Effect of Molasses Application Alone or Combined with Trichoderma asperellum T-34 on Meloidogyne spp. Management and Soil Microbial Activity in Organic Production Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Effect of Molasses on Meloidogyne spp. Reproduction and Soil Microbial Density
2.2. Effect of Molasses Alone or Combined with T34 Biocontrol on Meloidogyne spp. Reproduction and Soil Microbial Activity
2.3. Statistical Analyses
3. Results
3.1. Effect of Molasses on Meloidogyne spp. Reproduction and Soil Microbial Density
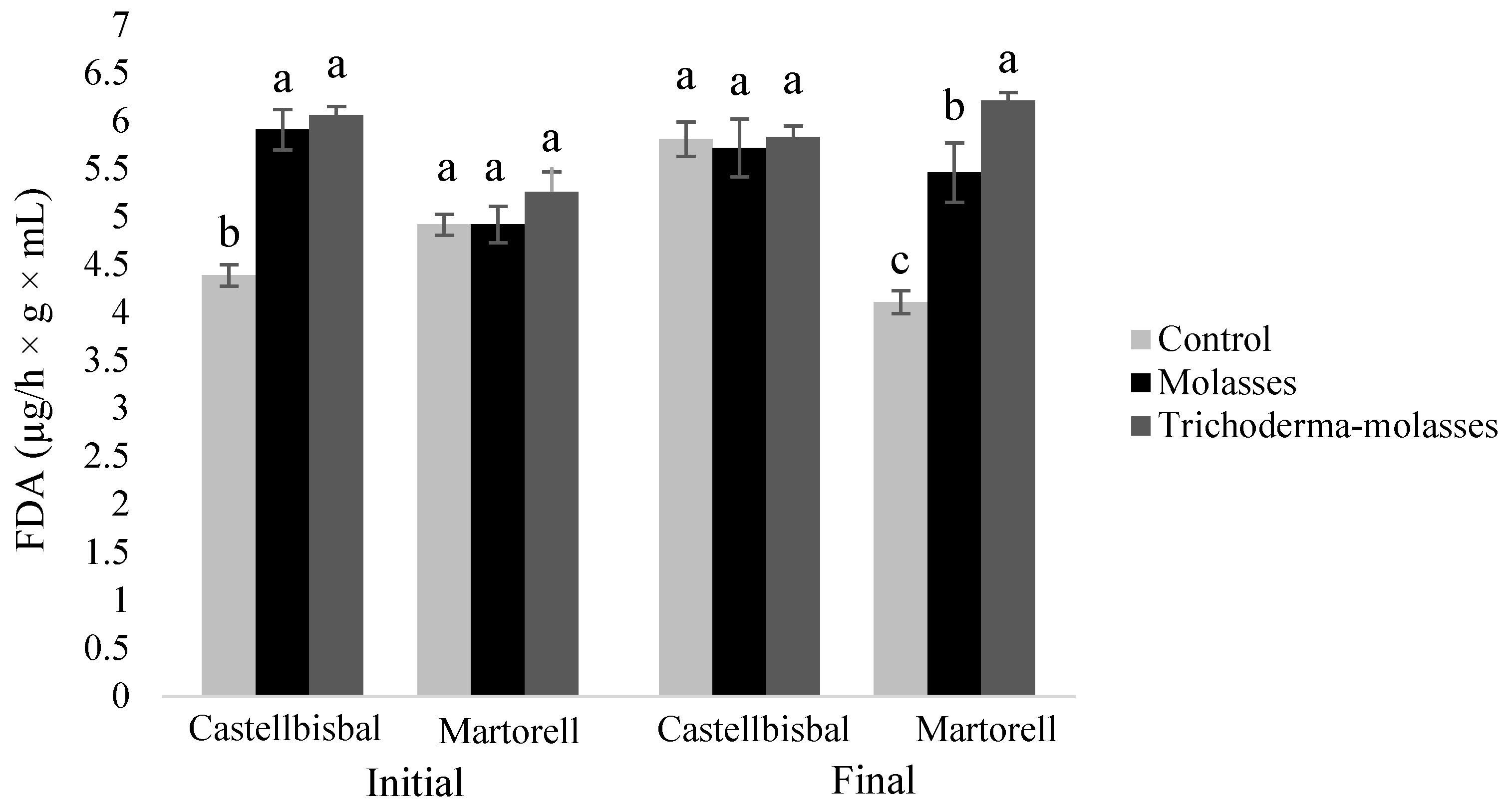
3.2. Effect of Molasses Alone or Combined with T34 Biocontrol on Meloidogyne spp. Reproduction and Soil Microbial Activity
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, J.T.; Haegeman, A.; Danchin, E.G.J.; Gaur, H.S.; Helder, J.; Jones, M.G.K.; Kikuchi, T.; Manzanilla-López, R.; Palomares-Rius, J.E.; Wesemael, W.M.L.; et al. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef]
- Hallmann, J.; Meressa, B.H. Nematode parasites of vegetables. In Plant Parasitic Nematodes in Subtropical and Tropical Agriculture; Sikora, R.A., Coyne, D., Hallmann, J., Timper, P., Eds.; CABI International: Wallingford, UK, 2018; pp. 346–410. [Google Scholar] [CrossRef]
- López-Gómez, M.; Giné, A.; Vela, M.D.; Ornat, C.; Sorribas, F.J.; Talavera, M.; Verdejo-Lucas, S. Damage functions and thermal requirements of Meloidogyne javanica and Meloidogyne incognita on watermelon. Ann. Appl. Biol 2014, 165, 466–473. [Google Scholar] [CrossRef]
- Vela, M.D.; Giné, A.; López-Gómez, M.; Sorribas, F.J.; Ornat, C.; Verdejo-Lucas, S.; Talavera, M. Thermal time requirements of root-knot nematodes on zucchini-squash and population dynamics with associated yield losses on spring and autumn cropping cycles. Eur. J. Plant Pathol. 2014, 140, 481–490. [Google Scholar] [CrossRef]
