lett. 2010, 13, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Piper, F.I.; Hoch, G.; Fajardo, A. Revisiting the relative growth rate hypothesis for gymnosperm and angiosperm species co-occurrence. Am. J. Bot. 2019, 106, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Urbina, I.; Sardans, J.; Beierkuhnlein, C.; Jenntsch, A.; Backhaus, S.; Grant, K.; Peñuelas, J. Shifts in the elemental composition of plants during a very severe drought. Environ. Exp. Bot. 2015, 111, 63–73. [Google Scholar] [CrossRef] [Green Version]
- Mouradi, M.; Bouizgarenb, A.; Farissi, M.; Latracha, L.; Qaddourya, A.; Cherki, G. Seed osmopriming improves plant growth, nodulation, chlorophyll fluorescence and nutrient uptake in alfalfa (Medicago sativa L.)—Rhizobia symbiosis under drought stress. Sci. Hortic. 2016, 213, 232–242. [Google Scholar] [CrossRef]
- Mariotte, P.; Cresswell, T.; Johansen, M.P.; Harrison, J.J.; Keitel, C.; Dijkstra, F.A. Plant uptake of nitrogen and phosphorus among grassland species affected by drought along a soil available phosphorus gradient. Plant Soil. 2020, 448, 121–132. [Google Scholar] [CrossRef]
- Evans, R.D.; Bloom, A.J.; Sukrapanna, S.S.; Ehleringer, J.R. Nitrogen isotope composition of tomato (Lycopersicon esculentum Mill. cv. T-5) grown under ammonium or nitrate nutrition. Plant Cell Environ. 1996, 19, 1317–1323. [Google Scholar] [CrossRef]
- Peuke, A.D.; Rennenberg, H. Carbon, nitrogen, phosphorus, and Sulphur concentration and partitioning in beech ecotypes (Fagus sylvatica L.): Phosphorus most affected by drought. Trees 2004, 18, 639–648. [Google Scholar] [CrossRef]
- Hacke, U.G.; Sperry, J.S.; Wheeler, J.K.; Castro, L. Scaling of angiosperm xylem structure with safety and efficiency. Tree Physiol. 2006, 26, 689–701. [Google Scholar] [CrossRef]
- Gessler, A.; Schaub, M.; McDowell, N.G. The role of nutrients in drought-induced tree mortality and recovery. New Phytol. 2017, 214, 513–520. [Google Scholar] [CrossRef] [Green Version]
- Schönbeck, L.; Gessler, A.; Schaub, M.; Rigling, A.; Hoch, G.; Kahmen, A.; Li, M.H. Soil nutrients and lowered source: Sink ratio mitigate effects of mild but not of extreme drought in trees. Environ. Exp. Bot. 2020, 169, 103905. [Google Scholar] [CrossRef]
- Bond, W.J. The tortoise and the hare: Ecology of angiosperm dominance and gymnosperm persistence. Bot. J. Linn. Soc. 1989, 36, 227–249. [Google Scholar] [CrossRef]
- Bowman, R.A.; Cole, C.V. Transformations of organic phosphorus substrates in soils as evaluated by NaHCO3 extraction. Soil Sci. 1978, 125, 49–54. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Follstad Shah, J.J. Ecoenzymatic stoichiometry and ecological theory. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 313–343. [Google Scholar] [CrossRef] [Green Version]
- Pretzsch, H.; Biber, P. Size-symmetric versus size-asymmetric competition and growth partitioning among trees in forest stands along an ecological gradient in central Europe. Can. J. For. Res. 2010, 40, 370–384. [Google Scholar] [CrossRef]
