1. Introduction
Plant cytoplasmic male sterility (CMS) is a natural, widespread phenomenon in higher plants that is characterized by maternal inheritance and the inability to generate pollen grains while still producing normal female gametes. CMS is an imperative driver of heterosis utilization in plants, as it enhances the purity of hybrid seeds by eliminating the need for artificial emasculation [
1,
2]. Certain nuclear genes, termed restorers-of-fertility (
Rf), can rescue CMS male fertility. CMS/
Rf systems have been successfully used for high-efficiency hybrid seed production in various crops [
3].
Maize is an important food and cash crop, and its demand is increasing around the world. The three-line system uses cytoplasmic male sterility lines, maintainer lines, and restorer lines, and it has significant application potential in commercial hybrid maize seed production [
4]. According to the characteristics of the mitochondrial genome and the pattern of fertility restoration, sterile maize cytoplasms can be divided into three major types: T (Texas), S(USDA), and C (Charrua) [
5,
6]. CMS-T sterile lines are no longer widely used in commercial hybrid seed production because of their susceptibility to
Bipolaris (
Helminthosporium)
maydis race T, which causes southern corn leaf blight [
7,
8]. S-type cytoplasmic sterility (CMS-S) is a form of gametophytic sterility, and its fertility can be easily affected by climatic factors [
9]. In comparison, C-type cytoplasmic sterility (CMS-C) is becoming one of the most attractive tools for hybrid maize-seed production due to its resistance to southern corn leaf blight and its stable sterility [
9]. Furthermore, CMS-C has a positive effect on grain yield [
10,
11].
The male sterility of CMS-C is caused by the chimeric mitochondrial gene
atp6c [
12], and its fertility restoration is controlled by multiple loci, including the major restorer-of-fertility (
Rf) and the quantitative trait locus (QTL). Currently, the major reported restorer genes for CMS-C include
Rf4 and
Rf5 [
13,
14].
Rf4 is located at the end of the short arm of chromosome 8, and
Rf5 is located at the long arm of chromosome 5 [
14,
15,
16]. Furthermore, an inhibitor gene, named
Rf-
I, which can constrain the function of
Rf5 but not
Rf4, was mapped to chromosome 7 [
17].
Rf5 has not been cloned yet, while
Rf4 has been proven to encode a bHLH transcription factor (GRMZM2G021276) [
18]. The allele of the
Rf4 restorer gene is also annotated as
Male Sterile23, an essential gene for tapetal development [
19]. Through the single-cell RNA sequencing of meiocytes and microspores, it was found that
Rf4 plays an important role in the maintenance of redox homeostasis in pollen formation [
20]. Comparative transcriptomic and proteomic analyses revealed that
Rf4 might boost energy generation to restore pollen fertility [
21,
22].
In addition to those major restorer genes, the male fertility recovery of maize CMS-C can be affected by several QTLs. Based on the study of weak restorer lines and translocation lines, some QTLs were found on the long arm of chromosome 3, the short arm of chromosome 4, the long arm of chromosome 5, and chromosome 9 [
23,
24]. Kohls et al. mapped three QTL loci with a high contribution rate that were distributed in bin 2.09, bin 3.06, and bin 7.03 [
25]. Recently, we detected a major locus,
qRf8-1, at the long arm of chromosome 8 in the inbred line A619 by QTL-seq [
26]. Additionally, we found that there may be minor restoration sites in the nuclear genome of male sterile lines. Although these loci cannot restore CMS-C when they exist alone, they can participate in fertility restoration through polymerization in the hybrid offspring of male sterile lines and tester lines [
27].
As a major restorer gene for CMS-C,
Rf4 has a powerful restoring ability and can restore almost all CMS-C lines. However, very few
Rf4-restorer lines have been identified so far, even though they have significant application potential in the utilization of CMS-C. Jaqueth et al. [
18] reported that the mutation of Y187F enables
Rf4 to restore the male fertility of CMS-C. Unexpectedly, we found that a few maize inbreds, such as 48-2 and Zi330, encoding phenylalanine at the functional site could not restore CMS-C lines [
27]. Therefore, the genotyping of the
Rf4 locus by analyzing its sequence features alone is not enough to determine the resilience of a maize line to CMS-C. In this study, to accurately identify strong
Rf4-restorer lines, various investigative methods, including sterility restoring–maintaining relationship determination, genetic analysis, molecular-marker mapping, and allelic tests were performed. Moreover, a Cleaved Amplified Polymorphism Sequences (CAPS) marker based on
Rf4 variations was developed for molecular-marker-assisted breeding.
