Spermidine Modify Antioxidant Activity in Cucumber Exposed to Salinity Stress
Abstract
:1. Introduction
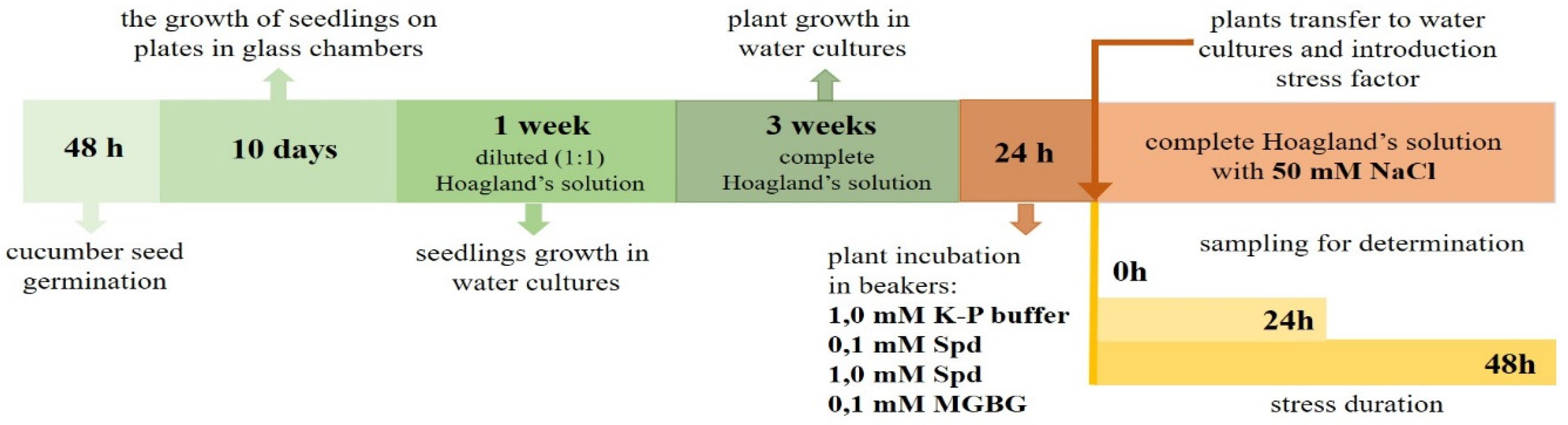
2. Materials and Methods
Plant Material and Treatment
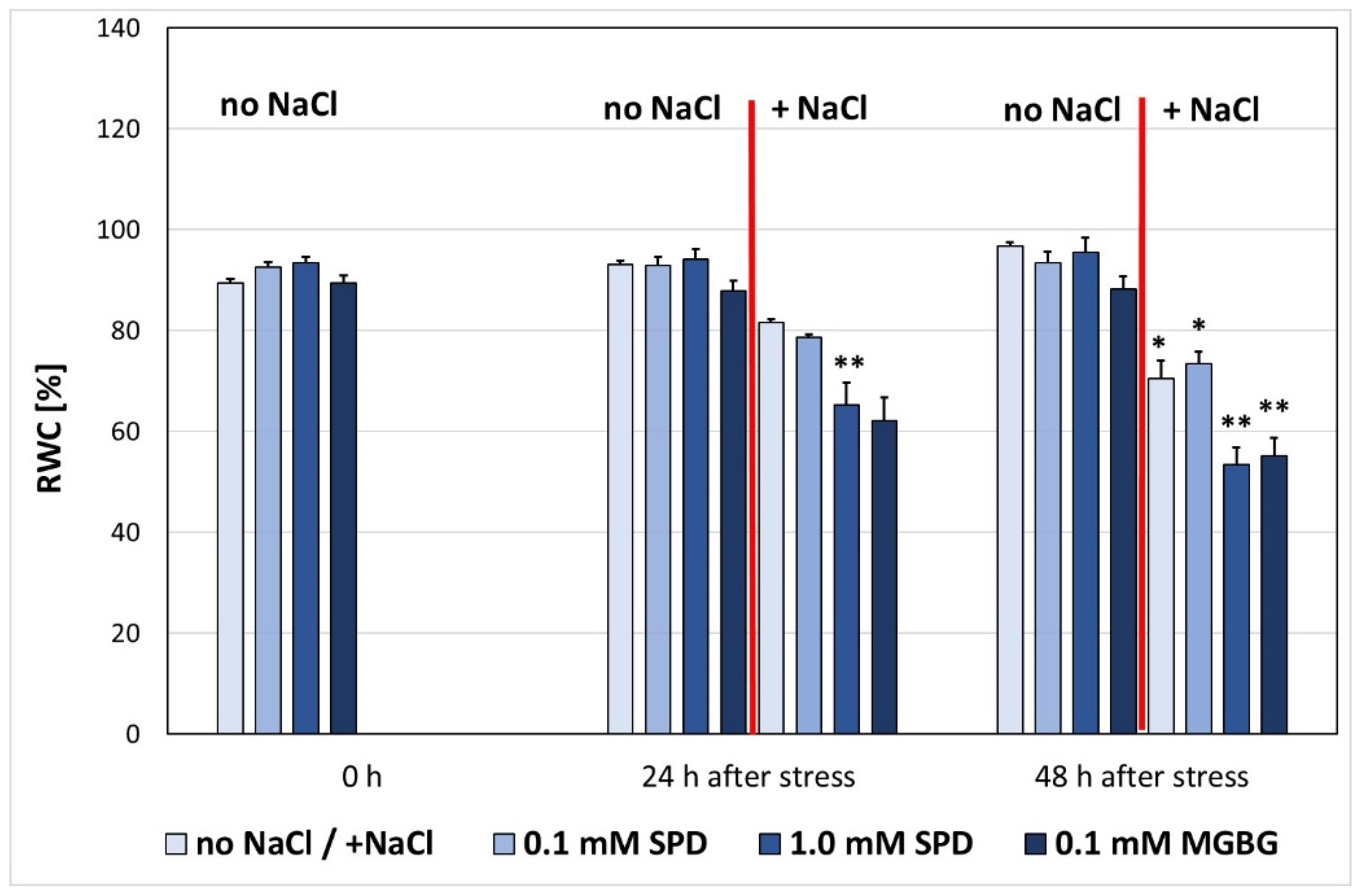
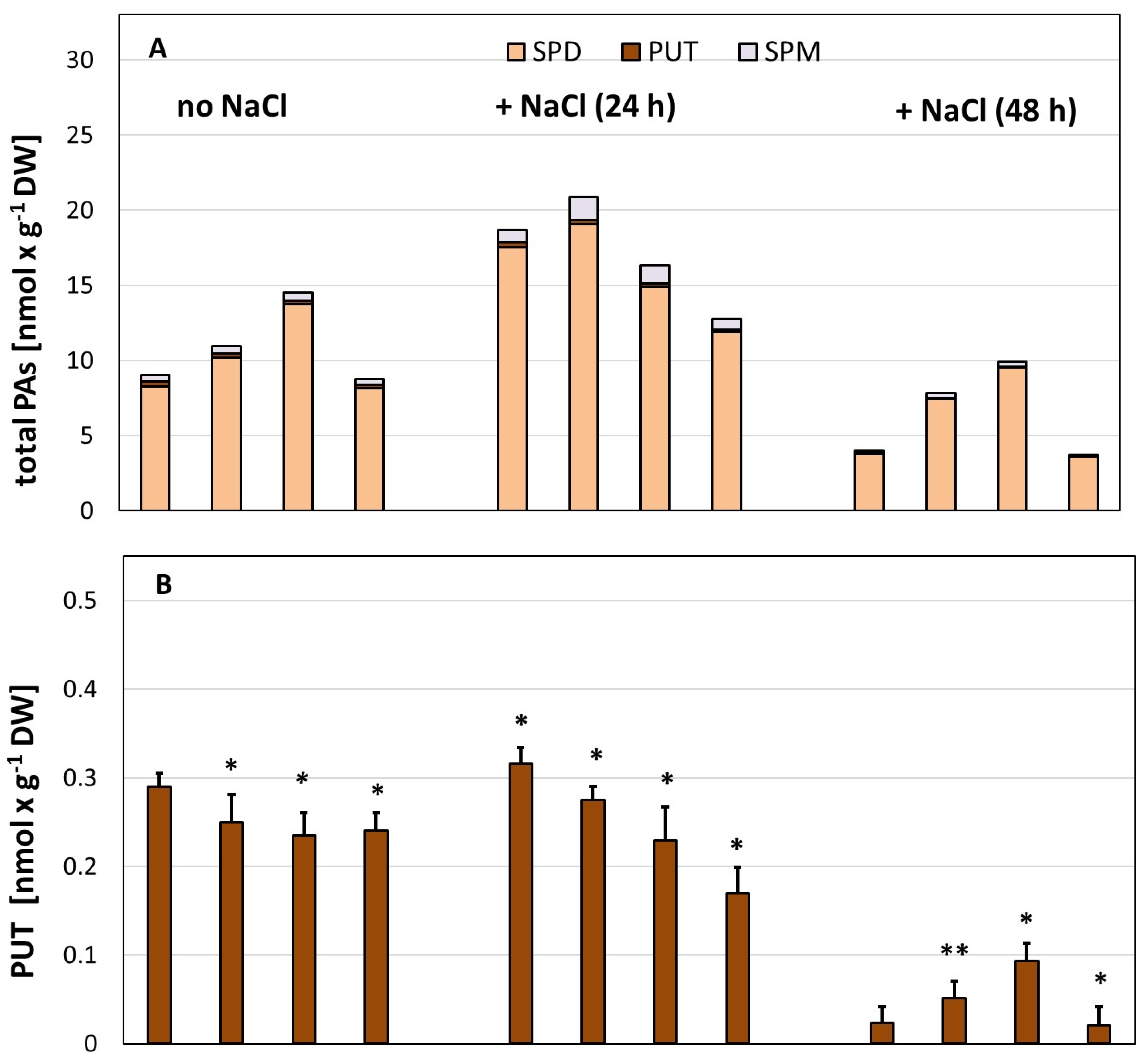
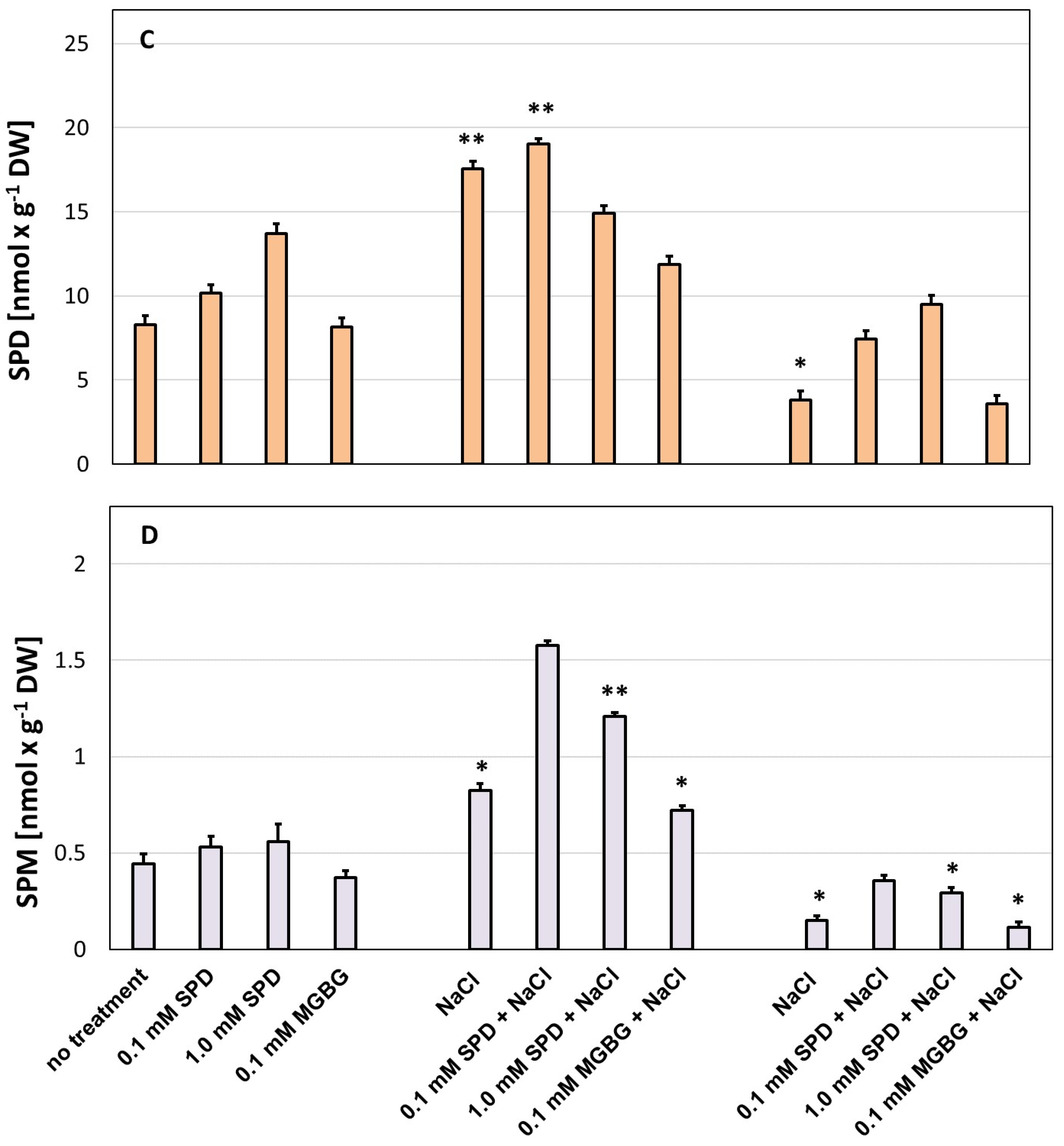
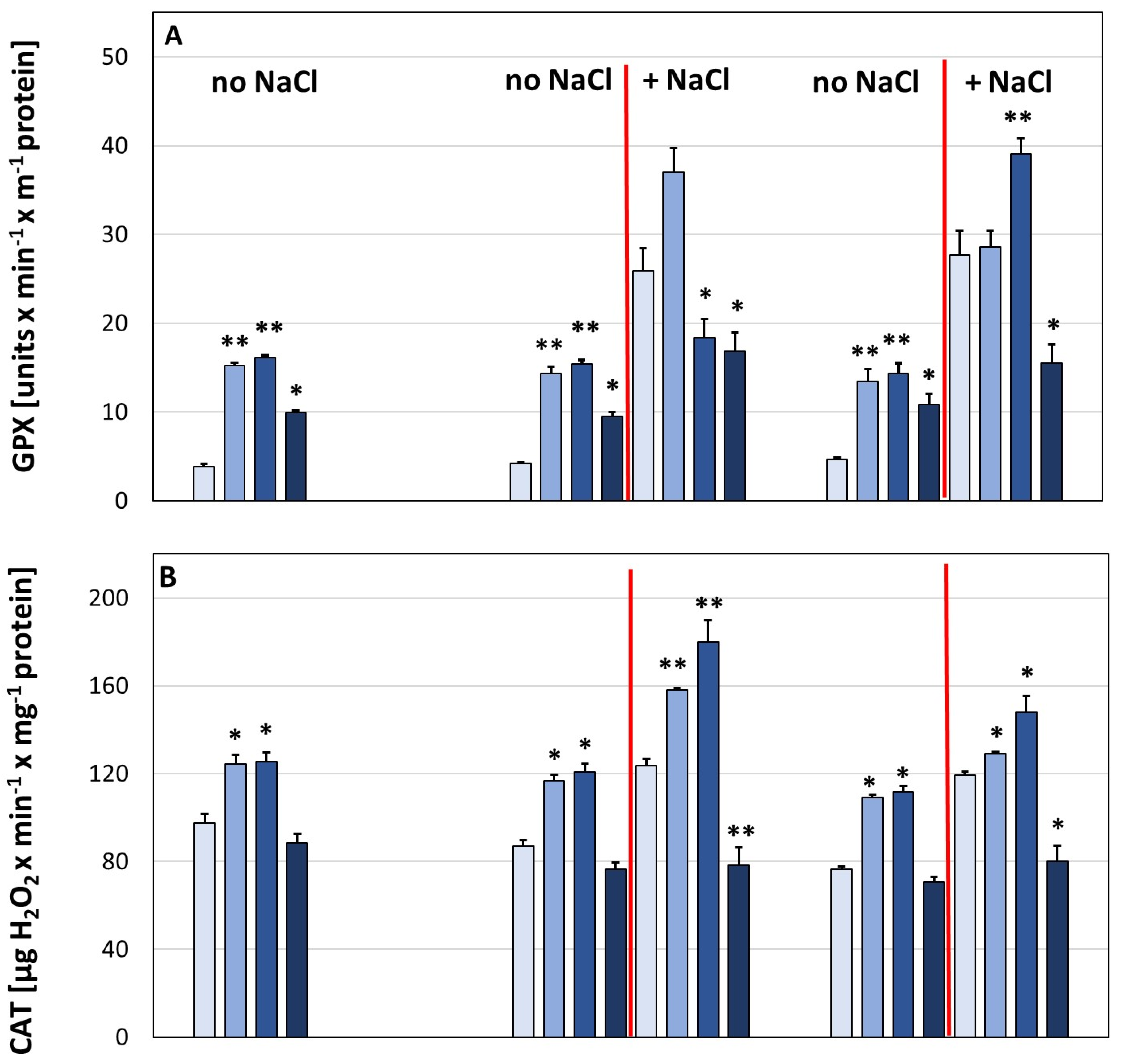
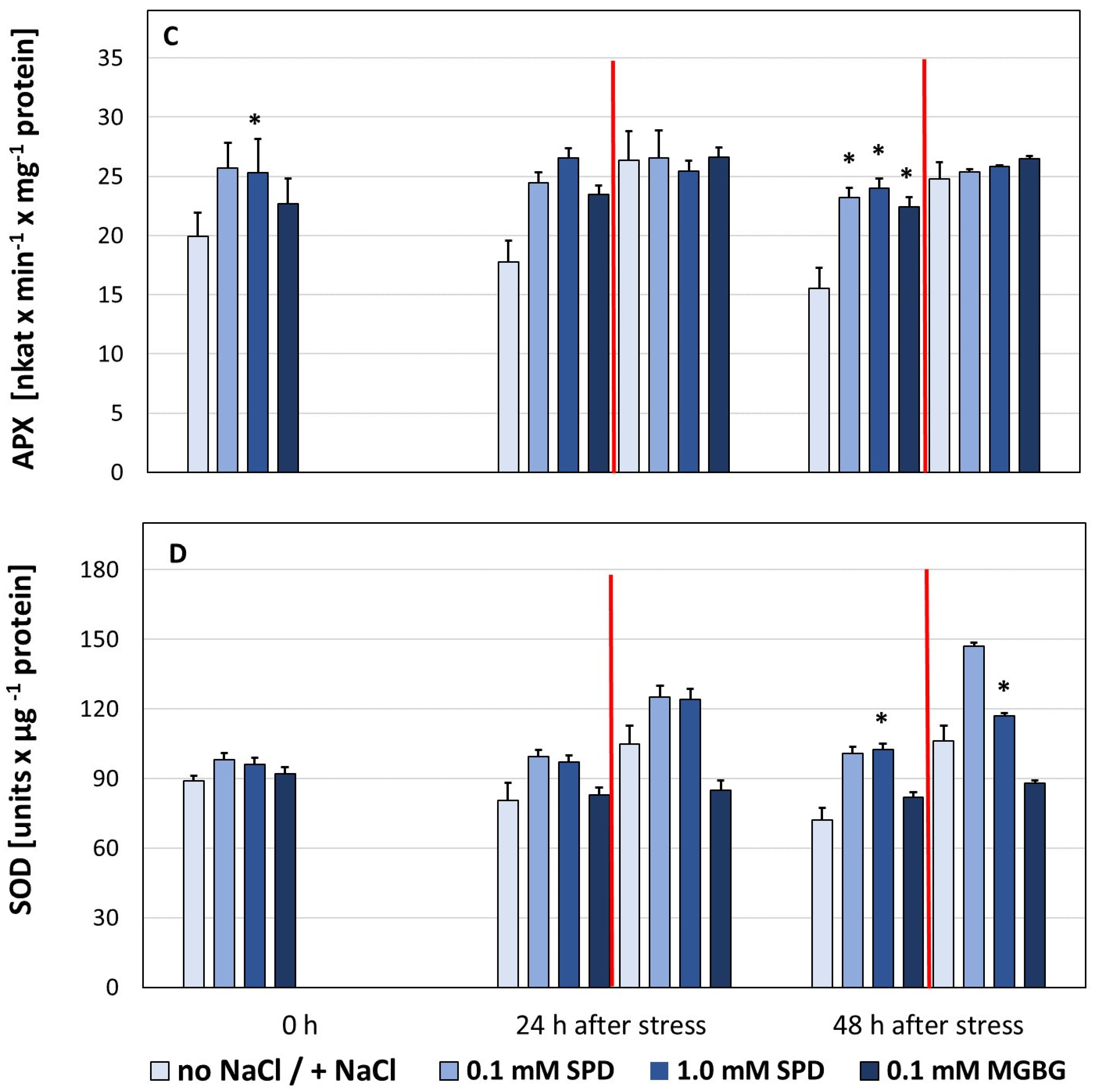
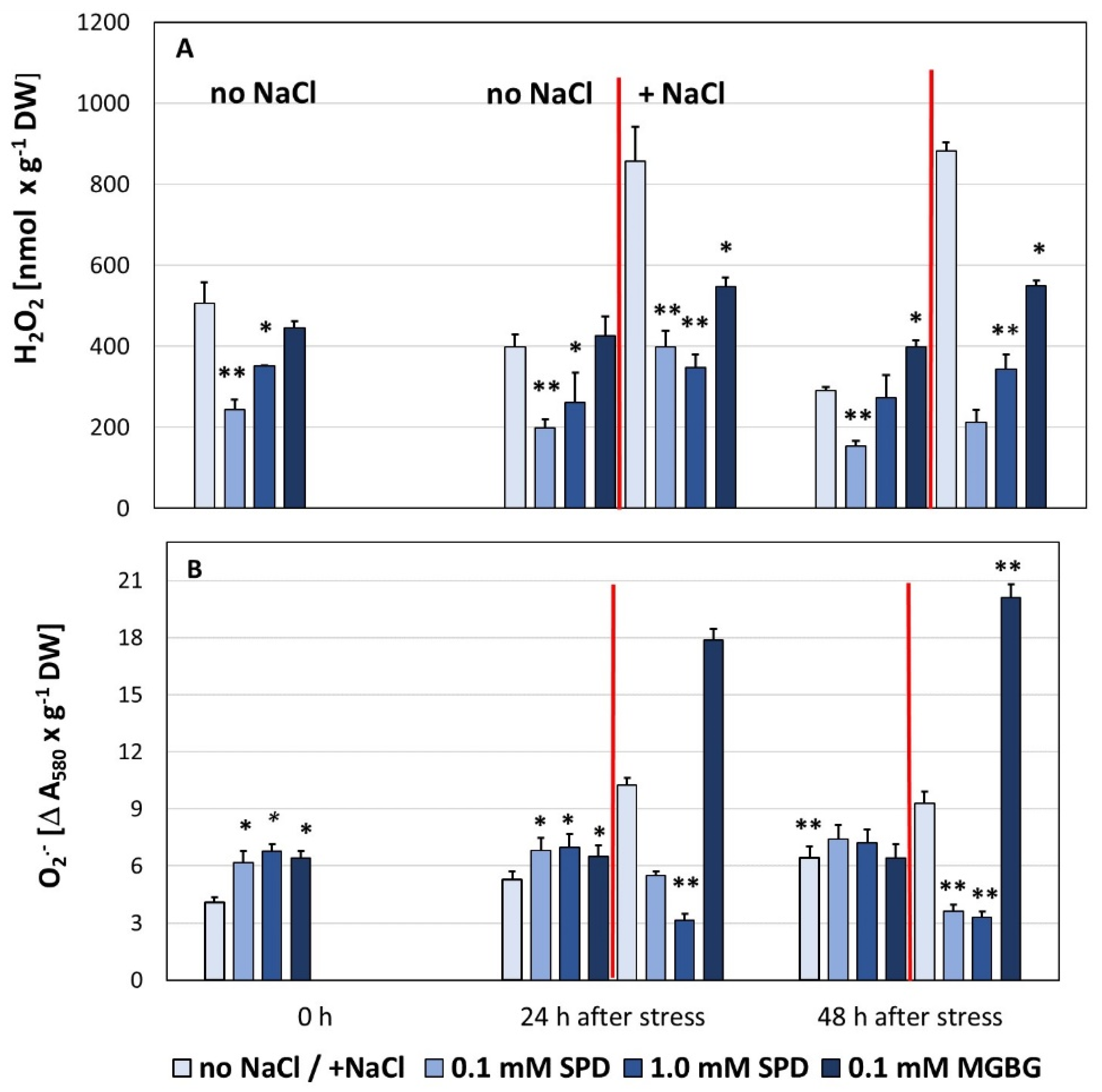
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bagni, N.; Tassoni, A. Biosynthesis, oxidation and conjugation of aliphatic polyamines in higher plants. Amino Acids 2001, 20, 301–317. [Google Scholar] [CrossRef] [PubMed]
- Tassoni, A.; Franceschetti, M.; Bagni, N. Polyamines and salt stress response and tolerance in Arabidopsis thaliana flowers. Plant Physiol. Biochem. 2008, 46, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Bouchereau, A.; Aziz, A.; Larher, F.; Martin-Tanguy, J. Polyamines and environmental challenges: Recent development. Plant Sci. 1999, 140, 103–125. [Google Scholar] [CrossRef]
- Kusano, T.; Yamaguchi, K.; Berberich, T.; Takahashi, Y. Advances in polyamine research in 2007. J. Plant Res. 2007, 120, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Groppa, M.D.; Benavides, M.P. Polyamines and abiotic stress: Recent advances. Amino Acids 2008, 34, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Todorova, D.; Katerova, Z.; Sergiev, I.; Alexieva, V. Role of Polyamines in Alleviating Salt Stress. In Ecophysiology and Responses of Plant under Salt Stress; Ahmad, P., Azooz, M.M., Prasad, M.N.V., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; Chapter 13; pp. 335–379. [Google Scholar]