- Reyer, C.; Lasch, P.; Mohren, G.M.J.; Sterck, F.J. Inter-specific competition in mixed forests of Douglas-fir (Pseudotsuga menziesii) and common beech (Fagus sylvatica) under climate change—A model-based analysis. Ann. For. Sci. 2010, 67, 805. [Google Scholar] [CrossRef]
- Ferrio, J.P.; Shestakova, T.A.; Castillo, J.; Voltas, J. Oak competition dominates in-terspecific interactions in growth and water-use efficiency in a mixed pine–oak medi-terranean forest. Forests 2021, 12, 1093. [Google Scholar] [CrossRef]
- Liu, Q.Y.; Xu, M.J.; Yuan, Y.; Wang, H.M. Interspecific competition for inorganic nitrogen between canopy trees and underlayer-planted young trees in subtropical pine plantations. For. Ecol. Manag. 2021, 494, 119331. [Google Scholar] [CrossRef]



| B. papyrifera | P. orientalis | |||
|---|---|---|---|---|
| p-Value | Pearson’s r | p-Value | Pearson’s r | |
| δ13C | 3.65 × 10−6 *** | 0.8648 | 2.20 × 10−7 *** | 0.9064 |
| N content | 0.0206 * | 0.5405 | 2.30 × 10−7 *** | −0.9059 |
| δ15N | 2.28 × 10−4 *** | 0.7634 | 0.0128 * | 0.5739 |
| P content | 0. 8149 | −0.0594 | 0.6883 | −0.1016 |
| PME | 6.24 × 10−5 *** | 0.8021 | 2.50 × 10−7 *** | 0.9048 |
| PDE | 2. 54 × 10−7 *** | 0.9046 | 6.07 × 10−9 *** | 0.9411 |
| gs | 2.79 × 10−4 *** | 0.7567 | 3.61 × 10−13 *** | 0.9828 |
| Leaf water content | 1.89 × 10−5 *** | 0.8316 | 4.71 × 10−7 *** | 0.8967 |
| p-Value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B. papyrifera | P. orientalis | |||||||||
| 1 d | 6 d | 11 d | 16 d | 21 d | 1 d | 6 d | 11 d | 16 d | 21 d | |
| δ13C | 0.689 | 0.964 | 0.440 | 0.012 | 0.655 | 0.689 | 0.964 | 0.440 | 0.012 | 0.655 |
| N content | 0.870 | 0.959 | 0.136 | 0.035 | 0.157 | 0.911 | 0.935 | 0.874 | 0.861 | 0.822 |
| δ15N | 0.675 | 0.865 | 0.059 | 0.006 | 0.072 | 0.676 | 0.619 | 0.878 | 0.001 | 0.800 |
| P content | 0.845 | 0.799 | 0.761 | 0.779 | 0.791 | 0.490 | 0.833 | 0.588 | 0.299 | 0.977 |
| PME | 0.426 | 0.091 | 0.264 | 0.568 | 0.587 | 0.542 | 0.611 | 0.036 | 0.710 | 0.617 |
| PDE | 0.815 | 0.817 | 0.058 | 0.013 | 0.334 | 0.815 | 0.817 | 0.058 | 0.013 | 0.334 |
| gs | 0.938 | 0.567 | 0.340 | 0.659 | 0.658 | 0.205 | 0.286 | 0.980 | 0.954 | 0.758 |
| Leaf water content | 0.873 | 0.930 | 0.010 | 0.040 | 0.073 | 0.585 | 0.951 | 0.010 | 0.212 | 0.695 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, K.; Wang, Y.; Wu, Y. Competition and Niche Differentiation of Water and Nutrients between Broussonetia papyrifera and Platycladus orientalis under Prolonged Drought Stress. Agronomy 2022, 12, 1489. https://doi.org/10.3390/agronomy12071489
Yao K, Wang Y, Wu Y. Competition and Niche Differentiation of Water and Nutrients between Broussonetia papyrifera and Platycladus orientalis under Prolonged Drought Stress. Agronomy. 2022; 12(7):1489. https://doi.org/10.3390/agronomy12071489
Chicago/Turabian StyleYao, Kai, Yanqing Wang, and Yanyou Wu. 2022. "Competition and Niche Differentiation of Water and Nutrients between Broussonetia papyrifera and Platycladus orientalis under Prolonged Drought Stress" Agronomy 12, no. 7: 1489. https://doi.org/10.3390/agronomy12071489