4. Discussion
The male fertility restoration mechanism of CMS-C in maize is very complex. In addition to the role of restorer genes, the nuclear–cytoplasmic interaction and nuclear–nuclear interaction between the sterile and restorer lines may affect the fertility restoration of maize CMS-C [
27]. The complexity of the fertility restoration of maize CMS-C makes it difficult to identify strong restorer lines. Currently, restorer lines are generally obtained via the continuous backcrossing of restorer genes. In order to enrich the germplasm resources of the CMS-C restorer lines, the authors of this study identified four
Rf4-restorer lines with different pedigrees, DAN598, PHT77, 78551S, and LH212Ht, through a series of genetic analyses combined with molecular markers. The identification of these restorer lines should significantly facilitate maize-breeding programs utilizing the C-type cytoplasm.
The nuclear genome of male sterile lines may have an influence on the fertility restoration function of restorer lines [
6]. For example, the inbred line 18Bai can restore the male fertility of the CMS-C line C48-2, but not CHZS; by contrast, the inbred line Zi330 can restore CHZS, but not C48-2 [
27]. At the same time, according to the results of previous studies [
13,
16,
24,
35,
36], Fengke1 shows one restorer gene for the CMS-C lines, CHZS and CENES (C-Ernanersi), and two pairs of restorer genes for CMo17 and C237. Similarly, Guang10-2 exhibits one restorer gene for CHZS and CENES and two
Rf genes for CMo17. In addition, the major fertility-restoring gene
Rf5 can only restore CMS-C lines lacking
Rf5-I [
17]. These results show that the restoring ability of restorer lines may be affected by the nuclear background of the sterile line. Hence, in the process of breeding or using restorer lines, the influence of the nuclear background of male sterile lines should be considered. In this experiment, DAN598, PHT77, 78551S, and LH212Ht were shown to completely restore the pollen fertility of CHZS, but their restoration function for other CMS-C lines may change. In future research, the recovery ability of these restorer lines needs to be further investigated.
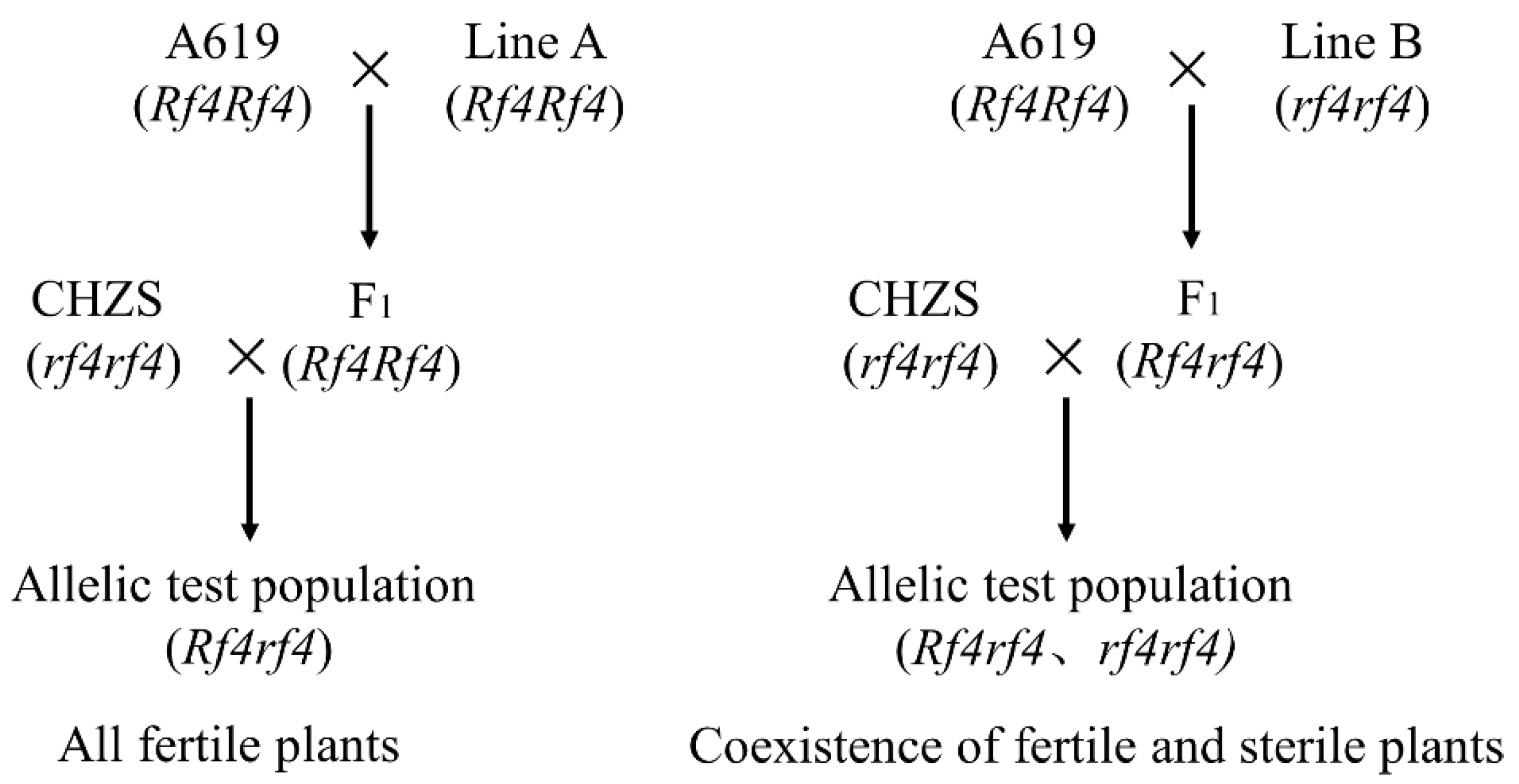
In this study, A619 (
Rf4Rf4) was used to determine the genotype alleles at the
Rf4 locus. The results of the work of Chen et al. [
13], Tang et al. [
14], and Sisco [
37] all showed that
Rf4 is the only restorer gene in A619. However, in our previous study, we found that A619 had two pairs of restorer genes in the fertility restoration of CMS-C C48-2 [
38]. According to Huang et al. [
36], the dominant restorer gene in A619 may be located on chromosome 7, and the inbred line A619 may possess another dominant restorer gene in addition to
Rf4. Furthermore, Sisco [
37] not only mapped the restorer gene