- Sharma, A.; Garg, N. Polyamines: A promising strategy for imparting salinity stress tolerance in legumes. In Abiotic Stress and Legumes; Singh, V.P., Singh, S., Tripathi, D.K., Prasad, S.M., Bhardwaj, R., Eds.; Devendra Kumar Chauhan Academic Press: Cambridge, MA, USA, 2021; pp. 137–174. ISBN 9780128153550. [Google Scholar]
- Ebeed, H.T.; Hassan, N.M.; Aljarani, A.M. Exogenous applications of Polyamines modulate drought responses in wheat through osmolytes accumulation, increasing free polyamine levels and regulation of polyamine biosynthetic genes. Plant Physiol. Biochem. 2017, 118, 438–448. [Google Scholar] [CrossRef]
- Liu, K.; Fu, H.; Bei, Q.; Luan, S. Inward potassium channel in guard cells as a target for polyamine regulation of stomatal movements. Plant Physiol. 2000, 124, 1315–1326. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Rodríguez, E.; Romero, L.; Ruiz, J.M. Accumulation of free polyamines enhances the antioxidant response in fruits of grafted tomato plants under water stress. J. Plant Physiol. 2016, 190, 72–78. [Google Scholar] [CrossRef]
- Isayenkov, S.V.; Maathuis, F.J.M. Plant Salinity Stress: Many Unanswered Questions Remain. Front. Plant Sci. 2019, 10, 80. [Google Scholar] [CrossRef] [Green Version]
- Legocka, J.; Kluk, A. Effect of salt and osmotic stress on changes in polyamine content and arginine decarboxylase activity in Lupinus luteus seedlings. J. Plant Physiol. 2005, 162, 662–668. [Google Scholar] [CrossRef]
- Zhu, J.K. Plant Salt Tolerance. Trends Plant Sci. 2001, 6, 66–71. [Google Scholar] [CrossRef]
- Zapata, P.J.; Serrano, M.; Pretel, M.T.; Amoros, A.; Botella, M.A. Changes in ethylene evolution and polyamine profiles of seedlings of nine cultivars of Lactuca sativa L. in response to salt stress during germination. Plant Sci. 2003, 164, 557–563. [Google Scholar] [CrossRef]