- Giné, A.; López-Gómez, M.; Vela, M.D.; Ornat, C.; Talavera, M.; Verdejo-Lucas, S.; Sorribas, F.J. Thermal requirements and population dynamics of root-knot nematodes on cucumber and yield losses under protected cultivation. Plant Pathol. 2014, 63, 1446–1453. [Google Scholar] [CrossRef]
- Giné, A.; Sorribas, F.J. Quantitative approach for the early detection of selection for virulence of Meloidogyne incognita on resistant tomato in plastic greenhouses. Plant Pathol. 2017, 66, 1338–1344. [Google Scholar] [CrossRef] [Green Version]
- Expósito, A.; Pujolá, M.; Achaerandio, I.; Giné, A.; Escudero, N.; Fullana, A.M.; Cunquero, M.; Loza-Alvarez, P.; Sorribas, F.J. Tomato and melon Meloidogyne resistant rootstocks improve crop yield but melon fruit quality is influenced by the cropping season. Front Plant Sci. 2020, 1742. [Google Scholar] [CrossRef]
- Djian-Caporalino, C. Root-knot nematodes (Meloidogyne spp.), a growing problem in French vegetable crops. EPPO Bull. 2012, 42, 127–137. [Google Scholar] [CrossRef]
- Talavera, M.; Sayadi, S.; Chirosa-Rios, M.; Salmeron, T.; Flor-Peregrin, E.; Verdejo-Lucas, S. Perception of the impact of root-knot nematode-induced diseases in horticultural protected crops of south-eastern Spain. Nematology 2012, 14, 517–527. [Google Scholar] [CrossRef]
- Sorribas, F.J.; Djian-Caporalino, C.; Mateille, T. Nematodes. In Integrated Pest and Disease Management in Greenhouse Crops; Gullino, M.L., Albajes, R., Nicot, P.C., Eds.; Springer: Cham, Switzerland, 2020; pp. 147–174. [Google Scholar] [CrossRef]
- EUROSTAT. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Organic_farming_statistics (accessed on 3 March 2022).