Rf4 to chromosome 8 but also inferred that
Rf4 had a duplicate on chromosome 3 in A619. Because A619 may contain an extra dominant restorer gene, its allelic test alone cannot directly identify
Rf4-containing restorer lines. Therefore, in this research, several F
2 populations were further used to estimate the number of restorer genes in DAN598, PHT77, 78551S, and LH212Ht to CHZS, and molecular markers were used to map them. This experimental design ensured that the fertility restoration of CHZS by the four materials was only controlled by
Rf4. The allelic test populations of the seven inbred lines (K10, PHW79, L127, L139, PHJ75, PHN82, and PHR55) showed full male fertility. This indicates that they contain dominant restorer genes; however, the restorer gene allele in A619 must be determined.
Similar to the (C48-2 × A619)F
2 population we observed [
27], the (CHZS × A619)F
2 in this study showed different male-fertility grade distribution patterns at different sites. Tracy et al. [
39] found that the proportion of fertile plants in the same cross population was significantly different in different years. Liu et al. [
40] pointed out that cool and high-humidity external conditions are more conducive to the fertility restoration of CMS-C stamens. Chen et al. [
41] argued that weak QTLs are more vulnerable to external conditions than major restorer genes. combined with our results, these studies indicate that the fertility recovery of CMS-C can be affected by environmental factors.
In this study, by comparing the
Rf4 genomic sequence between several male sterile lines (
rf4rf4) and restorer lines (
Rf4Rf4), we found that there are four amino acid variations in the coding region of
Rf4, of which the variation of the S1596 locus (TTT/TAC) tends to be more critical. The S1596 site encodes phenylalanine (TTT) in five restorer lines, and it encodes tyrosine (TAC) in four CMS-C lines. These results are consistent with previous reports and suggest that this amino acid residue encoded by S1596 is located within the core of the four-helix bundle, a region that is critical for stabilizing dimer conformation and influencing interaction partner selection [
18]. Functional CAPS markers are especially helpful for marker-assisted selection, and they are widely used in the breeding of various crops and the rapid selection of novel restorer lines [
42,
43]. With the S1596 variations, we successfully developed a CAPS molecular marker within
Rf4. Compared to the TaqMan marker developed by Jaqueth et al. [
18], the CAPS marker tends to be more rapid, economical and reliable in practical application. Based on our results, these molecular markers can be used for the preliminary screening of
Rf4-restorer lines; cross tests would then be required to further confirm the male-fertility-restoring ability of tester lines.