- Zapata, P.J.; Serrano, M.; Pretel, M.T.; Amorós, A.; Botella, M.A. Polyamines and ethylene changes during germination of different plant species under salinity. Plant Sci. 2004, 167, 781–788. [Google Scholar] [CrossRef]
- Zapata, P.J.; Serrano, M.; Pretel, M.T.; Botella, M.A. Changes in free polyamine concentration induced by salt stress in seedlings of different species. Plant Growth Regul. 2008, 56, 167–177. [Google Scholar] [CrossRef]
- Zhao, F.G.; Sun, C.; Liu, Y.L.; Zhang, W.H. Relationship between polyamine metabolism in roots and salt tolerance of barley seedlings. Acta Bot. Sin. 2003, 45, 295–300. [Google Scholar]
- Kirshnamurthy, R.; Bhagwat, K.A. Polyamines as modulators of salt tolerance in rice cultivars. Plant Physiol. 1989, 91, 500–504. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Zhang, Y.; Shi, Y.; Zhang, Z.; Zou, Z.; Zhang, H.; Zhao, J. Effect of exogenous spermidine on polyamine content and metabolism in tomato exposed to salinity–alkalinity mixed stress. Plant Physiol. Biochem. 2012, 57, 200–209. [Google Scholar] [CrossRef]
- Liu, J.H.; Wang, W.; Wu, H.; Gong, X.; Moriguchi, T. Polyamines function in stress tolerance: From synthesis to regulation. Front. Plant Sci. 2015, 6, 827. [Google Scholar] [CrossRef] [Green Version]
- Rathinapriya, P.; Pandian, S.; Rakkammal, K.; Balasangeetha, M.; Alexpandi, R.; Satish, L.; Rameshkumar, R.; Ramesh, M. The protective effects of polyamines on salinity stress tolerance in foxtail millet (Setaria italica L.), an important C4 model crop. Physiol. Mol. Biol. Plants 2020, 26, 1815–1829. [Google Scholar] [CrossRef]
- Simon-Sarkadi, L.; Kocsy, G.; Sebestyén, Z. Effect of salt stress on free amino acid and polyamine content in cereals. Acta Biol. Szeged. 2002, 46, 73–75. [Google Scholar]