- Briar, S.S.; Wichman, D.; Reddy, G.V. Plant-parasitic nematode problems in organic agriculture. In Organic Farming for Sustainable Agriculture; Nandwani, D., Ed.; Springer: Cham, Switzerland, 2016; pp. 107–122. [Google Scholar] [CrossRef]
- Hallmann, J.; Frankenberg, A.; Paffrath, A.; Schmidt, H. Occurrence and importance of plant-parasitic nematodes in organic farming in Germany. Nematology 2007, 9, 869–879. [Google Scholar]
- Akhtar, M.; Malik, A. Roles of organic soil amendments and soil organisms in the biological control of plant-parasitic nematodes: A review. Bioresour. Technol. 2000, 74, 35–47. [Google Scholar] [CrossRef]
- Oka, Y. Mechanisms of nematode suppression by organic soil amendments—A review. App. Soil Ecol. 2010, 44, 101–115. [Google Scholar] [CrossRef]
- McSorley, R. Overview of organic amendments for management of plant-parasitic nematodes, with case studies from Florida. J. Nematol. 2011, 43, 69. [Google Scholar]
- Renco, M. Organic amendments of soil as useful tools of plant parasitic nematodes control. Helminthologia 2013, 50, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Kabana, R.; King, P.S. Use of mixtures of urea and blackstrap molasses for control of root-knot nematodes in soil. Nematropica 1980, 10, 38–44. [Google Scholar]
- Vawdrey, L.L.; Stirling, G.R. Control of root-knot nematode (Meloidogyne javanica) on tomato with molasses and other organic amendments. Australas. Plant Pathol. 1997, 26, 179–187. [Google Scholar] [CrossRef]
- Walker, G.E. Effects of organic amendments, fertilisers and fenamiphos on parasitic and free-living nematodes, tomato growth and yield. Nematol. Mediterr. 2007, 35, 131–136. [Google Scholar]
- Pattison, A.B.; Badcock, K.; Sikora, R.A. Influence of soil organic amendments on suppression of the burrowing nematode, Radopholus similis, on the growth of bananas. Australas. Plant Pathol. 2011, 40, 385–396. [Google Scholar] [CrossRef]
- Khan, M.; Saifullah, I.A.; Abbas, A.; Khan, R.A. Effect of sugarcane molasses and ash on the organic management of root-knot nematode Meloidogyne javanica in tomato. J. Entomol. Zool. Stud. 2016, 4, 178–183. [Google Scholar]
- Baños, Y.S.; Concepción, A.D.B.; Lazo, R.C.; González, I.A.; Morejón, L.P. Efecto de enmiendas orgánicas y Trichoderma spp. en el manejo de Meloidogyne spp. Rev. Bras. Agroecol. 2010, 5, 224–233. [Google Scholar]
- Butler, D.M.; Kokalis-Burelle, N.; Muramoto, J.; Shennan, C.; McCollum, T.G.; Rosskopf, E.N. Impact of anaerobic soil disinfestation combined with soil solarization on plant–parasitic nematodes and introduced inoculum of soilborne plant pathogens in raised-bed vegetable production. Crop Protec. 2012, 39, 33–40. [Google Scholar] [CrossRef]
- Papavizas, G.C.; Dunn, M.T.; Lewis, J.A.; Beagle-Ristaino, J. Liquid fermentation technology for experimental production of biocontrol fungi. Phytopathology 1984, 74, 1171–1175. [Google Scholar] [CrossRef]
- Khan, M.R.; Majid, S.; Mohidin, F.A.; Khan, N. A new bioprocess to produce low cost powder formulations of biocontrol bacteria and fungi to control fusarial wilt and root-knot nematode of pulses. Biol. Control 2011, 59, 130–140. [Google Scholar] [CrossRef]