- Zapata, P.J.; Serano, M.; Garcia-Legaz, M.F.; Pretel, M.T.; Botella, M.A. Short term effect of salt shock on ethylene and polyamines depends on plant salt sensitivity. Front. Plant Sci. 2017, 8, 855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minocha, R.; Majumdar, R.; Minocha, S.C. Polyamines and abiotic stress in plants: A complex relationship. Front. Plant Sci. 2014, 5, 175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tailor, A.; Tandon, R.; Bhatla, S.C. Nitric oxide modulates polyamine homeostasis in sunflower seedling cotyledons under salt stress. Plant Signal. Behav. 2019, 14, 11. [Google Scholar] [CrossRef]
- Chen, D.; Shao, Q.; Yin, L.; Younis, A.; Zheng, B. Polyamine Function in Plants: Metabolism, Regulation on Development, and Roles in Abiotic Stress Responses. Front. Plant Sci. 2019, 9, 1945. [Google Scholar] [CrossRef] [PubMed]
- Malepszy, S.; Przybecki, Z.; Kowalczuk, C.; Filipecki, M. Genome sequencing makes a significant progress in plant breednig. Kosmos 2012, 3, 467–475. (In Polish) [Google Scholar]
- Wóycicki, R.; Witkowicz, J.; Gawroński, P.; Dąbrowska, J.; Lomsadze, A.; Pawełkowicz, M.; Śmiech, M. The genome sequence of the North-European cucumber (Cucumis sativus L.) unravels evolutionary adaptation mechanisms in plants. PLoS ONE 2011, 6, e22728. [Google Scholar]
- Weatherley, P.E. Studies in water relations of cotton plants. I. The field measurement of water deficits in leaves. New Phytol. 1950, 49, 81–97. [Google Scholar] [CrossRef]
- Flores, H.E.; Galston, A.W. Analysis of polyamines in higher plants by high performance liquid chromatography. Plant Physiol. 1982, 69, 701–706. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilising the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Hammerschmidt, R.; Nuckles, E.M.; Kuć, J. Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Physiol. Plant Pathol. 1982, 20, 73–82. [Google Scholar] [CrossRef]
- Aebi, H.E. Catalase. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; Verlag Chemie: Weinhem, Germany, 1983; Volume 3, pp. 273–287. [Google Scholar]
- Asada, K. Chloroplasts: Formation of active oxygen and its scavenging. In Methods in Enzymology; Packer, L., Ed.; Academic Press: New York, NY, USA, 1984; Volume 105, pp. 422–429. [Google Scholar]
- Smirnoff, N.; Colombe, S.V. Drought influences the activity of enzymes of the chloroplast hydrogen peroxide scavenging system. J. Exp. Bot. 1988, 39, 1097–1108. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–278. [Google Scholar] [CrossRef]
- Becana, M.; Aparicio-Tejo, P.; Irigoyen, J.J.; Sanchez-Diaz, M. Some enzymes of peroxide metabolism in leaves and root nodules of Medicago sativa. Plant Physiol. 1986, 82, 1169–1171. [Google Scholar] [CrossRef] [Green Version]
- Doke, N. Involvement of superoxide anion generation in the hypersensitive response of potato tuber tissues to infection with an incompatible race of Phytophthora infestans and the hyphal wall components. Physiol. Plant Pathol. 1983, 23, 345–357. [Google Scholar] [CrossRef]
- Ludwig, M.; Wilmes, P.; Schrader, S. Measuring soil sustainability via soil resilience. Sci. Total Environ. 2018, 626, 1484–1493. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Wang, Y.; Yan, Y.; Liu, Y.; Wang, J.; Chen, S. A Review on Plant Responses to Salt Stress and Their Mechanisms of Salt Resistance. Horticulturae 2021, 7, 132. [Google Scholar] [CrossRef]
- Wu, J.; Shu, S.; Li, C.; Sun, J.; Guo, S. Spermidine-mediated hydrogen peroxide signalling enhances the antioxidant capacity of salt-stressed cucumber roots. Plant Physiol. Biochem. 2018, 128, 152–162. [Google Scholar] [CrossRef]
- Chattopadhayay, M.K.; Tiwari, B.S.; Chattopadhyay, G.; Bose, A.; Sengupta, D.N.; Ghosh, B. Protective role of exogenous polyamines on salinity-stressed rice (Oryza sativa) plants. Physiol. Plant. 2002, 116, 192–199. [Google Scholar] [CrossRef]