- Sharon, E.; Chet, I.; Spiegel, Y. Improved attachment and parasitism of Trichoderma on Meloidogyne javanica in vitro. Eur. J. Plant Pathol. 2009, 123, 291–299. [Google Scholar] [CrossRef]
- Medeiros, H.A.D.; Araújo Filho, J.V.D.; Freitas, L.G.D.; Castillo, P.; Rubio, M.B.; Hermosa, R.; Monte, E. Tomato progeny inherit resistance to the nematode Meloidogyne javanica linked to plant growth induced by the biocontrol fungus Trichoderma atroviride. Sci. Rep 2017, 7, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Medina, A.; Fernandez, I.; Lok, G.B.; Pozo, M.J.; Pieterse, C.M.; van Wees, S.C. Shifting from priming of salicylic acid-to jasmonic acid-regulated defences by Trichoderma protects tomato against the root knot nematode Meloidogyne incognita. New Phytol. 2017, 213, 1363–1377. [Google Scholar] [CrossRef] [Green Version]
- Pocurull, M.; Fullana, A.M.; Ferro, M.; Valero, P.; Escudero, N.; Saus, E.; Gabaldón, T.; Sorribas, F.J. Commercial formulates of Trichoderma induce systemic plant resistance to Meloidogyne incognita in tomato and the effect is additive to that of the Mi-1.2 resistance gene. Front. Microbiol. 2020, 10, 3042. [Google Scholar] [CrossRef]
- Whitehead, A.G.; Hemming, J.R. A comparison of some quantitative methods for extracting small vermiform nematodes from soil. Ann. App. Biol. 1965, 55, 25–38. [Google Scholar] [CrossRef]
- Hussey, R.S.; Barker, K.R. Comparison of methods of collecting inocula of Meloidogyne spp.; including a new technique. Plant Dis. Rep. 1973, 57, 1025–1028. [Google Scholar]
- Ferris, H.; Roberts, P.A.; Thomason, I.J. Nematodes. In Integrated Pest Management for Tomatoes; University of California Statewide Integrated Pest Management Project, Ed.; Division of Agriculture and Natural Resources, University of California: Oakland, CA, USA, 1985; pp. 60–65. [Google Scholar]
- Zeck, W.M. Rating scheme for field evaluation of root-knot nematode infestations. Pflanzenschutz Nachr. 1971, 24, 141–144. [Google Scholar]
- Giné, A.; Bonmatí, M.; Sarro, A.; Stchiegel, A.; Valero, J.; Ornat, C.; Fernández, C.; Sorribas, F.J. Natural occurrence of fungal egg parasites of root-knot nematodes, Meloidogyne spp. in organic and integrated vegetable production systems in Spain. BioControl 2013, 58, 407–416. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Llorca, L.V.; Duncan, J.M. New media for the estimation of fungal infection in eggs of the cereal cyst nematode, Heterodera avenae Woll. Nematologica 1986, 32, 486–489. [Google Scholar] [CrossRef]
- Fernández, C.; Rodrıguez-Kábana, R.; Warrior, P.; Kloepper, J.W. Induced soil suppressiveness to a root-knot nematode species by a nematicide. Biol. Control 2001, 22, 103–114. [Google Scholar] [CrossRef]



| Sites | |||||
|---|---|---|---|---|---|
| Soil Characteristics | Sant Vicenç Dels Horts | Torrelles | Martorell | Castellbisbal | Begues |
| pH | 8.2 | 7.9 | 7.9 | 8.1 | 8.0 |
| E.C (dS m−1) | 0.44 | 0.43 | 0.61 | 0.22 | 0.33 |
| Organic Matter % | 1.8 | 2.5 | 3.0 | 3.3 | 3.9 |
| Texture (USDA) | Loam | Sandy clay loam | Sandy loam | Loam | Clay loam |
| N-NO3 (mg kg−1) | 20 | 11 | 11 | 12 | 34.7 |