- Duan, J.; Li, J.; Guo, S.; Kang, Y. Exogenous spermidine affects polyamine metabolism in salinity-stressed Cucumis sativus roots and enhances short-term salinity tolerance. J. Plant Physiol. 2008, 165, 1620–1635. [Google Scholar] [CrossRef]
- Puyang, X.; An, M.; Han, L.; Zhang, X. Protective effect of spermidine on salt stress induced oxidative damage in two Kentucky bluegrass (Poa pratensis L.) cultivars. Ecotoxycol. Environ. Saf. 2015, 117, 96–106. [Google Scholar] [CrossRef]
- Li, S.; Jin, H.; Zhang, Q. The effect of exogenous spermidine concentration on polyamine metabolism and salt tolerance inzoysiagrass (Zoysia japonica Steud) subjected to short-term salinity stress. Front. Plant Sci. 2016, 7, 1221. [Google Scholar] [PubMed] [Green Version]
- Roychoudhury, A.; Basu, S.; Sengupta, D.N. Amelioration of salinity stress by exogenously applied spermidine or spermine in three varieties of indica rice differing in their level of salt tolerance. J. Plant Physiol. 2011, 168, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.T.; Ye, T.T.; Chan, Z.I. Comparative proteomic and physiological analyses reveal the protective effect of exogenous polyamines in the bermudagrass (Cynodon dactylon) response to salt and drought stresses. J. Proteome Res. 2013, 12, 4951–4964. [Google Scholar] [CrossRef] [PubMed]
- Baniasadi, F.; Saffari, V.R.; Maghsoudi Moud, A.A. Physiological and growth responses of Calendula officinalis L. plants to the interaction effects of polyamines and salt stress. Sci. Hortic. 2018, 34, 312–317. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Peng, D.; Wang, X.; Peng, Y.; He, X.; Zhang, X.; Ma, X.; Huang, L.; Yan, Y. Corrigendum: Polyamine regulates tolerance to water stress in leaves of white clover associated with antioxidant defence and dehydrin genes via involvement in calcium messenger system and hydrogen peroxide signalling. Front. Physiol. 2016, 7, 52. [Google Scholar] [CrossRef]
- Moschou, P.N.; Paschalidis, K.A.; Delis, I.D.; Andriopoulou, A.H.; Lagiotis, G.D.; Yakoumakis, D.I.; Roubelakis-Angelakis, K.A. Spermidine exodus and oxidation in the apoplast induced by abiotic stress is responsible for H₂O₂ signatures that direct tolerance responses in tobacco. Plant Cell 2008, 20, 1708. [Google Scholar] [CrossRef] [Green Version]
- Andronis, E.A.; Moschou, P.N.; Imene, T.; Roubelakis-Angelakis, K.A. Peroxisomal polyamine oxidase and NADPH-oxidase cross-talk for ROS homeostasis which affects respiration rate in Arabidopsis thaliana. Front. Plant Sci. 2014, 5, 132. [Google Scholar] [CrossRef] [Green Version]
- Pál, M.; Szalai, G.; Janda, T. Speculation: Polyamines are important in abiotic stress signalling. Plant Sci. 2015, 237, 16. [Google Scholar] [CrossRef] [Green Version]
- Verma, S.; Mishra, S.N. Putrescine alleviation of growth in salt stressed Brassica juncea by inducing antioxidative defence system. J. Plant Physiol. 2005, 162, 669–677. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A revive. Ecotoxycol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef]
- Chourasia, K.N.; Lal, M.K.; Tiwari, R.K.; Dev, D.; Kardile, H.B.; Patil, V.U.; Kumar, A.; Vanishree, G.; Kumar, D.; Bhardwaj, V.; et al. Salinity Stress in Potato: Understanding Physiological, Biochemical and Molecular Responses. Life 2021, 11, 545. [Google Scholar] [CrossRef] [PubMed]