| P (mg kg−1) | 21 | 88 | 83 | 142 | 50 |
| K (mg kg−1) | 303 | 369 | 474 | 395 | 488 |
| Mg (mg kg−1) | 166 | 197 | 285 | 199 | 281 |
| Ca (mg kg−1) | 3088 | 2332 | 2814 | 1679 | 2429 |
| Na (mg kg−1) | 152 | 84 | 229 | 87 | 59 |
| Site | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | Treatment | Sant Vicenç Dels Horts | Torrelles | Martorell | Begues | ||||
| Genera 1 | Genera 2 | Genera 1 | Genera 2 | Genera 1 | Genera 2 | Genera 1 | Genera 2 | ||
| DSW (g) | Control | 6.5 ± 0.2 | 11.9 ± 3.2 | 9.3 ± 1.0 | 18.2 ± 1.4 | 9.9 ± 0.8 | 19.6 ± 0.9 | 12.4 ± 1.0 | 16.6 ± 0.7 |
| Molasses | 7.0 ± 0.6 | 12.3 ± 1.2 | 10.1 ± 0.7 | 18.1 ± 0.9 | 11.3 ± 0.3 | 19.6 ± 0.7 | 9.8 ± 0.8 * | 17.2 ± 0.5 | |
| FRW (g) | Control | 2.4 ± 0.3 | 7.3 ± 1.0 | 4.4 ± 1.3 | 25.0 ± 12 | 3.6 ± 0.6 | 12.0 ± 2.1 | 6.4 ± 1.1 | 21.0 ± 5.2 |
| Molasses | 3.6 ± 0.7 | 11.7 ± 1.1 * | 6.7 ± 1.6 | 26.9 ± 6.0 | 5.9 ± 0.9 * | 10.5 ± 1.6 | 9.5 ± 2.2 | 18.2 ± 1.8 | |
| GI | Control | 3.0 ± 0.0 | 3.8 ± 0.6 | 2.8 ± 0.2 | 4.4 ± 0.4 | 2.8 ± 0.2 | 3.6 ± 0.2 | 3.8 ± 0.2 | 5.0 ± 0.3 |
| Molasses | 3.2 ± 0.2 | 5.4 ± 0.2 * | 3.4 ± 0.3 | 4.6 ± 0.4 | 2.4 ± 0.2 | 3.4 ± 0.2 | 3.2 ± 0.2 | 5.4 ± 0.2 | |
| Eggs + J2 (×104) plant−1 | Control | 3.4 ± 0.3 | 14 ± 6.4 | 8.5 ± 0.6 | 56 ± 43 | 7.6 ± 0.8 | 47 ± 13 | 13 ± 1.3 | 88 ± 38 |
| Molasses | 6.0 ± 1.1 * | 60 ± 10 * | 11 ± 2.6 | 144 ± 22 | 8.0 ± 1.4 | 26.0 ± 5.3 | 10 ± 1.6 | 134 ± 26 | |
| Egg parasitism (%) | Control | 0 ± 0 | 2.3 ± 1.3 | 5.3 ± 1.9 | 0.4 ± 0.4 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 2.9 ± 1.5 |
| Molasses | 2.7 ± 0.6 | 0.8 ± 0.8 | 5.0 ± 0.5 | 0 ± 0 | 2.9 ± 1.5 | 1.9 ± 1.1 | 0 ± 0 | 0 ± 0 | |
| CFU bacteria (×104) | Control | 36 ± 5.0 | 72 ± 24 | 95 ± 26 | 79 ± 2.6 | 47 ± 0.1 | 77 ± 11 | 55 ± 26 | 27 ± 23 |
| Molasses | 34 ± 0.5 | 46 ± 7.0 | 48 ± 3.0 | 133 ± 56 | 30 ± 2 | 52 ± 23 | 29 ± 6 | 43 ± 7 | |
| CFU fungi (×103) | Control | 1.4 ± 0.6 | 3.6 ± 0.2 | 5.0 ± 0.4 | 8.8 ± 0.5 | 4.0 ± 0.3 | 7.9 ± 1.5 | 12 ± 0.01 | 8.8 ± 0.8 |
| Molasses | 3.0 ± 0.2 | 4.8 ± 0.2 | 3.3 ± 0.6 | 7.4 ± 0.6 | 7.8 ± 0.7 | 8.3 ± 3.2 | 11 ± 0.01 | 10 ± 3.7 | |
| Parameter | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Field | Treatment | Nematode Density (J2 250 cm−3 soil) | Galling Index | Nematode Reproduction (×103) † | Egg Parasitism (%) | ||||
| Pi | Pf | Tomato | Lettuce | Tomato | Lettuce | Tomato | Lettuce | ||
| Sant Vicenç dels Horts | Control | 349 ± 10.2 | 651 ±79 * | 4.0 ± 0.1 | 3.5 ± 0.1 * | 1983 ± 132 * | 11 ± 1 * | 5.6 ± 1.1 | 8.0 ± 1.4 |
| Molasses | 378 ± 14.8 | 434 ± 49 | 3.7 ± 0.1 | 3.0 ± 0.1 | 1284 ± 103 | 6 ± 0.4 | 6.5 ± 1.0 | 9.4 ± 1.6 | |
| Torrelles | Control | 627 ± 162 | 164 ± 41 | 4.8 ± 0.3 | 2.5 ± 0.5 | 66 ± 23 * | 3 ± 0.4 * | 3.9 ± 1.5 | 2.4 ± 0.9 |
| Molasses | 591 ± 116 | 188 ± 64 | 4.9 ± 0.3 | 3.1 ± 0.3 | 221 ± 57 | 6 ± 1 | 4.9 ± 1.1 | 6.8 ± 1.8 | |
| Martorell | Control | 154 ± 32 | 74 ± 35 | 3.8 ± 0.3 | 2.6 ± 0.2 | 254 ± 83 * | 13 ± 4 | 3.7 ± 1.2 | 4.4 ± 1.1 |
| Molasses | 147 ± 90 | 11 ± 5 | 2.8 ± 0.5 | 3.3 ± 0.7 | 35 ± 11 | 28 ± 13 | 0.1 ± 0.1 | 0 ± 0 | |
| Begues | Control | 23 ± 8 | 1305 ± 368 | 2.4 ± 0.4 | 5.6 ± 0.7 | 7 ± 3 | 32 ± 3 | nd | 2.4 ± 0.8 |
| Molasses | 14 ± 4 | 427 ± 185 | 2.0 ± 0.2 | 4.2 ± 0.6 | 3 ± 1 | 20 ± 4 | nd | 4.4 ± 1.4 | |
| Parameter | |||||
|---|---|---|---|---|---|
| Field | Treatment | Bacterial Density (CFU × 105) | Fungal Density (CFU × 103) | ||
| Initial | Final | Initial | Final | ||
| Sant Vicenç dels Horts | Control | 22.7 ± 5.4 | 51.8 ± 29.1 | 10.5 ± 4.3 * | 41.3 ± 4.4 |
| Molasses | 29.5 ± 6.3 | 29 ± 5.6 | 34.8 ± 3.0 | 31.7 ± 3.2 | |
| Torrelles | Control | 12.5 ± 3.3 | 13 ± 3.2 | 24.5 ± 2.2 * | 16.5 ± 2.0 |
| Molasses | 20.0 ± 2.0 | 10.4 ± 4.0 | 46.0 ± 6.8 | 11.0 ± 6.6 | |
| Martorell | Control | 2.7± 0.8 | 2.0 ± 0.8 | 2.7 ± 1.4 * | 10.3 ± 1.0 * |
| Molasses | 3.1 ± 1.6 | 1.9 ± 0.8 | 10.5 ± 2.1 | 2.0 ± 0.8 | |
| Begues | Control | 4.1 ± 1.0 | 3.0 ± 1.0 | 8.0 ± 1.4 | 18.0 ± 3.7 |
| Molasses | 2.3 ± 0.6 | 5.8 ± 1.8 | 9.9 ± 2.7 | 15.5 ± 1.0 | |
| Parameter | |||||||
|---|---|---|---|---|---|---|---|
| Field | Treatment | Nematode Density (J2 250 cm−3 soil) | Galling Index | Nematode Reproduction (×103) * | |||
| Pi | Pf | Tomato | Lettuce | Tomato | Lettuce | ||
| Castellbisbal | Control | 46 ± 13a | 513 ± 182a | 4.9 ± 0.5a | 4.0 ± 0.1a | 1455 ± 249a | 133 ± 8a |
| Molasses | 61 ± 17a | 10 ± 4b | 3.4 ± 0.4b | 3.2 ± 0.4b | 556 ± 196b | 63 ± 10b | |
| T34-molasses | 80 ± 40a | 178 ± 103ab | 4.3 ± 0.5ab | 4.1 ± 0.3a | 765 ± 208ab | 60 ± 9b | |
| Martorell | Control | 111 ± 50a | 57 ± 14a | 2.5 ± 0.4a | 2.4 ± 0.3a | 105 ± 38a | 8 ± 1a |
| Molasses | 158 ± 38a | 16 ± 9a | 2.0 ± 0.4a | 2.5 ± 0.2a | 102 ± 29a | 7 ± 2a | |
| T-34-molasses | 117 ± 60a | 26 ± 16a | 2.9 ± 0.2a | 2.1 ± 0.3a | 124 ± 30a | 6 ± 3a | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Expósito, A.; García, S.; Giné, A.; Escudero, N.; Herranz, S.; Pocurull, M.; Lacunza, A.; Sorribas, F.J. Effect of Molasses Application Alone or Combined with Trichoderma asperellum T-34 on Meloidogyne spp. Management and Soil Microbial Activity in Organic Production Systems. Agronomy 2022, 12, 1508. https://doi.org/10.3390/agronomy12071508
Expósito A, García S, Giné A, Escudero N, Herranz S, Pocurull M, Lacunza A, Sorribas FJ. Effect of Molasses Application Alone or Combined with Trichoderma asperellum T-34 on Meloidogyne spp. Management and Soil Microbial Activity in Organic Production Systems. Agronomy. 2022; 12(7):1508. https://doi.org/10.3390/agronomy12071508
Chicago/Turabian StyleExpósito, Alejandro, Sergi García, Ariadna Giné, Nuria Escudero, Sandra Herranz, Miriam Pocurull, Albert Lacunza, and Francisco Javier Sorribas. 2022. "Effect of Molasses Application Alone or Combined with Trichoderma asperellum T-34 on Meloidogyne spp. Management and Soil Microbial Activity in Organic Production Systems" Agronomy 12, no. 7: 1508. https://doi.org/10.3390/agronomy12071508
APA StyleExpósito, A., García, S., Giné, A., Escudero, N., Herranz, S., Pocurull, M., Lacunza, A., & Sorribas, F. J. (2022). Effect of Molasses Application Alone or Combined with Trichoderma asperellum T-34 on Meloidogyne spp. Management and Soil Microbial Activity in Organic Production Systems. Agronomy, 12(7), 1508. https://doi.org/10.3390/agronomy12071508