- Ahanger, M.A.; Qin, C.; Maodong, Q.; Dong, X.X.; Ahmad, P.; Abd_Allah, E.F.; Zhang, L. Spermine application alleviates salinity induced growth and photosynthetic inhibition in Solanum lycopersicum by modulating osmolyte and secondary metabolite accumulation and differentially regulating antioxidant metabolism. Plant Physiol. Biochem. 2019, 144, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ekİncİ, M.; Yildirim, E.; Dursun, A.; Mohamedsrajaden, N. Putrescine, Spermine and Spermidine Mitigated the Salt Stress Damage on Pepper (Capsicum annum L.) Seedling. YYU J. Agric. Sci. 2019, 29, 290–299. [Google Scholar]
| Traits | Significance on the Level α = 0.05 | Correlation Coefficient (r) |
|---|---|---|
| guaiacol peroxidase activity | significant | 0.69 |
| catalase activity | significant | 0.27 |
| ascorbate peroxidase activity | ns | 0.11 |
| superoxide dismutase activity | significant | 0.24 |
| relative water content | significant | −0.46 |
| hydrogen peroxide content | significant | −0.17 |
| superoxide anion | significant | −0.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korbas, A.; Kubiś, J.; Rybus-Zając, M.; Chadzinikolau, T. Spermidine Modify Antioxidant Activity in Cucumber Exposed to Salinity Stress. Agronomy 2022, 12, 1554. https://doi.org/10.3390/agronomy12071554
Korbas A, Kubiś J, Rybus-Zając M, Chadzinikolau T. Spermidine Modify Antioxidant Activity in Cucumber Exposed to Salinity Stress. Agronomy. 2022; 12(7):1554. https://doi.org/10.3390/agronomy12071554
Chicago/Turabian StyleKorbas, Agata, Jan Kubiś, Magdalena Rybus-Zając, and Tamara Chadzinikolau. 2022. "Spermidine Modify Antioxidant Activity in Cucumber Exposed to Salinity Stress" Agronomy 12, no. 7: 1554. https://doi.org/10.3390/agronomy12071554
APA StyleKorbas, A., Kubiś, J., Rybus-Zając, M., & Chadzinikolau, T. (2022). Spermidine Modify Antioxidant Activity in Cucumber Exposed to Salinity Stress. Agronomy, 12(7), 1554. https://doi.org/10.3390/agronomy12071554






